Elevated cytokine production restores bone resorption by human Btk-deficient osteoclasts
Abstract
Mutations in Bruton's tyrosine kinase (Btk) cause the B-cell disorder X-linked agammaglobulinaemia (XLA) in humans, but the effect of Btk deficiency in human bone health has not been investigated previously. In this study, we show that human Btk-deficient osteoclasts are defective at resorption activity in vitro owing to a dysregulation of the actin cytoskeletal function. Contrary to expectation, XLA patients did not exhibit increased bone density or alterations in serum markers of bone turnover, indicating that a potential compensation mechanism normalizes bone homeostasis. In contrast to the bone turnover markers, the levels of inflammatory cytokines interleukin 6 (IL-6), IL-1β, and tumor necrosis factor α (TNF-α) were significantly elevated in XLA patients' serum compared with control individuals. Supplementation of osteoclast cultures from normal and XLA subjects with serum from XLA patients or recombinant inflammatory cytokines IL-6, IL-1β, and TNF-α resulted in a stimulation of osteoclast activity in vitro, whereas the addition of cytokine-neutralizing antibodies inhibited this stimulatory effect, confirming that elevated inflammatory cytokines in XLA serum heightened osteoclast activity in vitro. This study provides novel evidence that Btk signaling is crucial for optimal actin cytoskeletal organization and lacunar resorption in isolated osteoclasts. In XLA patients, however, these inherent osteoclast defects are corrected by increased inflammatory cytokine levels, restoring osteoclast activity and leading to the normalization of bone density. © 2011 American Society for Bone and Mineral Research.
Introduction
Bruton's tyrosine kinase is a member of the Tec family of kinases that is expressed exclusively in hematopoietic lineages. Btk null mutations have been reported in mice (XID) and in humans (XLA), both of which result in profound deficiencies in B-cell development and function, leading to development of agammaglobulinaemia. Btk signals are also emerging as critical determinants of myeloid cell function.1-5 Osteoclasts are highly specialized multinucleated cells of myeloid origin that are responsible for resorbing bone, a process required for normal bone turnover. Excessive osteoclastogenesis results in pathologic osteolysis; therefore, osteoclast formation and activation must be tightly regulated for normal bone metabolism. In the Btk null mouse, myeloid progenitors are defective at forming osteoclasts in vitro, and indeed, mice deficient in both functional Btk and the closely related kinase Tec have higher bone density because osteoclasts are virtually absent.6 Btk and Tec have been shown to integrate two intracellular pathways critical to osteoclast precursor maturation: RANK, a receptor of the tumor necrosis factor (TNF) family that interacts with its ligand RANKL, and the ITAM-domain containing receptors. These pathways coordinate the sustained expression of NFATc1, a transcription factor essential for osteoclast formation.7 Likewise XID mice, carrying a point mutation in Btk (R28C) that renders it inactive, display incomplete osteoclast differentiation that is thought to arise from a problem with fusion of mononuclear precursors.8
While Btk and related Tec family kinases are clearly emerging as being of central importance in controlling osteoclast formation, it is likely that Btk is also important for osteoclast activity because it has been shown to interact with numerous mediators of actin reorganization such as Vav, WASP, and the Src family kinases. Btk interacts directly with actin in other cell types such as mast cells and platelets.9, 10 Since the formation of an F-actin ring is essential for osteoclast bone resorption, this may well be affected by the aberrant Btk activity. Defective Btk in XID mice results in impaired activation of WASP and Vav,11, 12 molecules that play well-recognized roles in actin remodeling and osteoclast activity.13 The cytoskeleton is also required for efficient transport of intracellular proteins and enzymes to the osteoclast basolateral membrane, where coordinated and highly localized release of H+ ions and proteases, notably cathepsin K, results in the ordered excavation of deep pits on the bone surface.
In humans, X-linked agammaglobulinaemia (XLA) is a relatively rare but severe immunodeficiency that occurs as a result of the absence of Btk or the production of functionally defective protein resulting in abnormal B-cell development.14, 15 XLA patients have serious health problems related to recurrent pulmonary infections and require regular replacement immunoglobulin therapy and often prophylactic antibiotics.16, 17 These repeated infections may result in elevated levels of inflammatory cytokines in XLA patient serum, although a systemic inflammatory profile of XLA patients has yet to be reported. Chronic and systemic inflammation in both mouse models and human disease is very commonly associated with low bone mass (ie, osteoporosis) because inflammatory cytokines greatly stimulate osteoclastic bone resorption and also may impair osteoblast activity. Indeed, inflammatory cytokine ablation reduces the severity of bone lesions in inflammatory arthritis.18 As such, XLA patients therefore might be expected to be at risk of osteoporotic fractures, particularly older individuals. However, the requirement for Btk in osteoclast development as identified in XID mice8 and the Btk/Tec double-deficient mice6 suggests conversely that XLA patients should be predisposed to osteopetrosis. Therefore, we have examined osteoclast formation and function in vitro using precursors from the peripheral blood mononuclear cells of XLA patients in the presence and absence of inflammatory cytokines, and we have performed ultrasound bone density analysis and determined serum markers of bone turnover in the XLA patients.
