Vitamin D Supplementation in Young White and African American Women
ABSTRACT
There is limited information on the effects of vitamin D on serum 25 hydroxyvitamin D (25OHD) in young people and none on African Americans. The main objective of this trial was to measure the effect of different doses of vitamin D3 on serum 25OHD and serum parathyroid hormone (PTH) in young women with vitamin D insufficiency (serum 25OHD ≤ 20 ng/mL (50 nmol/L). A randomized double-blind placebo-controlled trial of vitamin D3 was conducted in young white and African American women, age 25 to 45 years. A total of 198 healthy white (60%) and African American (40%) women were randomly assigned to placebo, or to 400, 800, 1600, or 2400 IU of vitamin D3 daily. Calcium supplements were added to maintain a total calcium intake of 1000 to 1200 mg daily. The primary outcomes of the study were the final serum 25OHD and PTH levels at 12 months. The absolute increase in serum 25OHD with 400, 800, 1600, and 2400 IU of vitamin D daily was slightly greater in African American women than in white women. On the highest dose of 2400 IU/d, the mixed model predicted that mean 25OHD increased from baseline 12.4 ng/mL (95% confidence interval [CI], 9.2–15.7) to 43.2 ng/mL (95% CI, 38.2–48.1) in African American women and from 15.0 ng/mL (95% CI, 12.3–17.6) to 39.1 ng/mL (95% CI, 36.2–42.0) in white women. There was no significant effect of vitamin D dose on serum PTH in either race but there was a significant inverse relationship between final serum PTH and serum 25OHD. Serum 25OHD exceeded 20 ng/mL in 97.5% of whites on the 400 IU/d dose and between 800 and 1600 IU/d for African Americans. The recommended dietary allowance (RDA) suggested by the Institute of Medicine for young people is 600 IU daily. The increase in serum 25OHD after vitamin D supplementation was similar in young and old, and in white and African American women. © 2014 American Society for Bone and Mineral Research.
Introduction
Vitamin D plays a potent role in bone and mineral metabolism. Severe deficiency occurs when serum 25 hydroxyvitamin D (25OHD) is less than 10 ng/mL and may be associated with osteomalacia. Vitamin D insufficiency, defined as a serum 25OHD ≤ 20 ng/mL leads to secondary hyperparathyroidism1 that causes increased bone loss, osteoporosis, and increased fracture risk. Vitamin D deficiency occurs most commonly in very young and very old people but is less common during the teenage years.2-5 In adolescents the effects of vitamin D insufficiency may be more subtle and may result in inhibition of the development of peak bone mass.6, 7
The largest input of vitamin D into the body is from sunlight; diet is a smaller contributor except in areas where there is a very high intake of fish. Vitamin D from diet and sunlight is not biologically active and has to be converted into two metabolites, first in the liver to 25OHD and second in the kidney to the active metabolite 1,25 dihydroxyvitamin D (1,25(OH)2D).8 The principal physiological functions of 1,25(OH)2D) on mineral metabolism are to increase absorption of calcium and phosphorus in the gut, maintain normocalcemia, and provide an adequate amount of calcium and phosphorus for normal mineralization of bone.
Various studies have been conducted that show African Americans have lower serum 25OHD levels compared to whites.9, 10 This could be attributed to the increased melanin pigmentation that interferes with absorption of ultraviolet B light and formation of vitamin D in the skin11 or result from lower vitamin D absorption in the intestine. In a recent work we did not find a difference between older whites and African Americans in serum 25OHD levels after supplementation with six doses of vitamin D,12, 13 suggesting that lower serum 25OHD in African Americans is due to lower skin production and that vitamin D absorption from the gut is similar. The recent Institute of Medicine (IOM) report with its new recommendations for vitamin D and calcium intake noted that nearly all data was collected on white women and that there was no significant information for vitamin D and calcium in other ethnic groups.1 There has been only one study that directly compared the effect of vitamin D on serum 25OHD in white and African American women and performed in older women, average age 68 years, and there was no difference in the serum 25OHD after vitamin D.12 There have been no comparative studies in younger adults of vitamin D supplementation in white and African American women.
The main objective of this 1-year treatment trial was to study the effect of increasing doses of vitamin D3 on serum 25OHD and serum parathyroid hormone (PTH) in younger white and African American women with vitamin D insufficiency; ie, serum 25OHD ≤20 ng/mL (50 nmol/L).
