N-cadherin Restrains PTH Activation of Lrp6/β-catenin Signaling and Osteoanabolic Action
ABSTRACT
Interaction between parathyroid hormone/parathyroid hormone–related peptide receptor 1 (PTHR1) and low-density lipoprotein receptor–related protein 6 (Lrp6) is important for parathyroid hormone (PTH) signaling and anabolic action. Because N-cadherin has been shown to negatively regulate canonical Wnt/β-catenin signaling, we asked whether N-cadherin alters PTH signaling and stimulation of bone formation. Ablation of the N-cadherin gene (Cdh2) in primary osteogenic lineage cells resulted in increased Lrp6/PTHR1 interaction in response to PTH1-34, associated with enhanced PTH-induced PKA signaling and PKA-dependent β-catenin C-terminus phosphorylation, which promotes β-catenin transcriptional activity. β-catenin C-terminus phosphorylation was abolished by Lrp6 knockdown. Accordingly, PTH1-34 stimulation of Tcf/Lef target genes, Lef1 and Axin2, was also significantly enhanced in Cdh2-deficient cells. This enhanced responsiveness to PTH extends to the osteo-anabolic effect of PTH, as mice with a conditional Cdh2 deletion in Osx+ cells treated with intermittent doses of PTH1-34 exhibited significantly larger gains in trabecular bone mass relative to control mice, the result of accentuated osteoblast activity. Therefore, N-cadherin modulates Lrp6/PTHR1 interaction, restraining the intensity of PTH-induced β-catenin signaling, and ultimately influencing bone formation in response to intermittent PTH administration. © 2014 American Society for Bone and Mineral Research.
Introduction
Parathyroid hormone (PTH), whose main physiologic function is to maintain serum calcium homeostasis and stimulate bone resorption, can also exert a bone anabolic effect if administered intermittently.1, 2 The classic model of PTH signal transduction in bone-forming cells views PTH binding to the PTH receptor 1 (PTHR1) to activate two signaling cascades, the Gs/protein kinase A (PKA) and Gq/protein kinase C (PKC).3 However, the downstream consequences of PTHR1 activation are more complex than a simple bipartite signaling flow would predict, as these two pathways intersect other signaling networks, including the Wnt/β-catenin system, a major driver of skeletal development and maintenance.4 Lipoprotein receptor–related protein 5 or 6 (Lrp5 or Lrp6) act as co-receptors for Frizzled (Fzl) upon binding of a Wnt ligand resulting in activation of β-catenin and Tcf/Lef-mediated gene transcription.5 Abnormal expression of members of Wnt/β-catenin pathway lead to a wide variety of skeletal phenotypes and diseases.6 Specifically, interference with Lrp5 or Lrp6 expression or function affects osteoblast differentiation, skeletal development, and postnatal bone maintenance.7, 8 Likewise, β-catenin is essential at specific steps of osteoprogenitors' progression to matrix-producing osteoblasts.9, 10 Recent work has shown that Lrp6 is required for PTH signaling and bone anabolic effect.11, 12 Specifically, PTH binding to PTHR1/Lrp6 complex leads to activation of β-catenin signaling, culminating in upregulation of Tcf/Lef-mediated transcription and stimulation of bone formation.11-14 Interestingly, PTH-induced activation of β-catenin and Tcf/Lef transcription are largely reduced upon inhibition of PKA,13 and PTH enhances β-catenin/PKA interaction in osteoblastic cells.11 Collectively, these findings suggest that PKA and β-catenin signaling systems intersect after activation by PTH.
β-catenin is also an important component of adherens junctions,15 where it stabilizes the adhesion structure by linking the cell adhesion molecules, cadherins, to the actin cytoskeleton.16 N-cadherin is abundantly present in osteogenic lineage cells, and in vivo ablation or overexpression of the N-cadherin gene (Cdh2) in osteogenic cells impairs postnatal bone acquisition.17, 18 In addition to their role in cell-cell adhesion, cadherins are recognized as modulators of intracellular signaling networks.19, 20 Indeed, an inverse relationship between adhesion and signaling has been proposed, hinging upon cadherin abundance.21 According to this model, cadherins can keep β-catenin bound at the cell surface in adherens junctions by decreasing the pool of cytoplasmic β-catenin available for signaling.15 An alternative model of N-cadherin interference with Wnt/β-catenin has been proposed in osteoblasts, where N-cadherin binds to Lrp5 or Lrp6 via axin. This interaction keeps either receptor tethered to N-cadherin and promotes β-catenin degradation.18, 22 N-cadherin/axin/Lrp5/6 interaction also prevents ERK1/2 and PI3K/Akt signaling, resulting in reduced osteoblast proliferation, differentiation, and survival.23 Conversely, disruption of this interaction allows activation of such signaling cascades, ultimately resulting in increased cell proliferation and differentiation.24 Because Lrp6 is involved in PTH signaling and anabolic effect, we hypothesized that N-cadherin may modulate PTH responses by regulating Lrp6 availability to participate in PTH/PTHR1 signaling. This premise would be consistent with observations that Lrp5 and Lrp6 are involved in signaling activated by other G-protein–coupled receptors, aside from PTHR1.14, 25 To test this hypothesis, we studied PTH signaling and anabolic effect in mice with Cdh2 ablation in Osx+ cells.26 Results show that, as predicted by the above model, N-cadherin modulates PTH-induced signaling and bone formation by functioning as a “buffer” for Lrp6 availability.