Methods
Tissue culture reagents
Complete medium
RPMI 1640 containing 100 U/mL of penicillin/streptomycin and 10% heat-inactivated fetal calf serum (FCS) and 0.2 mM L-glutamate. Serum also was isolated from clotted blood collected from XLA patients and control donors. All cell culture incubations were performed at 37°C in a humidified atmosphere containing 5% CO2.
Isolation of PBMCs from XLA patients and control donors
Diagnosis of XLA depends on the presence of mutations in the gene encoding Btk that result in a truncated form of Btk or its complete absence (Table 1). Blood samples were collected immediately prior to intravenous immunoglobulin therapy from patients without any evidence of acute infection, although most of the patients had chronic bronchiectasis of variable severity. Ethical permission for the study was obtained from the local Royal Free Ethics Committee. Blood samples from XLA patients and control donors were collected into lithium heparin vacutainers. Each blood sample was mixed with an equal volume of Hank's balanced salt solution (HBSS), and the peripheral blood mononuclear cells (PBMCs) were prepared by density separation using Ficoll-Hypaque centrifugation.
| Patient ID | Age (years) | Description of mutation |
|---|---|---|
| 1 | 51 | R641H |
| 2 | 46 | R641H |
| 3 | 54 | R641H |
| 4 | 28 | C1583-1880 Deleted |
| 5 | 38 | C1583-1880 Deleted |
| 6 | 26 | Y425X C→A stop exon 14 |
| 7 | 46 | A582V |
| 8 | 56 | M587L A→C at1891 exon 18 |
| 9 | 34 | R562P |
| 10 | 37 | R255X C→A at 895 stop exon 8 |
| 11 | 38 | Q293X C→T 1009 Gh stop exon 10 |
| 12 | 49 | 285 Insertion T frameshift exon 3 |
| 13 | 41 | 908 + 5 G→A aberrant splicing skips exon 9 |
| 14 | 24 | 2026 Deleted A frameshift exon 18 |
| 15 | 46 | 2026 Deleted A frameshift exon 18 |
| 16 | 44 | Y598C |
| 17 | 37 | P619S exon 18 |
| 18 | 50 | E567K G→A at 1831 exon 17 |
| 19 | 33 | R641H G→A at 2054 exon 19 |
| 20 | 45 | R641H G→A at 2054 Exon 19 |
- Note: Twenty patients were used in this study, and their respective mutations are shown in terms of the amino acid substitution or insertion of a stop codon.
Sorting of CD14+ population from the PBMCs of XLA patients and control donors
Total PBMCs isolated from the peripheral blood of patients with XLA and control donors were incubated with MACS CD14 MicroBeads, and cell sorting was performed using the MidiMACS magnet and LS columns as per manufacturer's instructions (Miltenyi Biotec, Surrey, UK).
Plating of XLA cell preparations
XLA CD14+ cells and control CD14+ cells were seeded (2 × 105 cells/well) into 96-well tissue culture plates for tartrate-resistant acid phosphatase (TRACP) assays and dentine assays (described below). The cells then cultured in complete medium containing monocyte colony-stimulating factor (M-CSF; 25 ng/mL) and increasing concentrations of receptor activator of NF-κB ligand (RANKL; 0 to 20 ng/mL).
Plating of cells for serum supplementation, cytokine neutralization antibodies, or recombinant cytokine treatment
Monocytes from buffy coat PBMC preparations from XLA or heathy control donors were seeded (1 × 106 cells/mL) into flasks containing complete medium and M-CSF (25 ng/mL). After 3 days, the cells were scraped and seeded (2 × 105 cells/well) into 96-well tissue culture plates. The cells were cultured in complete medium containing M-CSF (25 ng/mL) and RANKL (20 ng/mL) and supplemented with 5% (heat-inactivated) healthy control serum or XLA patient serum in the presence or absence of IgG isotype control antibody or neutralizing antibodies to either interleukin 6 (IL-6), TNF-α, or IL-1β (10 µg/mL) (R&D Systems, Oxford, UK). XLA and control donor monocytes also were prepared in complete medium containing M-CSF (25 ng/mL) and RANKL (20 ng/mL) with or without recombinant cytokines IL-1β, TNF-α, and IL-6 (0.001 to 1 ng/mL) (R&D Systems) either independently or combined.
In vitro osteoclast TRACP assay
Expression of the osteoclast-associated enzyme tartrate-resistant acid phosphatase (TRACP) was assessed at 7 days of culture on cell preparations in 96-well plates (3 wells/treatment). The cells were fixed in 4% formaldehyde and permeabilized in 50:50 (v/v) ethanol-acetone and allowed to air dry. Histochemical staining of the preparations was then performed as described previously.19 The number of TRACP+ mononuclear cells and/or multinucleated cells containing three or more nuclei (MNCs) were counted at ×20 magnification.