Subjects and Methods
Study design and subject population
The study design was a 1-year prospective randomized placebo-controlled trial aimed at establishing the dose of vitamin D3 required to increase serum 25OHD and normalize serum PTH in 97.5% of study subjects. A total of 198 healthy young women, 119 white and 79 African American met the inclusion and exclusion criteria and entered into study.
The main inclusion criteria were age 25 to 45 years old and serum 25OHD levels ≤20 ng/mL (50 nmol/L). Exclusion criteria were as follows: pregnant, significant comorbidities, history of cancer except skin cancer within the last 10 years, uncontrolled type I diabetes ± significant proteinuria or fasting blood sugar >140 mg in type II diabetes, active kidney stone disease or history of kidney stones more than two times previously, chronic renal failure (serum creatinine >1.4 mg/dL), evidence of chronic liver disease, alcoholism, severe vitamin D deficiency (ie, serum 25OHD <5 ng/mL [<12.5 nmol/L], BMI >45 kg/m2, serum calcium ≥10.3 mg/dL (>2.575 mmol/L), 24-hour urine calcium >290 mg (7.25 mmol) on two baseline tests, or a T-score < –3 on spine or hip (specific to race). Additional exclusion criteria were use of bone active drugs: fluoride, PTH or derivatives, calcitonin, estrogen during the last 6 months, chronic high-dose corticosteroid therapy (>10 mg/d), bisphosphonates for more than 3 months in the past, anticonvulsants, or high-dose thiazide therapy (>37.5 mg/d). Recruitment was obtained from local population by advertising the study in local newspapers, church bulletins, hair salons, and on social media.
The institutional review board at Creighton University, Omaha, NE, USA, approved the study protocol. All participants signed and received a copy of an informed consent form. A data safety and monitoring board was established before the study started.
Randomization and treatment
White and African American women who met the eligibility criteria were randomly assigned to one of four vitamin D dose groups (400, 800, 1600, or 2400 IU/d) or placebo. The study design was double-blind; ie, all the study participants as well as staff that assessed primary outcomes were blinded to the treatment, and only the statistician had access to the treatment code. The randomization method was randomized blocks with block sizes of four and eight, stratified by race and screening serum 25OHD level (<15 versus ≥15 ng/mL for whites and <12 versus ≥12 ng/mL for African Americans). The study statistician generated the randomization list with SAS software (SAS Institute Inc., Cary, NC, USA). Screening occurred throughout the year from January 2008 to January 2010.
Vitamin D3 in the 400 IU, 800 IU, 1600 IU, or 2400 IU capsules and matching placebo capsules were manufactured specifically for this study by Douglas Labs (Pittsburgh, PA, USA). The actual vitamin D3 concentrations in the capsules were measured independently in Dr. Hector DeLuca's laboratory located at the University of Wisconsin (Madison, WI, USA) every 6 months over 3 years. There was no significant change in potency over the time period. The actual content of vitamin D3 was 503 IU in the 400 IU capsule, 910 IU in the 800 IU capsule, 1532 IU in the 1600 IU capsule, and 2592 IU in the 2400 IU capsule. Every participant was advised to take one vitamin D3 capsule in the morning after breakfast. Before the study randomization each subject collected a 7-day dietary record. From this the dietician estimated the average vitamin D and calcium intake. Based on the analysis, calcium supplements 200 mg elemental (Citracal; Bayer Health-Care, Morristown, NJ, USA) were given to maintain a total calcium intake between 1000–1200 mg/d and subjects were advised to take calcium capsules in divided doses. A central medication log was maintained of all study drugs dispensed to the subjects. At 3-month, 6-month, 9-month, and 12-month visits compliance was calculated by counting the pills returned and dispensing new study pills. Information was also collected on concomitant medications and over-the-counter supplements at each visit. Subjects were not allowed to take other vitamin D supplements during the study, those who wanted to take multivitamins were provided with multivitamins without vitamin D (Kirkman multivitamin without vitamins A&D; Oregon, WA, USA) at no cost.