Materials and Methods
Transgenic mice
Osx-GFP::Cre (Osx-Cre),10 Cdh2f/f,27 and Osx-Cre::Cdh2f/f26 mice have been previously described. Osx-Cre::Cdh2f/f mice were a generous gift by Daniel Link, Washington University in St. Louis. Lrp6f/f mice were a generous gift from Bart Williams, Van Andel Research Institute at Grand Rapids, MI. All the mouse lines used for this study were produced in a C57BL/6 background, and littermate mice were used as control. Genotyping was performed by PCR on genomic DNA extracted from mouse tails using the HotSHOT method.28 Primers to detect Cdh2fl allele have been described previously.17 Primers to detect Osx-GFP::Cre allele were: F, 5′-catcaaggtgaacttcaag-3′ and R, 5′-gctgtcacttggtcgtg-3′. They yield one PCR product at 583 bp corresponding to Osx-GFP::Cre-specific amplicon. Primers to detect Lrp6f/f allele were: E2, 5′-GCATGTGATTGGCTTGGAGA-3′; Neo, 5′-GCCTTCTATCGCCTTCTTGAC-3′; and WT, 5′-CAGACAGACATCCAGGAA-3′. They yield two PCR products at 550 bp and 450 bp corresponding to the flox or wild-type allele, respectively. Mice were fed regular chow ad libitum and housed in a room maintained at constant 25°C temperature on a 12-hour light/dark cycle. All procedures were approved by the Animal Studies Committee of Washington University.
Bone microstructure
Changes in bone microstructure, before and after vehicle or PTH treatment, were determined by in vivo microcomputed tomography (vivaCT 40, Scanco Medical AG, Brüttisellen, Switzerland), as previously described.26
Bone histology and histomorphometric analysis
Bone samples were prepared as previously described.29 Quantitative histomorphometry was performed using a commercial software (OSTEO 2010 V10.3.6, Bioquant, Nashville, TN, USA), and standard bone parameters were determined as described before.29
Cell culture
Bone marrow stromal cells (BMSC) were harvested and cultured as described previously.26 Once confluent, BMSC were seeded in a 6-well plate at 1,000,000 cells per well and cultured for 5 days in media with mineralization cocktail (50 µg/mL ascorbic acid and 10 µM β-glycerophosphate). Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Quantitative real-time PCR and primers
Messenger RNA (mRNA) was isolated from BMSC exposed to vehicle, PTH1-34, or Wnt3a for 6 hours in serum-free α-MEM. Messenger RNA was then extracted with NucleoSpin RNA II (Macherey-Nagel Inc., Bethlehem, PA, USA, #740955.250) according to manufacturer's instructions, and was reverse transcribed into cDNA using RNA to cDNA EcoDry Premix Double Primed (Clontech, Mountain View, CA, USA, #ST0335). Data were normalized to Cyclophilin, analyzed with ΔΔCT method, and expressed relative to the average of vehicle-treated Cdh2f/f or Cdh2-cKO samples, as described previously.30 SYBR Gene expression assays (Applied Biosystems, Carlsbad, CA, USA, #4309155) was used for JunB (F, 5′-TCACGACGACTCTTACGCAG-3′; R, 5′-CCTTGAGACCCCGATAGGGA-3′), Lef-1 (F, 5′-TGTTTATCCCATCACGGGTGG-3′; R, 5′-CATGGAAGTGTCGCCTGACAG-3′), and Axin-2 (F, 5′-ATGTGTGGATACGCTGGACTT-3′; R, 5′-TTCTTGATGCCATCTCGTATG). Taqman Gene expression assays (Applied Biosystems, #4444557) was used for Cdh2 (Applied Biosystems, #Mm01162490_m1) and RANKL (Applied Biosystems, #Mm00441908_m1).
Statistics
Group means were analyzed by analysis of variance (ANOVA) followed by post hoc analysis for multiple group comparisons with the level of significance for comparison set at p < 0.05. Analyses were performed using SigmaPlot Vs11.0 (Systat, San Jose, CA, USA). Data are presented as mean ± SD.