In vitro osteoclast resorption assay
Functional evidence of osteoclast differentiation and activation was determined by a lacunar resorption assay on dentine disks (3 dentines/treatment). After the cells had been cultured on the dentine for 21 days, the cells were removed in 1 M ammonium hydroxide. The dentine slices were rinsed in dH2O and stained with 0.5% (w/v) toluidine blue. Resorption pits were examined by light microscopy, and the average area of lacunar resorption of three fields of view on 3 dentines/treatment was measured using Velocity image analysis software (Perkin Elmer, Waltham, MA, USA).
Confocal analysis
Three-day M-CSF-treated XLA and control donor PBMCs were plated at 2 × 105 cells/well on dentine slices. Cells were treated with RPMI 1640 and cultured in complete medium containing M-CSF (25 ng/mL) and RANKL (0 to 20 ng/mL) for 10 days. The cells were washed in PBS and fixed in 4% paraformaldehyde and permeabilized in 0.25% Triton X-100. The preparations were blocked in 10% goat serum and then incubated with either mouse antihuman cathepsin K (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 hours at room temperature. After washing, the preparations were incubated with Alexa-phalloidin (Molecular Probes, Invitrogen Ltd., Paisley, UK) to visualize F-actin. After a final washing step, the preparations were mounted with coverslips using antifade Vecta mountant (Invitrogen Ltd., Paisley, UK).
Western blot analysis
XLA PBMCs, control PBMCs, and B-cell-depleted PBMCs were plated (5 × 106 cells/well) into 6-well plates and cultured in the presence of M-CSF alone (25 ng/mL) or in the presence of M-CSF (25 ng/mL) and RANKL (20 ng/mL). At various time points, the cells were harvested into lysis buffer (25 mM HEPES, pH 7.0, 150 mM NaCl, and 1% Nonidet P-40) containing protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Nuclei and cell debris were removed by centrifugation. The lysates were electrophoresced on 8% SDS-PAGE, followed by electrotransfer of proteins onto polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in 10% Marvel/PBS/0.05% Tween and probed for 2 hours with mouse antihuman Btk (Becton Dickinson UK Ltd., Oxford, UK) or antihuman cathepsin K (Santa Cruz Biotechnology) at 1 µg/mL. After washing, the membranes were incubated with antimouse horseradish peroxidase (GE Healthcare, UK Ltd, Little Chalfont, Buckinghamshire, UK) at 1:5000. After washing, the membranes were developed using enhanced chemiluminescence according to the manufacturer's instructions (Amersham Biosciences, UK Ltd, Little Chalfont, Buckinghamshire, UK). In order to ensure that equivalent protein was loaded in each lane, the blots were reprobed with antibodies to α-tubulin (Sigma-Aldrich).
Human XLA patient bone density: Qualitative ultrasound
Quantitative ultrasound (QUS) is a cost effective method to measure bone mineral density (BMD) and has been used successfully for more than a decade in predicting the risk of osteoporotic fractures.20 The CUBAclinical ultrasound scanner (Siemens Healthcare, Camberley, Surrey, UK) was kindly provided to us by the British College of Osteopathic Medicine. We used QUS of the heel to carry out a pilot study to investigate the BMD of 7 XLA patients (ages 36 to 53 years). Demographic data, weight, and current medications were extracted from the medical record. All the patients in the study were ambulatory. None of the patients had any history of fractures, and patients receiving any additional medication potentially affecting bone metabolism (eg, steroids) were not included in the study. The broadband ultrasound attenuation (BUA) values, which reflects the frequency dependence of ultrasound attenuation in the frequency range 200 to 600 kHz, were combined for left and right heels and plotted against normative data for BUA values performed in age-matched healthy individuals.
Biochemical analysis of serum markers of bone turnover
Serum samples, once isolated, were frozen immediately at –80°C. Osteoprotegerin (OPG) (Biomedica Group, Vienna, Austria), and active isoform 5b of tartrate-resistant acid phophatase (TRACP5b; Immunodiagnostic Systems Ltd., Boldon, Tyne and Wear, UK) was measured by ELISA. Samples were transported on dry ice to the Royal Liverpool University Hospital and the Royal Free Hospital for biochemical analysis of various bone turnover markers, including serum C-terminal telopeptide of type 1 collagen (CTX), bone-specific alkaline phosphatase (ALP), procollagen type 1 N-propeptide (P1NP), and osteocalcin.
Measurement of serum cytokines by ELISA
Concentrations of human TNF-α, IL-1β, and IL-6 (R&D Systems) were measured by ELISA. The absorbance was read and analyzed at 450 nm on a spectrophotometric ELISA plate reader (Labsystems Multiskan Biochromic, Labsystems, Uxbridge, UK) using the DeltaSoft II.4 software program (DeltaSoft, Inc., Hillsborough, NJ, USA). Results are expressed as the mean concentration of duplicate cultures ± SD.
Statistical analysis
Each TRACP and bone-resorption assay experiment was carried out in triplicate. Data are presented as the mean number of TRACP+ MNCs per field of view (at ×200 magnification) ± SEM. Statistical analysis on measurements was performed using a Mann-Whitney U test. Values less than p = .05 were considered significant (*p < .05; **p < .005; ***p < .0005). Statistical analyses on ELISA assays or experiments using serum were performed using unpaired t tests. Values less than p = .05 were considered significant.