At baseline subjects underwent a medical history; previously validated questionnaires were used for smoking history, alcohol use, caffeine intake, depression scale, sun exposure, physical activity, and fall and fracture history/incidence. Fasting blood samples were collected at all visits: baseline, 3, 6, 9, and 12 months, between 7:00 a.m. and 10:00 a.m. After serum separation samples were stored frozen at −70°C until analysis. A comprehensive panel including serum calcium, creatinine, complete blood count, and lipid profile was performed at baseline and 12 months. A basic metabolic panel was done at 3, 6, and 9 months. Serum 25OHD, serum PTH, and serum N-telopeptides (NTx) were collected at baseline, 6 months, and 12 months. A 24-hour urine was collected at baseline and every 3 months for measurement of calcium and creatinine. Serum and urine chemistries were measured at the Creighton University Clinical Chemistry Laboratory. Serum 25OHD at screening was measured by radioimmunoassay (RIA) after an acetonitrile extraction in the Bone Metabolism Laboratory using kits manufactured by Diasorin, Inc. (Stillwater, MN, USA). The minimum detection range reported from Diasorin and in our laboratory is 5 ng/mL. The interassay variation is 9.8% and intraassay variation is 9.2% in our laboratory. The Bone Metabolism Laboratory does participate in the vitamin D External 159 Quality Assessment Scheme (DEQAS),7 which is an international program for monitoring the accuracy of serum 25OHD assays; our results were within ± 1 SD of the all-laboratory trimmed mean. Serum intact PTH was measured by Diasorin immunoradiometric assay. The lower limit of detection for serum PTH in our laboratory is 1.0 pg/mL. The interassay variation is 4.1% and intraassay variation is 2.9%. Dietary intake of calcium and vitamin D at baseline was collected from 7-day food diaries and analyzed by a trained dietician.
Primary outcomes
The primary outcomes are presented in this work and results of secondary outcomes will follow in subsequent works.
The primary outcomes were the dose response of serum 25OHD and serum PTH levels after vitamin D3 supplementation for 12 months. Serum 25OHD and serum PTH were measured at baseline, 6 months, and 12 months. Secondary outcomes in this work are serum calcium and 24-hour urine calcium. Data on adverse events was collected at each visit. An adverse event was defined as any side effect that occurred while the participant was in trial.
The normal range for serum calcium is 8.5 to 10.2 mg/dL. For study purposes elevated serum calcium was defined as fasting serum calcium >0.3 mg/dL above the upper limit of normal at any visit; ie, ≥10.6 mg/dL (2.65 mmol/L). If the serum calcium level was found to be elevated, then serum calcium analysis was repeated within 2 weeks. If the repeat value was confirmed as high, calcium supplements were stopped and serum calcium was repeated again within 1 week. If serum calcium still remained high then the study drug was stopped.
For study purposes elevated urine calcium was defined as 24-hour urine calcium >400 mg (10 mmol) at any of the follow-up visits. If hypercalciuria (>400 mg) developed during the treatment period then a 24-hour urine calcium was repeated within 2 weeks. If hypercalciuria persisted then calcium supplements were discontinued and dietary calcium rechecked. A repeat 24-hour urine was performed in 2 to 4 weeks and if elevation persisted then the study drug was discontinued.
Sample size justification
The primary endpoints for sample size calculation were serum 25OHD and PTH at the 12-month visit. For serum 25OHD from our previous studies we estimated the placebo group would have a 12-month 25OHD level of 15.6 ng/mL with a SD = 3.3 ng/mL and the 2400 IU/d dose group was estimated to have a 12-month level of 40 ng/mL (SD = 10.7 ng/mL). Using the larger SD, we would expect a 2.3-SD difference between the dose groups. For serum PTH, based on our previous studies, the placebo group is expected to have a 12-month mean serum PTH of 34.6 pg/mL and the 2400 IU/d dose group mean serum PTH of 26.3 pg/mL with an SD difference of 9.8, giving an estimated effect of 0.84 SD difference between dose groups. Assuming that the means for the dose groups are approximately equally-spaced in this range, a 0.05 level of significance, and a total dropout rate of 10%; 20 subjects randomized to each dose group for each race will provide over 90% power to detect a 2.3-SD difference between the doses for serum 25OHD and a 0.84-SD difference for serum PTH. There is 80% power to detect a 0.84-SD difference between the race groups, assuming a clinically significant difference between the races to be 9 ng/mL (SD = 10.7) for serum 25OHD and a 0.20-SD difference for PTH, assuming a clinically significant difference of 2 pg/mL (SD = 9.8).
Statistical analysis
Analysis included all subjects that were randomized. For subjects who dropped out or were removed from the study, their data was included in the analysis if available. Subject characteristics at baseline were compared between the dose groups and by race.