Results
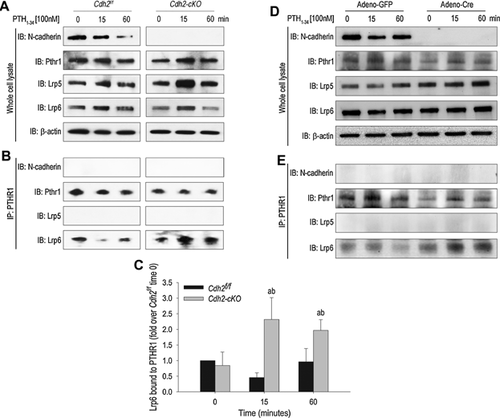
Absence of N-cadherin enhances PTHR1/Lrp6 interaction upon PTH stimulation in osteoblasts
BMSC harvested from Osx-Cre::Cdh2f/f (Cdh2-cKO) mice were exposed to either PTH1-34 (100 nM) or vehicle. BMSC from Cdh2f/f mice were used as control. Western blots (WB) analyses from whole-cell lysates cells show expression of N-cadherin, PTHR1, Lrp5, and Lrp6 but absence of N-cadherin in Cdh2-cKO lysates (Fig. 1A), confirming effective Cdh2 ablation. Absence of N-cadherin did not affect the abundance of any of these receptors (Fig. 1A). In immunoprecipitates using a PTHR1 antibody, we detected bands reactive to Lrp6 but not Lrp5 or N-cadherin antibodies (Fig. 1B), suggesting that PTHR1 interacts with Lrp6 but not Lrp5 or N-cadherin. Intriguingly, the intensity of the Lrp6 band in the PTHR1 immunoprecipitates increased after 15- and 60-minute exposure to PTH1-34 in Cdh2-cKO, contrasting with a modest decrease of Lrp6/PTHR1 complexes in control BMSC at 15 minutes, and return to basal levels by 60 minutes (Fig. 1B, C). These results were reproduced by in vitro Cdh2 ablation using an adenovirus vector expressing Cre (Adeno-Cre) in Cdh2f/f BMSC. A stronger Lrp6 band was observed in PTHR1 immunoprecipitates of Adeno-Cre–transduced cells in response to PTH1-34, relative to BMSC transduced with a GFP-expressing vector (Adeno-GFP) (Fig. 1D, E). These results suggest that in the absence of N-cadherin, PTHR1/Lrp6 interaction is enhanced in response to PTH1-34.
Enhanced β-catenin C-terminus phosphorylation by PTH1-34 in N-cadherin–deficient osteoblastic cells
We next determined the consequences of Cdh2 ablation on downstream events activated in response to PTH1-34. Immunoblots of Cdh2f/f whole-cell lysates revealed that CREB phosphorylation increased 15 and 60 minutes after PTH1-34 exposure, returning to basal levels by 120 minutes (Fig. 2A, B). On the other hand, CREB phosphorylation increased continuously in Cdh2-cKO, and at 120 minutes it was significantly higher than in Cdh2f/f cells (Fig. 2A, B). Total CREB abundance was unaffected in either cell genotype, whereas N-cadherin abundance tended to decrease with time (Fig. 2A), an effect also noted in previous experiments (Fig. 1A, D). Importantly, β-catenin phosphorylation at S675 increased in a time-dependent manner in response to PTH1-34 in control and Cdh2-cKO cells, whereas total β-catenin abundance was not affected (Fig. 2C). S675 is targeted by PKA, and phosphorylation at this site promotes activation of β-catenin downstream signaling.31 Quantitative densitometric analysis revealed that although p-β-catenin (S675) was less abundant in mutant relative to control cells at baseline (Fig. 2C), it increased to a significantly larger extent in Cdh2-cKO than in control cells upon PTH1-34 exposure (Fig. 2D).