Results
Increased TRACP+ cell formation occurs in Btk-deficient osteoclasts cultured on plastic
Human monocyte/macrophages have been shown to express the Tec family kinases Btk, Tec, and Bmx.21 To assess whether Btk is present throughout osteoclast development, human osteoclast cultures from peripheral blood mononuclear cells of normal subjects were evaluated, and the expression of Btk was shown to remain constant throughout osteoclast differentiation in vitro (Fig. 1A). To investigate whether the lack of Btk in human osteoclast differentiation in vitro was the same as that observed in the Btk-deficient mice, osteoclasts were generated from CD14+ monocytes isolated from XLA patient and control PBMCs. The CD14+ cells were cultured on plastic in the presence of M-CSF and increasing concentrations of RANKL, and the average number of TRACP+ mononuclear cells and multinucleated cells (MNCs) were assessed after 7 days (Fig. 1B, C). TRACP+ cells did not form in the absence of RANKL. There was an elevation in the number of TRACP+ mononuclear cells in XLA cultures compared with control cultures, statistically significant at all concentrations of RANKL (1, 5, and 20 ng/mL; Fig. 1D). Furthermore, at the higher doses of RANKL (5 and 20 ng/mL), the average number of TRACP+ cells containing three or more nuclei (MNCs) formed in XLA cultures was significantly increased compared with those formed in control cultures (Fig. 1E). No difference in the average number of nuclei per TRACP+ MNC was observed, and osteoclasts containing 5 or 7 nuclei were abundant in both XLA and control cultures. There were no detectable differences in either the size or morphology of TRACP+ MNCs formed in XLA patient cultures and donor controls, suggesting that when cultured on plastic, the lack of Btk does not affect the fusion efficiency of human osteoclasts in the same way that has been reported for murine osteoclasts.8 To determine whether the absence of Btk affected F-actin-rich podosome belt formation, we assessed XLA patient and control osteoclasts cultured in glass chamber slides; no differences were observed in F-actin ring formation between XLA-derived cells and controls (Fig. 1F, G).
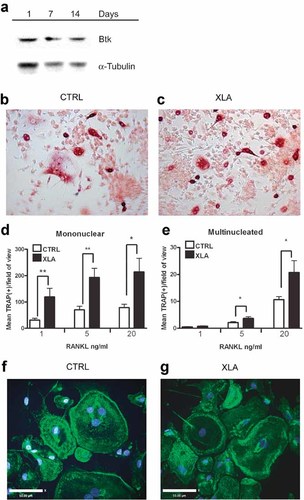
Btk-deficient osteoclasts cultured on plastic. (A) Protein extracts were prepared from 5 × 106 cells/well after 1, 7, and 14 days of culture in the presence of M-CSF (25 ng/mL) and RANKL (20 ng/mL). Western blots were probed using antibodies to either Btk or α-tubulin as a loading control and visualized using ECL. (B) Control and (C) XLA peripheral blood CD14+ cells cultured for 7 days in the presence M-CSF (25 ng/mL) and RANKL (20 ng/mL). Cells were fixed and stained for TRACP+ (red). (D) Enumeration of TRACP+ mononuclear cell and (E) TRACP+ multinucleated cell formation after 7 days of culture of either donor or XLA patient peripheral blood CD14+ cells in the presence M-CSF (25 ng/mL) and RANKL (1, 5, or 20 ng/mL) (n = 5). Data are presented as the mean (± SEM) number of TRACP+ cells per field of view. Statistical analysis was performed using a Mann-Whitney U test. *p < .05; **p < .005. Control (F) and XLA (G) osteoclasts generated in glass chamber slides for 10 days in the presence of M-CSF (25 ng/mL) and RANKL (20 ng/mL). Confocal images of osteoclasts stained for actin cytoskeleton with phalloidin (green) and nuclei with DAPI (blue). Bar = 50.00 µm.
Btk-deficient osteoclast activity is reduced owing to impaired actin ring formation
Osteoclastic bone resorption depends on the formation of an F-actin ring structure known as the sealing zone; this is distinct from the F-actin-rich belt structures that form when cells are cultured on plastic or glass. Therefore, the effect of Btk absence on F-actin ring-sealing zone formation was assessed in XLA patient and control osteoclasts cultured on dentine. The number and size of the F-actin ring-sealing zone structures were reduced significantly in XLA patient cultures compared with controls, suggesting that Btk is required for substrate-dependent actin reorganization (Fig. 2A–C).