Mixed effects models were used to estimate dose-response curves for serum 25OHD and PTH. Dose (as continuous) and time (as categorical, baseline, 6 months, and 12 months) were included as fixed effects with random subject effects. Quadratic and cubic terms were explored for dose as well as interactions with dose, and log transformations for outcome. Models that were linear were determined to fit well for both serum 25OHD and PTH. A logarithmic transformation was required for serum PTH but not for serum 25OHD. Model fit was examined by looking at various residual plots. One-thousand bootstrapped samples were used to determine the 95% prediction limits for the 12-month serum 25OHD levels. The recommended daily allowance (RDA) is estimated as the dose at which the 95% prediction lower limit is above a serum 25OHD of 20 ng/mL, at this dose 97.5% of women would be predicted to have serum 25OHD >20 ng/mL. The estimate of the average requirement (EAR), the dose at which serum 25OHD is >20 ng/mL in 50% of the subjects, was found similarly.
Missing serum 25OHD and PTH caused by loss of follow-up are possibly related to the subject's dose (non-ignorable missingness). Within the mixed effects models we tested for missing completely at random (MCAR) following the method of Park and Lee.14 Briefly, this method uses indicator variables for the missing data pattern and the coefficients for these indicator variables are tested to determine which are significantly different from zero. The missing data mechanism should not be assumed to be MCAR if any of these indicator variables are significantly different from zero.
The mixed model method was used to fit a generalized linear mixture model that is appropriate when the MCAR assumption is violated, to examine factors associated with outcome.15 In this approach, the change in outcome over time is assumed to be dependent on the dropout time.
Multivariate mixed effects models were also examined. The models adjust for known covariates based on clinical experience: season at baseline, age, BMI category (<25, 25–29.9, ≥30), calcium intake, smoking status, alcohol use, and serum creatinine. Interactions between dose and covariates were explored and interactions significant at the 0.10 levels were retained in the model. SAS software was used for the statistical analysis. R version 2.11.0 was used to create graphical displays. Values of p <0.05 were considered statistically significant.
Results
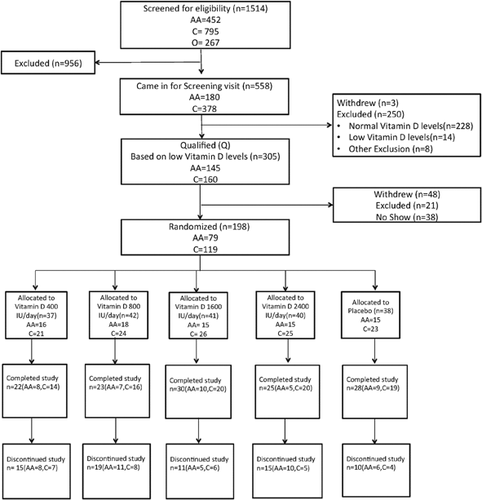
A total of 198 subjects were randomized in the study; 128 subjects (65%) completed the study and 70 subjects withdrew after randomization. The reasons for withdrawal were as follows: lost to follow-up, 48; health issues, 8; scheduling issues, 6; personal reason, 7; and noncompliant, 1. Details of the recruitment, randomization, and distribution of the subjects (CONSORT) are shown in Fig. 1. The baseline characteristics for the complete study population are shown in Table 1 and are separated by race group in Table 2.
| All subjects (n = 198) | Placebo (n = 38) | Dose 400 IU/d (n = 37) | Dose 800 IU/d (n = 42) | Dose 1600 IU/d (n = 41) | Dose 2400 IU/d (n = 40) | |
|---|---|---|---|---|---|---|
| Age (years) | 36.7 (5.9) | 36.4 (6.1) | 35.6 (6.6) | 37.7 (5.5) | 36.4 (5.9) | 37.2 (5.4) |
| BMI (kg/m2) | 30.2 (6.6) | 30.9 (6.7) | 31.0 (8.1) | 31.1 (5.8) | 28.4 (5.4) | 30.0 (6.6) |
| Race, n (%) | ||||||
| African American | 79 (40%) | 15 (39%) | 16 (43%) | 18 (43%) | 15 (37%) | 15 (38%) |