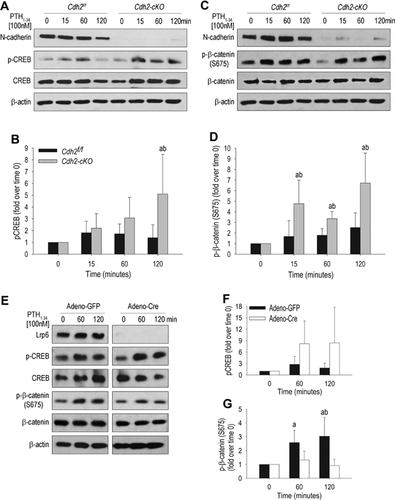
Although total β-catenin was unaffected in response to PTH1-34 (Fig. 2C, E), nuclear β-catenin abundance increased after 2-hour exposure to PTH1-34 in MC3T3-E1 cells and continued to increase at later time points (Supplemental Fig. S1A). This result is consistent with previous reports of enhanced β-catenin nuclear levels in response to PTH1-34 in MC3T3-E1 cells and primary osteoblasts.11 Interestingly, in immunoblots using an antibody that recognizes nonphosphorylated β-catenin at serine 33/37 and threonine 43 (S33/S37/T43), we found no difference in β-catenin abundance in the presence or in the absence of PTH1-34 and/or N-cadherin (Supplemental Fig. S1B, C). Furthermore, in the presence of the PKA inhibitor H-89,32 PTH1-34 failed to increase both pCREB and p-β-catenin (S675) in control and Cdh2-cKO cells (Supplemental Fig. S1D–F). Densitometric quantification confirmed that PTH1-34 increased p-β-catenin (S675) to a larger magnitude in Cdh2-cKO than in control cells, an event that was inhibited in the presence of H-89 (Supplemental Fig. S1F). To determine whether the enhanced PTH1-34 induced β-catenin S675 phosphorylation in N-cadherin–deficient cells is linked to increased Lrp6/PTHR1 interaction, we ablated Lrp6 in BMSC isolated from Lrp6fl/fl mice using Adeno-Cre. PTH1-34 upregulation of CREB phosphorylation still occurred in the absence of Lrp6 (Fig. 2E, F). Conversely, as others' data would predict,13, 14 Lrp6 deletion prevented PTH-induced β-catenin S675 phosphorylation (Fig. 2E, G). Therefore, PTH1-34-induced β-catenin phosphorylation at S675 requires Lrp6, and activation of the PKA pathway is necessary but not sufficient for this effect.
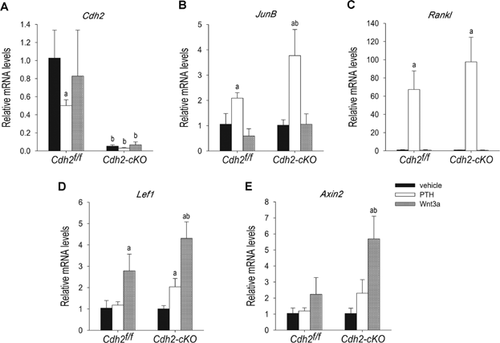
Enhanced induction of Tcf/Lef-dependent gene expression by PTH1-34 in Cdh2-ablated osteoblasts
To determine the consequences of N-cadherin loss on PTH-induced gene expression, we next examined mRNA abundance of known CREB and Tcf/Lef target genes in control and Cdh2-ablated cells and in in vitro recombined Cdh2f/f cells. In keeping with the trend observed with the immunoblot studies, Cdh2 mRNA were reduced in PTH1-34-treated Cdh2f/f BMSC, whereas Wnt3a had no effect (Fig. 3A). Such a decrease in Cdh2 mRNA was not found in Adeno-GFP–transduced Cdh2f/f cells (Supplemental Fig. S2A). We then examined mRNA levels of JunB and Rankl, two known CREB-signaling target genes.33, 34 As expected, PTH1-34, but not Wnt3a, upregulated JunB and Rankl mRNA. Importantly, the effect of PTH1-34 on JunB mRNA was significantly larger in Cdh2-cKO than in Cdh2f/f cells (Fig. 3B). The same trend was observed for PTH1-34 stimulation of Rankl mRNA in BMSC cells (Fig. 3C) and in vitro recombined cells (Supplemental Fig. S2C); however, these trends were not statistically significant. PTH1-34 did not affect JunB mRNA in adenovirally transduced cells (Supplemental Fig. S2B). Expression of Lef-1 and Axin-2 mRNA, two known Tcf/Lef target genes,35, 36 was not affected by PTH1-34 in control cells. Conversely, PTH1-34 elicited a two-fold increase in Lef1 and Axin2 mRNA in Cdh2-cKO BMSC (Fig. 3D, E). The effect of PTH1-34 on Lef1 and Axin2 mRNA was less evident in Adeno-Cre–deleted cells (Supplemental Fig. S2D). Wnt3a induced a significantly larger increase of Lef1 and Axin2 mRNA relative to PTH1-34, and this response was also more robust in the absence than in the presence of N-cadherin (Fig. 3D, E, and Supplemental Fig. S2D, E). Consistent with previous reports,13 PTH1-34 stimulated the activity of a Tcf/Lef-luciferase reporter in MC3T3-E1 cells, although this effect was far less potent than that produced by Wnt3a (Supplemental Fig. S2F). As predicted by the PKA dependency of PTH1-34-induced activation of β-catenin signaling, PTH1-34 failed to activate Tcf/Lef-luciferase in the presence of H-89, which by itself reduced basal reporter activity (Supplemental Fig. S2F).