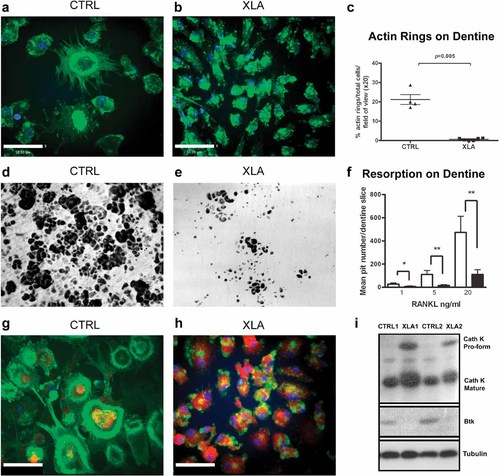
Btk-deficient osteoclasts are functionally defective when cultured on dentine. All experiments were carried out in the presence of M-CSF (25 ng/mL) and RANKL (20 ng/mL) unless otherwise stated. Control (A) and XLA (B) confocal images of osteoclasts cultured on dentine for 10 days, stained for actin cytoskeleton with phalloidin (green) and nuclei with DAPI (blue). Bar = 50.00 µm. (C) Enumeration of F-actin rings as a percentage of total cells per field of view at ×20 magnification (n = 13). (D, E) Bright-field images of toluidine blue–stained dentine slices showing lacunar pit formation following 21 days of culture of age-matched control donor or XLA peripheral blood CD14+ cells. (F) Quantification of pit number after culture in the presence M-CSF (25 ng/mL) and RANKL (1, 5, or 20 ng/mL) was performed using Velocity image-analysis software (n = 13). Data are presented as the mean (± SEM) pit number per dentine. Statistical analysis was performed using a Mann-Whitney U test. *p < .05; **p < .005. Confocal images of (G) control and (H) XLA osteoclasts on day 10 of culture on dentine, stained for cathepsin K (red) actin cytoskeleton with phalloidin (green) and nuclei with DAPI (blue). Bar = 50.00 µm. (I) Western blot of cathepsin K protein and Btk protein and α-tubulin loading control of 10-day-old cultures of control and XLA osteoclasts on plastic.
As a consequence of actin ring defects, it was likely that these cells also would exhibit reduced lacunae pit formation on dentine. CD14+ cells, sorted from XLA PBMCs and controls, were cultured in the presence of M-CSF and increasing concentrations of RANKL on dentine for 21 days. Resorption pits were unable to form in the absence of RANKL (data not shown). The mean number of resorption pits created by osteoclasts from sorted CD14+ XLA monocytes was reduced significantly compared with control monocytes at RANKL concentrations of 5 ng/mL (p = .008) and 20 ng/mL (p = .013), thus demonstrating that Btk is required for optimal lacunar resorption activity on dentine (Fig. 2D–F).
The formation of an actin ring-sealing zone allows the formation of an acidified compartment (pH.4) between the bone substrate and the cell that is optimized for cleavage of collagen by cathepsin K. Since bone resorption is defective in XLA osteoclasts, we investigated whether there also were effects on cathepsin K expression. Cathepsin K was visible in both donor and XLA osteoclasts cultured on dentine (Fig. 2G, H). Confocal microscopic images showed that osteoclasts derived from XLA monocytes were abnormally burdened with cathepsin K compared with those of control donors. Similarly, Western blot analysis confirmed that XLA patient osteoclast cultures contained significantly increased protein levels of both the proform and mature form of cathepsin K compared with healthy controls (Fig. 2I).
XLA patient bone density and markers of bone turnover remain normal while levels of TNF-α, IL-1β, and IL-6 in XLA serum are elevated
In light of the in vitro osteoclast resorption defects, it would be predicted that XLA patients would exhibit an osteopetrotic phenotype akin to that observed in the Btk-deficient mice. Bone density was measured by qualitative ultrasound (QUS) of the heel in the XLA patients; the normative data are presented as means for each age-matched grouping ± SD. The QUS analysis is shown as combined BUA values of 7 patients (ages 25 to 53 years) compared with the average BUA values of age-matched controls. These data suggest that patients with XLA have neither increased nor decreased bone mass compared with age-matched controls (Fig. 3A). We also were unable to detect any significant differences in the markers of bone remodeling, such as CTX and P1NP, between XLA patients and controls (Fig. 3B–G). These data suggest that despite the inherent defects in osteoclast function, bone density in XLA patients remains normal, suggesting that other factors may be able to rectify XLA osteoclast defects in vivo.
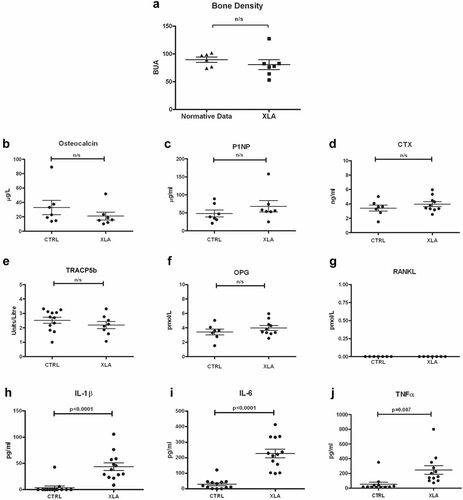
XLA patients have normal bone density. Scatter plots showing (A) the mean BUA (dB/MHz) values of 7 XLA patients (ages 25 to 53 years) compared with the average BUA values of age-matched normative controls. (B–G) Markers of bone turnover in the serum or plasma of XLA (n = 6 to 10 patients) and control donor samples were assessed. (B) Osteocalcin, (C) procollagen type 1 N-propeptide (P1NP), (D) C-terminal telopeptide of type 1 collagen (CTX), (E) the active isoform 5b of tartrate-resistant acid phophatase (TRACP5b), (F) osteoprotegerin (OPG), and (G) soluble RANKL. Cytokine levels of (H) IL-1β, (I) IL-6, and (J) TNF-α were measured in the serum of XLA patients (n = 13) and age-matched healthy controls (n = 13). Statistical analysis was performed using an unpaired t test; the results are shown as means ± SEM.