| White | 119 (60%) | 23 (61%) | 21 (47%) | 24 (57%) | 26 (63%) | 25 (62%) |
| Smoking status, n (%) | ||||||
| Current smoker | 36 (18%) | 5 (13%) | 8 (22%) | 7 (17%) | 9 (22%) | 7 (18%) |
| Former smoker | 35 (18%) | 5 (13%) | 8 (22%) | 7 (17%) | 7 (17%) | 8 (20%) |
| Never smoker | 127 (64%) | 28 (74%) | 21 (57%) | 28 (66%) | 25 (61%) | 25 (62%) |
| Alcohol use, n (%) | ||||||
| No | 54 (27%) | 11 (29%) | 13 (35%) | 9 (21%) | 8 (20%) | 13 (32%) |
| Yes | 144 (73%) | 27 (71%) | 24 (65%) | 33 (79%) | 33 (80%) | 27 (68%) |
| Serum calcium (mg/dL) | 9.2 (0.3) | 9.1 (0.3) | 9.2 (0.3) | 9.2 (0.3) | 9.2 (0.3) | 9.1 (0.3) |
| 24-Hour urine calcium (mg) | 138.1 (77.4) | 131.9 (86.6) | 144.9 (73.2) | 120.9 (74.6) | 158.2 (80.2) | 135.2 (70.2) |
| Serum creatinine (mg/dL) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.7 (0.1) | 0.7 (0.1) | 0.8 (0.1) |
| Serum alkaline phosphatase (IU/L) | 59.2 (17.5) | 61.1 (17.7) | 60.4 (13.1) | 59.2 (18.1) | 60.4 (20.7) | 55.1 (17.1) |
| Serum glucose (mg/dL) | 94.8 (15.8) | 93.7 (8.4) | 92.1 (9.6) | 99.3 (25.7) | 95.5 (16.7) | 92.9 (9.8) |
| Serum AST (IU/L) | 19.9 (5.5) | 20.6 (6.2) | 19.9 (5.2) | 18.8 (3.8) | 20.0 (6.2) | 20.3 (6.0) |
| Serum ALT (IU/L) | 17.6 (9.4) | 18.6 (9.1) | 17.4 (7.5) | 16.2 (6.0) | 18.1 (11.8) | 17.8 (11.3) |
| Serum 25OHD (ng/mL) | 13.4 (4.5) | 12.7 (4.1) | 13.1 (4.2) | 13.8 (4.3) | 13.3 (5.1) | 14.1 (4.8) |
| Serum PTH (pg/mL) | 36.1 (13.7) | 36.7 (12.1) | 33.0 (11.8) | 38.3 (16.6) | 35.9 (14.6) | 36.4 (12.4) |
| Dietary vitamin D intake/d (mg) | 100 (72) | 108 (82) | 102 (69) | 89 (75) | 113 (84) | 91 (43) |
| Dietary calcium intake/d (mg) | 655 (262) | 677 (275) | 627 (248) | 599 (249) | 681 (282) | 692 (254) |
| Current drug use | ||||||
| Thiazide use | 12 (6%) | 2 (5%) | 1 (3%) | 2 (5%) | 3 (7%) | 4 (10%) |
| Diuretic use | 1 (0.5%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
- Values are mean (SD) or n (%).
- BMI = body mass index; AST = aspartate aminotransferase; ALT = alanine aminotransferase; 25OHD = 25 hydroxyvitamin D; PTH = parathyroid hormone.
| Total | African American | White | |
|---|---|---|---|
| Age (years) | 36.7 (5.9) | 35.1 (6.0) | 37.7 (5.6) |
| BMI (kg/m2) | 30.2 (6.6) | 32.5 (6.7) | 28.8 (6.1) |
| Smoking status, n (%) | |||
| Current smoker | 36 (18%) | 11 (14%) | 25 (21%) |
| Former smoker | 35 (18%) | 12 (15%) | 23 (19%) |
| Never smoker | 127 (64%) | 56 (71%) | 71 (60%) |
| Alcohol use, n (%) | |||
| No | 54 (27%) | 26 (33%) | 28 (24%) |
| Yes | 144 (73%) | 53 (67%) | 91 (76%) |
| Serum calcium (mg/dL) | 9.2 (0.3) | 9.1 (0.3) | 9.2 (0.3) |
| 24-Hour urine calcium (mg) | 138.1 (77.4) | 114.8 (73.2) | 153.6 (76.5) |
| Serum creatinine (mg/dL) | 0.8 (0.1) | 0.8 (0.1) | 0.7 (0.1) |
| Serum alkaline phosphatase (IU/L) | 59.2 (17.5) | 61.3 (19.4) | 57.9 (16.2) |
| Serum glucose (mg/dL) | 94.8 (15.8) | 93.3 (10.8) | 95.8 (18.4) |
| Serum AST (IU/L) | 19.9 (5.5) | 20.4 (6.0) | 19.6 (5.2) |
| Serum ALT (IU/L) | 17.6 (9.4) | 17.9 (11.5) | 17.4 (7.7) |
| Serum 25OHD level, screening (ng/dL) | 13.4 (4.5) | 11.6 (4.1) | 14.6 (4.4) |
| Serum PTH (pg/mL) | 36.1 (13.7) | 40.9 (16.3) | 32.9 (10.5) |
| Dietary vitamin D intake/d (mg) | 100 (72) | 96 (79) | 103 (66) |
| Dietary calcium intake/d (mg) | 655 (262) | 503 (159) | 756 (268) |
| Current drug use | |||
| Thiazide use | 12 (6%) | 8 (10%) | 4 (3%) |
| Diuretic use | 1 (0.5%) | 0 (0%) | 1 (0.8%) |
- Values are mean (SD), n = 198.