Accentuated trabecular bone accrual in response to intermitted PTH1-34 treatment in Cdh2-cKO mice
Our in vitro findings suggest that ablation of Cdh2 in bone-forming cells enhances responsiveness to PTH and Wnt signals. Because the Wnt/β-catenin pathway is important for PTH anabolic action,12, 37-39 we next asked whether absence of N-cadherin alters responsiveness to intermittent administration of PTH in vivo (iPTH). Consistent with previous reports,17, 26 trabecular bone mass was more sparse in Cdh2-cKO mice compared with Cdh2f/f littermates at baseline (Fig. 4Ai, ii). Accordingly, trabecular bone volume relative to total bone volume (BV/TV), bone mineral density (BMD), trabecular number (Tb.N), and trabecular thickness (Tb.Th) were all lower in mutant compared with control mice at baseline (Fig. 4B–E). In both genotypes, trabecular bone mass, BV/TV, BMD, and Tb.Th increased, whereas Tb.N decreased in response to PTH1-34 (Fig. 4Aiii, iv, B–E). Nonetheless, the percent changes in all these parameters were significantly greater in Cdh2-cKO relative to Cdh2f/f littermates (Fig. 4B–E). Because of the differences between genotypes at baseline, we also compared the absolute changes induced by PTH1-34 in the two groups of mice. For all parameters examined, the effect of iPTH administration was significantly larger in Cdh2-cKO relative to Cdh2f/f littermates (Supplemental Fig. S3A–C).
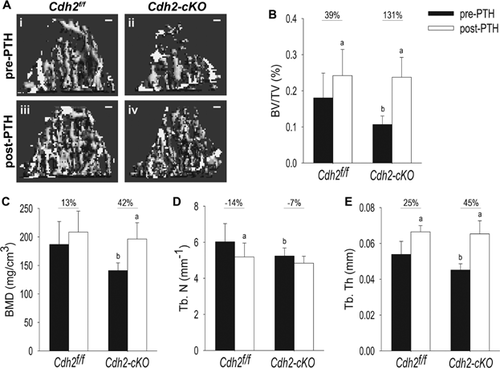
Delayed cortical postnatal bone accrual has been previously reported in Osx-GFP::Cre (Osx-Cre) mice, a defect that resolves by 12 weeks of age.40 To control for a possible effect of the Osx-Cre transgene and for body growth during the month of treatment, 3-month-old male Cdh2f/f, Cdh2-cKO, and Osx-Cre mice were administered vehicle for 4 weeks; no significant changes in trabecular BV/TV, BMD, or Tb.Th were detected at the end of the treatment regimen, although there was a nonsignificant upward trend in the Cdh2-cKO group (Supplemental Fig. S4A–D). Because Osx-Cre mice had higher bone mass, density, and trabecular thickness than the other groups, it is unlikely that the larger changes effected by iPTH in the Cdh2-cKO mice had been conditioned by the Osx-Cre transgene; in fact, the effect might have been dampened.
Histological analysis confirmed more rarefied metaphyseal trabeculation in vehicle-treated Cdh2-cKO mice relative to controls (Fig. 5Ai, ii). Also consistent with vivaCT data, Cdh2-cKO exhibited a larger percent bone gain in trabecular BV/TV in response to iPTH treatment compared with Cdh2f/f mice (Fig. 5Aiii, iv, B). Osteoblast surface was only modestly decreased in vehicle-treated Cdh2-cKO mice compared with control, and a nonsignificant upward trend was observed in response to iPTH only in mutant mice (Fig. 5C). Further, iPTH treatment significantly increased osteocyte number to a similar extent in both mouse groups (Fig. 5D), and we found no differences in trabecular osteoclast number between genotypes and no effect of iPTH treatment (Supplemental Fig. S5A, B). However, serum cross-linked C-telopeptide (CTX), a biochemical marker of bone resorption, significantly and similarly increased in response to iPTH in both groups (Supplemental Fig. S5C). There were no effects of vehicle in either genotype.