XLA patients do not receive corticosteroids, the long-term use of which can promote bone loss, as part of their treatment regime. However, XLA patients are susceptible to low-grade pulmonary and gastric infections despite replacement immunoglobulin therapy, and hence it is possible that infection-induced inflammatory cytokines may contribute to osteoclast activation in the absence of Btk. The levels of IL-1β, IL-6, and TNF-α were found to be significantly elevated in XLA serum (p = .0001, p < .0001, and p = .007, respectively) compared with donor serum (Fig. 3H–J). XLA serum contained increased IL-1β 44.0 ± 7.2 pg/mL (mean ± SEM) compared with controls (3.8 ± 3.2 pg/mL). IL-6 also was found to be elevated to 227.1 ± 28.3 pg/mL compared with controls (29.00 ± 9.2 pg/mL). TNF-α XLA levels were increased to 246.3 ± 58.63 pg/mL compared with controls (53.7 ± 27.6 pg/mL). In many of the control serum samples, the values of IL-1β and IL-6 were below the detectable limits of the ELISA. Additionally, the serum levels of TGF-α, sIL-6 receptor, IL-10, IL-8, and interferon γ (IFN-γ) also were evaluated and shown to be comparable between the two groups or below the level of detection for the assay (Supplemental Fig. 1).
XLA serum stimulates osteoclast activity in vitro
To determine whether factors present in the serum of XLA patients could modify osteoclast function in vitro, control osteoclast cultures were supplemented with either 5% XLA serum or 5% control serum. There was a significant increase (10-fold) in F-actin ring formation on dentine in the presence of XLA serum (Fig. 4A–C). 3D opaquely enhanced images, using Velocity image analysis software, demonstrate actin and cathepsin K protein density and localization in relation to the dentine surface. Using this type of analysis, it was observed that osteoclastic pit depth was increased in the presence of XLA serum compared with control serum, with cathepsin K expression (red) clearly visible below the actin ring (green) and within the dentine (Fig. 4D, E). The percentage area of bone resorption by control-derived osteoclasts also was increased in the presence of XLA serum compared with control serum (Fig. 4F–H).
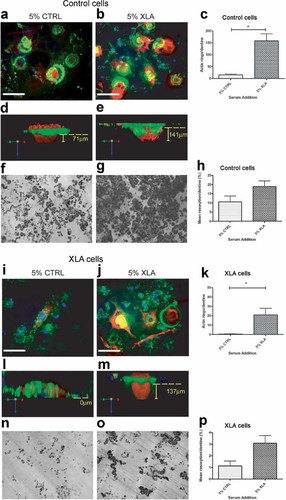
Serum of XLA patients enhances osteoclast activity. All experiments were carried out in the presence of M-CSF (25 ng/mL) and RANKL (20 ng/mL) and cultured for 10 days for confocal analysis or 21 days for resorption quantification. Donor or XLA osteoclast cultures were supplemented with either 5% control serum or 5% XLA serum. (A,B,D,E,I,J,L,M) Confocal images representing cathepsin K (red), actin cytoskeleton with phalloidin (green), and nuclei with DAPI (blue). (D,E,L,M) 3D opaquely enhanced images, using Velocity image-analysis software, in order to demonstrate protein density and localization. (D,E) F-actin ring encircling cathepsin K enzyme within cell body and lacunae. (L) Dysregulated actin and absence of lacunae in XLA monocytes in the presence of 5% control serum are restored in the presence of (M) 5% XLA serum. Yellow dotted line indicates upper surface of dentine. Yellow vertical bars indicate depth of resorption pits. White bar = 50.00 µm. Enumeration of F-actin rings per dentine (C, K) or resorption pits per dentine (H, P) of control (n = 7) or XLA (n = 3) monocytes supplemented with 5% control serum (n = 7) or 5% XLA serum (n = 7). Statistical analysis performed using the paired t test. Results are shown as means ± SEM. *p < .05.
To determine whether the defect in XLA osteoclasts could be corrected by culture with XLA serum, the experiments described earlier were repeated with XLA monocytes. The addition of 5% XLA serum to XLA monocyte–derived osteoclasts, as compared with 5% control serum, significantly restored F-actin ring formation on dentine (60-fold increase; Fig. 4I–K). The inability of Btk-deficient osteoclasts to form actin rings was clearly observed in the cross-sectional composite images showing a disrupted actin ring and the failure to form a resorption pit while the cathepsin K is still clearly visible within the cell (Fig. 4L). Following the addition of XLA serum, Btk-deficient osteoclasts were able to form actin rings and secrete cathepsin K to promote bone resorption (Fig. 4M). Likewise, there was an increase in lacunar bone resorption following the addition of XLA serum to the XLA osteoclast cultures compared with control serum (Fig. 4N–P).