- BMI = body mass index; AST = aspartate aminotransferase; ALT = alanine aminotransferase; 25OHD = 25 hydroxyvitamin D; PTH = parathyroid hormone.
Mean compliance of vitamin D in white women was 88% and in African American women was 84%. Mean compliance of calcium in white women was 91% and in African American women it was 82%.
Serum 25OHD results in white and African American women
A mixed-effects model was used to estimate the dose-response curve of serum 25OHD. In fitting the model, 198 subjects had serum 25OHD levels at baseline, 142 at 6 months, and 129 at 12 months. The model fit was determined to fit well. Significant interaction terms were present between race, dose, and time (p = 0.019), between dose and race (p = 0.011), and time and race (p = 0.0059). Because there are significant interactions with race in the model, the dose-response curves for African American and white women were estimated separately.
A total of 119 white subjects had serum 25OHD levels at baseline, 97 at 6 months, and 90 at 12 months (Fig. 2); 79 African American women had serum 25OHD levels at baseline, 45 at 6 months, and 39 at 12 months (Fig. 3). The model included significant fixed effects for time, dose, and the dose by time interaction. In white women, the slopes did not differ significantly between the 6-month and 12-month curves (p = 0.35), but the intercepts did marginally (p = 0.045); in African American women neither the slopes (p = 0.33) nor intercepts (p = 0.88) differed significantly (Supplementary Table 1). Based on the confidence interval (CI) for the slope of the dose-response curve, which was greater in the African American women than white women, one could conclude that there was a greater dose response in African Americans. However, African American women started at lower levels of serum 25OHD and their greater response to vitamin D supplementation resulted in 12-month serum 25OHD levels that were similar to white women at the higher doses. On the highest dose of vitamin D 2400 IU/d, mean serum 25OHD increased from a baseline of 15.0 (95% CI, 12.3–17.6) ng/mL to 39.1 (95% CI, 36.2–42.0) ng/mL in white women and from 12.4 (95% CI, 9.2–15.7) ng/mL to 43.2 (95% CI, 38.2–48.1) ng/mL in African American women.
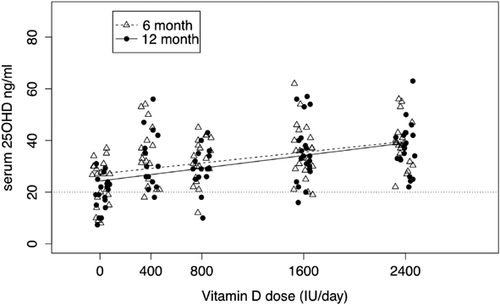
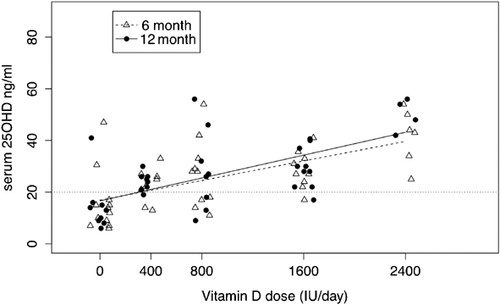
If a serum 25OHD of 20 ng/mL is used as an indicator for the RDA for vitamin D then the prediction limits estimated the RDA in white women to be 400 IU/d and between 800 and 1600 IU/d in African American women. If the interpolation of the prediction limits is used in African Americans then the RDA is 1200 IU daily. The EAR was 400 IU/d in both groups.
The mixed effects models of dose response were adjusted for clinically important covariates: age, BMI, total calcium intake (diet + supplements), smoking status, alcohol use, and serum creatinine. Interactions between dose and covariates were explored and nonsignificant interactions were excluded. Indicator variables for dropout time (6 or 12 months) were included in the multivariate model to account for the non-ignorable missing pattern.14, 15
Serum creatinine and the interaction between dose*BMI and dose*age are significant covariates in the white multivariate model An increase in age from 25 to 45 years predicted a decrease in 12-month serum 25OHD after vitamin D of 6 ng/mL in the older women by age 45 years. The BMI effect predicts an increase in serum 25OHD of 3.3 (95% CI, 0.016–6.6) ng/mL in subjects with BMI <25 compared to those with a BMI ≥30.