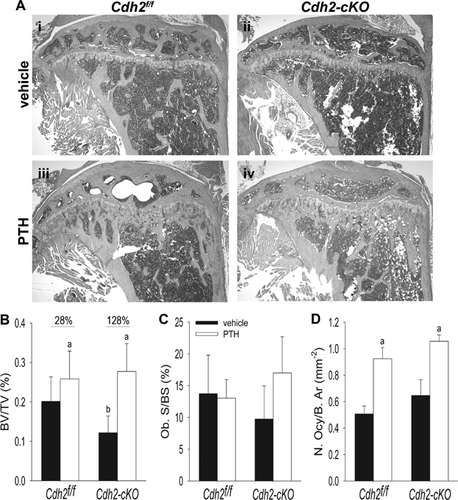
Accentuated trabecular bone formation, but not cortical response, to anabolic treatment with PTH1-34 in conditionally Cdh2-ablated mice
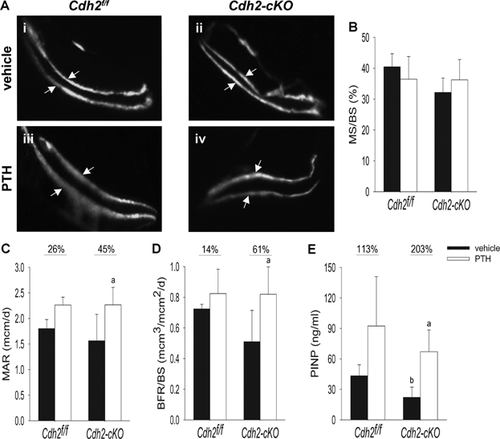
Consistent with significant bone mass accrual in the trabecular compartment, dynamic bone histomorphometry revealed that iPTH increased the distance between the two calcein labels, relative to vehicle-treated mice, in both control and Cdh2-cKO animals (Fig. 6Ai, iii and ii, iv, respectively), indicative of enhanced bone formation. We observed no significant differences in mineralizing surfaces between PTH1-34 and vehicle-treated animals in either genotype (Fig. 6B), suggesting that, at least in these animals, iPTH stimulated bone formation by promoting activation of existing osteoblasts rather than by increasing their number. Mineral apposition rate (MAR) and bone formation rate per bone surface (BFR/BS) were higher in iPTH-treated mice relative to vehicle-treated animals in both genotype groups. However, these differences were larger and statistically significant only between PTH1-34-treated and vehicle-treated Cdh2-cKO mice (Fig. 6C, D). Similar patterns were observed with serum P1NP, a biochemical marker of bone formation, which was significantly lower in vehicle-treated Cdh2-cKO mice relative to control but increased in PTH1-34-treated mice. Again, statistically significant differences upon PTH treatment were only observed between Cdh2-cKO groups (Fig. 6E).
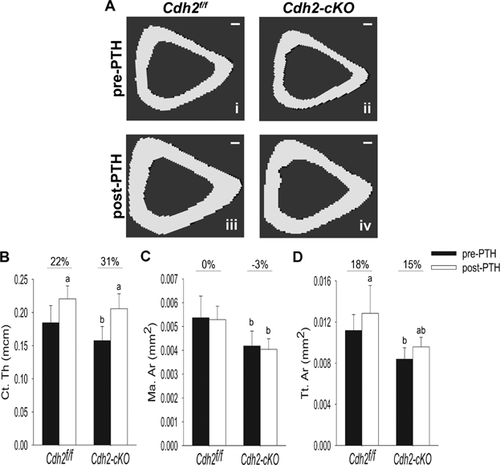
As previously reported,17 long bones of Cdh2-cKO mice were slightly smaller than control littermates in their diaphyseal cross section (Fig. 7Ai, ii). Intermittent PTH1-34 treatment increased cortical thickness in both Cdh2f/f and Cdh2-cKO animals relative to vehicle-treated groups (Fig. 7Aiii, iv, B). The iPTH regimen did not alter marrow area (Fig. 7C), but it increased total tissue area in both genotypes (Fig. 7D). Because all three cortical parameters were reduced in Cdh2-cKO relative to Cdh2f/f mice, as we previously reported,17 absolute differences between PTH1-34 -treated and vehicle-treated mice were calculated, revealing no differences between genotypes (Supplemental Fig. S6A–C). Vehicle treatment did not affect any of the cortical parameters in Cdh2f/f, Cdh2-cKO, or Osx-Cre animals (Supplemental Fig. S7A–D), thus excluding interference of the Osx-Cre transgene or body growth during the month of treatment on the observed effect of iPTH. Furthermore, dynamic histomorphometry revealed that neither Cdh2 ablation nor iPTH treatment affected endocortical mineralizing surfaces (Ec. MS/BS) (Supplemental Fig. S8A), mineral apposition rate (Ec. MAR) (Supplemental Fig. S8B), or bone formation rate (Ec. BFR/BS) (Supplemental Fig. S8C). Conversely, the mineralizing surfaces in the periosteum of either genotype were increased in a similar fashion in response to iPTH (Supplemental Fig. S8D). Mineral apposition rate (Ps. MAR) and bone formation rate (Ps. BFR/BS) at the periosteum showed similar non-significant upward trends in iPTH-treated mice relative to vehicle-treated animals in both genotype groups (Supplemental Fig. S8E, F).
Discussion
Our findings identify N-cadherin as a regulator of PTH responsiveness in bone, where it functions to restrain the hormone's activation of Lrp6/β-catenin signaling and stimulation of trabecular bone accrual in vivo. Therefore, our work unveils a novel role of N-cadherin as a signaling modulator for a key regulator of bone and mineral homeostasis.