It should be noted that the actin rings and resorption pits observed in control monocytes supplemented with 5% XLA serum were more numerous than those formed by XLA monocytes supplemented with XLA serum, indicating that the factors within the serum could only partially restore osteoclast activity in this isolated in vitro cell system (Fig. 4C, K, H, P). Overall, these data suggest that XLA patient serum is highly osteoclastogenic, and despite defects in Btk signaling activity in XLA osteoclasts, the presence of XLA-derived serum factors is sufficient to partially restore actin ring formation and resorptive activity in vitro.
Inflammatory cytokines in XLA serum stimulate osteoclast activity even in the absence of Btk
To determine whether the inflammatory cytokine levels detected in XLA patient serum were sufficient to induce osteoclast differentiation and activation, neutralizing antibodies to the individual cytokines TNF-α, IL-1β, and IL-6 were added to the osteoclast cultures in the presence of either 5% donor serum or 5% XLA patient serum. The number of actin rings per dentine (mean ± SEM) in cultures of healthy donor osteoclasts in the presence of XLA serum was greatly inhibited by the addition of neutralizing antibodies to all three cytokines (5.0 ± 1.6) or anti-IL-1 alone (8.5 ± 0.9) compared with addition of the isotype control antibody (27.6 ± 4.6) (Fig. 5A–D). Likewise, XLA monocyte–derived osteoclasts in the presence of XLA serum were significantly inhibited by the addition of neutralizing antibodies to all three of the cytokines (0.0 ± 0.0) or anti-IL-1 alone (0.0 ± 0.0) compared with the isotype control (8.0 ± 2.0) (Fig. 5E–H). These results support our hypothesis that inflammatory cytokines present in XLA serum are capable of driving increased osteoclastogenesis in vitro.
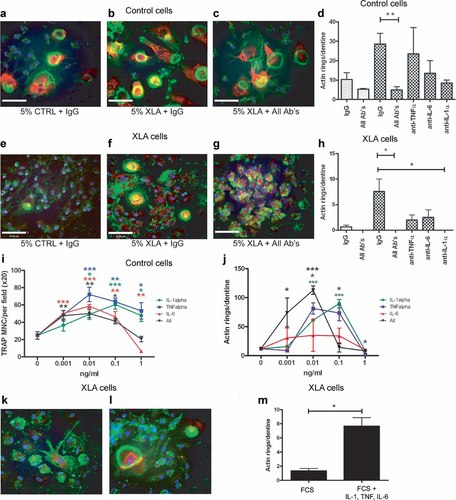
Inflammatory cytokines in XLA serum stimulate osteoclast activity even in the absence of Btk. All experiments were carried out in the presence of M-CSF (25 ng/mL) and RANKL (20 ng/mL) and cultured for 10 days for confocal analysis of actin ring formation. Neutralizing antibodies were used at 10 µg/mL. Colors used in confocal images represent cathepsin K (red), actin cytoskeleton with phalloidin (green), and nuclei with DAPI (blue). Control or XLA monocyte–derived osteoclasts were supplemented with either (A, E) 5% control, (B, F) 5% XLA serum plus IgG isotype antibodies, or (C, G) 5% XLA serum plus anti-TNF-α, anti-IL-6, and anti-IL-1β. Quantification of the mean (± SEM) actin ring number per dentine of (D) control osteoclasts or (H) XLA-derived osteoclasts in the presence the antibodies. Enumeration of (I) TRACP+ MNC formation and (J) actin ring formation of control osteoclasts in the presence of exogenous TNF-α, IL-6, or IL-1β or a combination of all three in a dose range from 0.001 to 1 ng/mL. Actin ring formation of XLA-derived osteoclasts in 10% FCS (K) without or (L) with the addition of recombinant IL-1β, TNF-α, and IL-6 all at 10 pg/mL. (M) Enumeration of actin rings formed in the presence and absence of the cytokines. Data are presented as the mean (± SEM) number of TRACP+ cells per field of view. Statistical analysis performed using the paired t test. *p < .05.
It is surprising that despite the low picogram per milliliter concentrations of cytokine present in the serum samples, there is a significant effect on osteoclast activity. To confirm that these cytokines can indeed promote osteoclast formation and activity at low concentrations, normal osteoclast cultures were supplemented with a dose range of IL-1β, TNF-α, or IL-6, either individually or combined, from 1 pg/mL to 1 ng/mL. The cytokine addition resulted in increased TRACP+ cell formation between 1 and 100 pg/mL for all of the cytokines tested (Fig. 5I), and actin ring formation also was increased significantly by the cytokine addition, with the exception of IL-6 (Fig. 5J). At 1 ng/mL (Fig. 5I, J) and higher concentrations (data not shown), these cytokines inhibited both formation and activation of the human osteoclast cultures.
Furthermore, we demonstrate that actin ring formation can be partially restored in XLA-derived osteoclasts by the addition of a cocktail of all three cytokines (Fig. 5K–M).