Serum PTH results in white and African American women
There was insufficient serum for PTH in 2 white subjects at 6 months, for 1 subject at 12 months, and for 1 African American subject at 6 months. In both races we found that log serum PTH differs significantly over time (African Americans p = 0.0002, whites, p < 0.0001), but does not differ significantly with dose (African Americans = 0.83, whites = 0.63, p = 0.63). However, there was a significant correlation between serum PTH and serum 25OHD in both white and African American women (Figs. 4 and 5). The decrease in serum PTH was maximal at 6 months and did not decrease further between 6 and 12 months. A mixed effects model was used to examine the relationship between serum PTH and serum 25OHD and showed that serum 25OHD is a significant predictor of serum PTH (Supplementary Table 2).
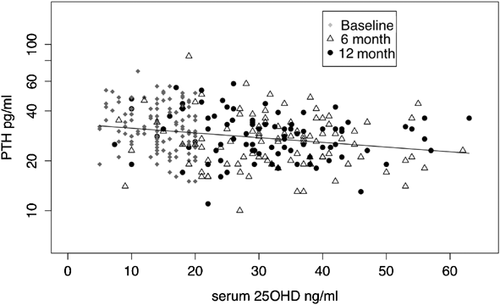
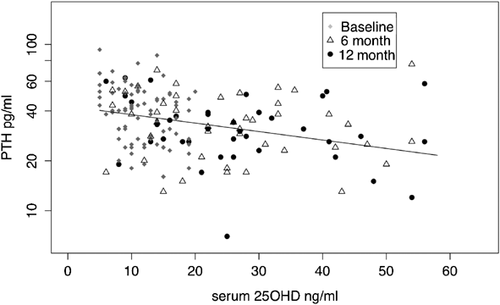
In the white multivariate model for serum PTH there was a significant interaction between dose and smoking status and dose*age. Current smokers have significantly higher serum PTH than never-smokers. BMI was a marginal predictor of serum PTH in the multivariate model (p = 0.064).
24-Hour urine calcium
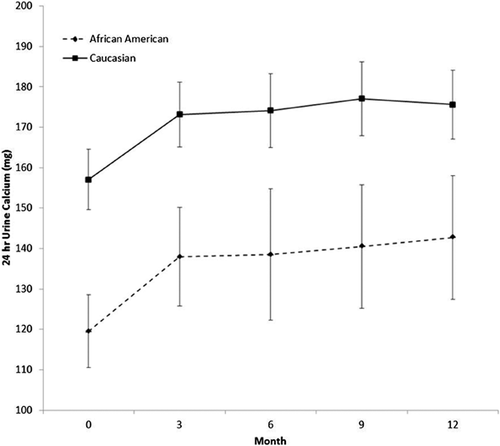
On average, urine calcium levels in whites are 41 mg higher than in African Americans (95% CI, 21–61; p < 0.0001). There was no race*time interaction (p = 0.99), indicating that the change over time was similar for both races. There was a marginal effect of time (p = 0.058). Baseline tends to be lower than the others (but not significantly so, if you adjust for multiple comparisons). The other time points were not significantly different from each other (all p > 0.4) (Fig. 6).
Safety and adverse events
There were a total of five serious adverse events in 4 subjects: internal bruising and bleeding due to an auto accident; a subarachnoid hemorrhage followed by craniotomy and resection of a hemangioma; maxillary hypoplasia surgery; and a broken ankle and tibia. None of these events were attributed to study treatment.
Hypercalcemia, defined as serum calcium ≥10.3 mg/dL, occurred in only 1 African American subject in the 400 IU/d group at 6 months and was 10.4 mg/dL; there were no elevated values in white subjects. Mean 24-hour urine calcium increased in both white and African American women (Fig. 6). Hypercalciuria, defined as 24-hour urine calcium >400 mg, occurred in 1 African American and 2 white women. Twenty-four-hour urine calcium >300 mg occurred in 25 white (27%) and 9 African American women (21%). Any high test result was repeated after 2 weeks and all repeat values fell below <300 mg except for 1 subject. One of the subjects with hypercalciuria discontinued because she refused a retest at the 9-month visit. There was no correlation between the vitamin D dose and hypercalciuria. No renal stones were reported. There were no significant changes in other clinical chemistry tests.