Because cadherins regulate the pool of Lrp5/6 available for signaling,18, 41 it is likely that the increased abundance of Lrp6/PTHR1 complexes we observed in the absence of N-cadherin reflects increased Lrp6 availability for interaction with PTHR1. Indeed, we did not find N-cadherin in PTHR1-containing complexes, suggesting that Lrp6 interaction with N-cadherin or PTHR1 is mutually exclusive; consequently, N-cadherin interference with PTHR1 signaling is indirect. Lrp5/6 and N-cadherin interact via an axin bridge through their intracellular C-terminus domains,18 whereas Lrp6 binds to PTHR1 through its extracellular domain.13 Although the reason why N-cadherin-bound Lrp6 is unable to interact with PTHR1 remains to be clarified, conformational changes upon binding to the N-cadherin-axin complex may change Lrp6 affinity for PTHR1, or sequestration of Lrp6 within adherens junctions may hinder access of PTHR1 to Lrp6. However, we cannot exclude that N-cadherin, Lrp6, and PTHR1 form a complex that is not stable enough to be detected by co-immunoprecipitation. Although loss of N-cadherin increased Lrp6/PTHR1 interaction, we found no evidence of Lrp5/PTHR1 interaction in primary BMSC and MC3T3-E1 cells (data not shown), which was instead reported to occur in rat UMR-106 osteosarcoma and HEK 293 cells exposed to PTH1-84.13 In that study, however, protein overexpression systems were used,13 whereas we studied interactions among endogenously expressed proteins. The different cell systems and PTH analogs may also represent a source of variance because PTHR1 undergoes different conformational changes that induce distinct responses depending on the ligand and cell type.42, 43
Our results demonstrate that in the absence of N-cadherin, PTH activation of PKA/CREB is enhanced, leading to increased β-catenin transcriptional activation upon phosphorylation of S675 by PKA (Fig. 8). This observation is consistent with previous findings of enhanced PKA/β-catenin interaction in response to PTH1-34.11 Our results also suggest that activation of β-catenin signaling is via noncanonical mechanisms and does not involve β-catenin's destruction complex. It is likely that PTH-induced β-catenin phosphorylation at S675 affects only the small fraction of β-catenin available for nuclear translocation and signaling, so that changes in this fraction are not sufficient to alter total β-catenin abundance,31 which is mostly accounted by the β-catenin within adherens junctions at the cell surface.44, 45 Our data also demonstrate that both PKA activity and Lrp6 are required for PTH-induced activation of β-catenin because β-catenin phosphorylation at S675 can be blocked by both PKA inhibition and Lrp6 silencing. These results are consistent with observations that Lrp6 ablation renders Tcf/Lef-dependent gene transcription unresponsive to PTH.12, 13 On the other hand, PTH can still induce CREB phosphorylation in the absence of Lrp6, suggesting that CREB activation does not depend on formation of Lrp6/PTHR1 complex. This bipartite signaling flow activated by PTH is consistent with multiple reports of PTH activation of both CRE and Tcf/Lef target genes.11, 13, 37, 46 Accordingly, Cdh2 ablation enhances Tcf/Lef target gene (Lef1 and Axin2) expression upon PTH stimulation, consistent with previous reports that N-cadherin modulates Wnt/β-catenin signaling in osteoblasts.18 Thus, we propose a model whereby N-cadherin keeps β-catenin and Lrp5/6 at the plasma membrane, thus reducing availability of Lrp5/6 for interaction with PTHR1 and Frizzled (Fzl). In N-cadherin–deficient osteoblasts, more Lrp5 and Lrp6 are available to interact with PTHR1 or Fzl, leading to enhanced β-catenin/Tcf/Lef transcriptional activity in response to PTH. Activation of β-catenin by PTH requires Lrp6 but is also contributed to by PKA-dependent phosphorylation at S675 (Fig. 8).