Discussion
There are numerous processes controlling the ability of osteoclasts to effectively resorb calcified matrices. It is essential that a sealing zone is made with a thick F-actin ring to isolate the underlying bone from surrounding areas. Some of the signaling molecules involved in F-actin ring-sealing zone formation include Src, Vav, WASP, and dynamin, all of which have been shown to interact with Btk.22, 23 In this study using osteoclasts derived from human XLA patients, there was significant inhibition of both F-actin ring formation and lacunar resorption on dentine substrate in vitro (Fig. 2). However, Btk expression was not required for podosome formation on glass (Fig. 1F, G), suggesting that Btk-independent pathways of actin reorganization also exist. WASP is essential for F-actin ring formation in osteoclasts. It links upstream signals to actin polymerization and branching by stabilizing Arp2/3 complexes via phosphoinositide activation.13 Competitive inhibition of phosphoinositides following osteoclast αvβ3 receptor engagement results in disruption of F-actin ring formation and resorption function, with an increased propensity for formation of F-actin-enriched patchlike structures.24 These reported effects closely resemble what we have observed here in XLA patient osteoclasts. Since Btk associates directly with and phosphorylates WASP,25 it is possible that Btk is important for actin-dependent functions of osteoclasts cultured on dentine. Other actin-dependent processes such as phagocytosis and chemotaxis are impaired in XLA-derived cells.26 Overall, these data show that Btk is necessary for effective organization of cytoskeletal proteins, formation of F-actin rings, and resorptive functions on dentine.
Surprisingly, despite the obvious defects in human Btk-deficient osteoclasts in vitro, the measurements of bone turnover markers and heel ultrasound showed that the XLA patients had no significant bone abnormalities. This is in contrast to the observations in Btk/Tec-deficient mice, which are osteopetrotic. Unlike Btk-deficient mice, which are kept in clean animal facilities, XLA patients are exposed to a myriad of environmental pathogens that may alter bone homeostasis. We found significantly raised levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β) in XLA compared with control serum, cytokines well documented as being of particular importance in bone pathophysiology.27
TNF-α is among the most potent of the osteoclastogenic cytokines implicated in the osteolysis associated with chronic inflammatory diseases such as rheumatoid arthritis, orthopedic implant loosening, and periodontal disease.28-30 TNF-α induces a relative increase in the ratio of RANKL to OPG in stromal and osteoblastic cells.31-33 Numerous researchers have confirmed the synergism between TNF-α and RANKL in osteoclast formation, whereas others provide evidence to suggest that TNF-α stimulates macrophages directly to differentiate into osteoclasts by a mechanism independent of RANKL.34, 35 IL-6 stimulates osteoclast activity and bone resorption by an indirect mechanism, stimulating the expression of RANKL and IL-1 by osteoblasts.36 It is of particular importance in the development of generalized bone loss associated with conditions such as Chrohn's disease and ulcerative colitis,37 and blocking IL-6 receptor inhibits osteoclast formation in vitro and in vivo.38 IL-1 has a direct and potent stimulatory effect on osteoclast resorption activity both in vitro and in vivo39, 40 and has been shown to act via a Src-TRAF6 complex to regulate cytoskeletal organization.41 We found that serum from XLA patients contained enhanced levels of inflammatory cytokines compared with serum from control donors (Fig. 3H–J) and that the addition of 5% serum to either XLA to control osteoclast cultures significantly enhanced the osteoclastogenic activity compared with 5% serum from controls in vitro (Fig. 4). The addition of these three cytokines in combination was sufficient to restore actin ring formation in XLA osteoclasts (Fig. 5K–M). The enhanced actin ring formation in the presence of XLA serum was ameliorated by the addition of neutralizing antibodies to each cytokine, especially IL-1, with all the antibodies in combination providing the most significant reduction (Fig. 5A–H). These cytokines also have been shown to increase RANKL production by osteoblasts and fibroblasts,33, 42 adding to the production of factors that promote osteoclast formation and activation. Thus the increased cytokine production in these patients is likely to restore osteoclast activity and maintain bone homeostasis by both direct and indirect actions on osteoclasts.
In this study we show that Btk is required for efficient osteoclast activation in humans. In murine Btk-deficient osteoclasts, there was a failure in both formation and activation,8 giving rise to an osteopetrotic phenotype that reflects the osteoclast defect.6 XLA patients exhibited normal bone turnover parameters, suggesting that the studies in pathogen-free mouse models may not address the complexity of Btk deficiency under environmental stress conditions. We have determined that humans with Btk deficiency, who lack B cells and thus antibody production, have an elevated systemic inflammatory status, most likely as a consequence of chronic pulmonary or gastric infections.16, 17 We postulate that this systemic overproduction of TNF-α, IL-6, and IL-1β cytokines induces an increase in osteoclast activity that is sufficient to compensate for the inherent osteoclast Btk signaling defect and normalize the bone density of XLA patients. In conclusion, these findings demonstrate that Btk plays a role not only in human innate and humoral immune responses but also in osteoclast-specific functions, all of which have transitive implications on skeletal health.
Disclosures
All the authors state that they have no conflicts of interest.
Acknowledgements
This work was funded by Arthritis Research UK.