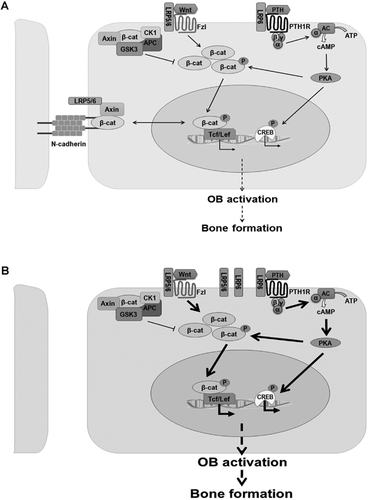
The increased sensitivity of Cdh2-ablated osteoblasts to PTH activation of PKA and β-catenin signals translates into in vivo effects. Intermittent PTH1-34 treatment induced a larger net trabecular bone accrual in Cdh2-cKO mice relative to control mice, by both histomorphometric and µCT-based microstructural analyses. Indeed, at the end of iPTH treatment, bone mass and all the other trabecular structural parameters were similar in Cdh2-cKO and Cdh2f/f mice, despite a significantly lower baseline in trabecular bone mass, number, and thickness in N-cadherin–deficient mice. Therefore, iPTH “normalizes” trabecular bone mass in Cdh2-cKO mice. We propose that the accentuated bone anabolic effect of iPTH in Cdh2-cKO mice is related to the accentuated β-catenin/Tcf/Lef signaling brought about by N-cadherin deficiency (Fig. 8) because Lrp6 is necessary for PTH anabolic effect12 and Lrp6 silencing prevents β-catenin but not PKA/CREB activation by the hormone (our data). Consistent with others' reports,47, 48 we also observed increased cortical thickness and total cortical area in response to iPTH, suggestive of increased periosteal bone apposition, without changes in endocortical remodeling. Bone accrual occurred at a similar extent in both genotypes, so that most cortical parameters still remained significantly different in Cdh2-cKO relative to control mice after iPTH treatment. Because N-cadherin is expressed in periosteal cells,26 and these cells are targeted by Osx-GFP::Cre,49 it is unlikely that the selective modulatory action of N-cadherin on trabecular rather than cortical bone is attributable to a lack of Cdh2 recombination in periosteal cells. Instead, these results suggest that N-cadherin selectively modulates bone remodeling rather than bone modeling, which in postnatal life is driven primarily by periosteal bone formation.50
The accentuated increase in trabecular bone mass in iPTH-treated Cdh2-cKO mice is most likely related to accentuated activation of pre-existing osteoblasts because we found no significant changes in osteoblast surface and mineralizing surface per bone surface after iPTH treatment, despite a significant increase in mineral apposition and bone formation rate. Accentuated osteoblast activation is consistent with enhanced β-catenin signaling in Cdh2-ablated mice because β-catenin signaling is crucial for osteoblast differentiation and function.9, 10 On the other hand, N-cadherin deficiency did not alter the increased bone resorption response we observed after iPTH treatment. The discrepancy between the significant increase in serum CTX and lack of changes in osteoclast surface may suggest that, in this study, iPTH activated pre-existing osteoclasts rather than recruiting new osteoclasts. Moreover, the large increase in osteocyte number upon iPTH may reflect decreased osteoblast/osteocyte apoptosis in response to iPTH.51 Notably, the effect of iPTH on osteocyte number was not altered by Cdh2 ablation in Osx+ cells, consistent with downregulation of N-cadherin during normal osteoblast differentiation and lack of N-cadherin in osteocytes.26, 52 Decreased N-cadherin abundance with osteoblast differentiation may in part explain the discrepancy with the findings of Bromberg and colleagues, who did not observe any effect of Cdh2 ablation on PTH anabolic response.53 They used Col2.3-Cre to drive Cdh2 recombination, therefore targeting primarily differentiated osteoblasts, whereas we used Osx, which targets an earlier and broader population of osteoblastic and other bone marrow cells.54 Different treatment regimens and doses of PTH may also contribute to the apparent discrepant findings.53
In summary, we found that N-cadherin regulates Lrp6 availability to interact with PTHR1, thus modulating the intensity of PTH-induced β-catenin activation and ultimately influencing PTH stimulation of bone formation. Our work supports the premise that N-cadherin in osteoblasts represents a potential pharmacologic target for enhancing the bone anabolic effect of iPTH and Wnt signaling stimulators.
Disclosures
RC receives research support from Pfizer, Inc. and Amgen, and holds stock of Amgen, Eli Lilly, and Merck & Co. GM is cofounder of Confluence Life Sciences. All other authors state that they have no conflicts of interest.
Acknowledgments
We thank Drs Marcus Watkins and Susan Grimston for the useful discussions and technical assistance; Drs Daniel Link and Adam Greenbaum, Washington University in St. Louis, for providing their input and the Osx-Cre::Cdh2f/f mice; and Dr Bart Williams, Van Andel Research Institute, Grand Rapids, MI, for providing the Lrp6f/f mice. We also thank the Musculoskeletal Histology, Morphometry, and the Structure and Strength Cores of Washington University Musculoskeletal Research Center. This work was supported by grants from NIH/NIAMS R01-AR055913 (to RC) and NIH/NIAMS P30-AR057235 (Washington University Musculoskeletal Research Center).
Authors' roles: LR designed and performed the research, analyzed the data, and wrote the manuscript. JK assisted with static histomorphometric analysis, performed cortical dynamic histomorphometric analysis, and assisted with genotyping. S-YJ performed trabecular dynamic histomorphometric analysis. JL assisted with gene expression analysis and genotyping. VS designed and performed preliminary experiments for the conception of this study. GM and RC contributed to the research design, supervised the entire experimentation, data analysis and interpretation, and contributed to manuscript editing. LR and RC take full responsibility for data integrity and analysis. This work was performed in partial fulfillment for the doctoral thesis of LR.