Discussion
This is the first randomized controlled vitamin D dose-response study in a younger population of white and African American women. The increase in serum 25OHD levels after vitamin D supplementation is similar at 6 months to that at 12-months in both race groups. The dose-response curves differed by race, but both had a linear response in the dose range tested in this study. African American women had lower baseline serum 25OHD levels and the absolute increase in serum 25OHD after vitamin D supplementation was greater than in the white women, but final serum 25OHD levels were similar in both races at higher doses.
There have been very few long-term dose-response studies in white women of the same age group as in this study. In a study of 112 white women, aged 19 to 35 years, randomly assigned to placebo or 800 IU/d vitamin D3 for 1 year, mean serum 25OHD levels increased by 14 ng/mL from 25 ng/mL to 39 ng/mL.16 In a study performed in winter in Antarctica, where exposure to ultraviolet B (UV-B) rays is zero, 55 men and women aged 39 to 44 years were given vitamin D doses 400, 1000, or 2000 IU/d; mean serum 25(OH) D levels increased from baseline of 18 ng/mL to 23, 25, and 28 ng/mL, respectively.17 In two placebo-controlled studies of young men and women between 29 and 41 years old in Boston (MA, USA), 1000 IU/d of vitamin D2 or D3 increased serum 25OHD from 18 to 28 ng/mL and from 20 to 28 ng/mL, whereas the control group showed no change; these increases are similar to those in the present study.18, 19
Similar responses to vitamin D were found in white and African American women and men, average age 48 years, who were given varying doses of vitamin D ranging from 2000 to 4000 IU/d. In African Americans serum 25OHD increased from 16 to 38 ng/mL on a mean dose of 3880 IU/d and in whites serum 25OHD increased from 23 to 39 ng/mL on a mean dose of 2840 IU/d.20 In older women, mean age 68 years, final serum 25OHD was 42 ng/mL after 2400 IU/d and 47 ng/mL after 4800 IU/d in whites, and 39 ng/mL after 2400 IU/d and 50 ng/mL after 4800 IU/d in African Americans.12
The increase in serum 25OHD was minimally dependent on BMI in white women, the increase in serum 25OHD was 3.3 ng/mL greater in women with low BMI (<25 kg/m2) compared to women with a BMI ≥30 kg/m2. There were very few obese women in this study. Similar results were seen in our older women study in which serum 25OHD was 7 ng/mL higher in the lowest BMI group (<25 kg/m2) compared to the BMI >30 kg/m2 group.The BMI effect is more obvious in thinner women and the difference in serum 25OHD is very small between the BMI groups 25–29.9 kg/m2 and >30 kg/m2.12, 21
Other findings from this study are the decreases in serum PTH after vitamin D. In the young women there was no obvious dose-response effect on serum PTH; this could be attributed in part to the wide interindividual variability in serum 25OHD after vitamin D. However, when serum PTH is examined with final serum 25OHD as the dependent variable there is a significant inverse correlation between serum 25OHD and PTH, similar to that seen in many cross-sectional studies.22
With regard to the safety of vitamin D and calcium, hypercalcemia occurred in 1 young white subject and 1 young African American subject (0.8%) and hypercalciuria (>300 mg) occurred in 21% of young white women and 11% of young African Americans. The hypercalcemia and hypercalciuria events were not related to the vitamin D dose, and it seems likely that the calcium supplement is the cause. In the younger women the incidence of hypercalciuria and hypercalcemia is lower than that reported in older women.12, 13
One reason for the lower incidence of hypercalciuria in African Americans compared to whites is that mean baseline 24-hour urine calcium excretion is lower and remains significantly lower after vitamin D and calcium supplementation. Two previous studies performed in young African American and white subjects showed similar results; baseline 24-hour urine calcium was significantly lower in African Americans before and after calcitriol administration.11, 23 Explanations that have been suggested for the lower urine calcium in African American women are as follows: bone is more resistant to the resorbing effects of PTH and 1,25 dihydroxyvitamin D, there may be increased renal tubular reabsorption of calcium due to secondary hyperparathyroidism,23, 24 and possibly there is intestinal resistance to the effect of calcitriol on calcium absorption.25
The strengths of our study include its design and that it had adequate power to detect differences in 25OHD levels across a broad range of dose groups. Most vitamin D studies have been single-dose studies and did not use a dose-response design. This is the first long-term, multiple-dose–response, controlled trial carried out in a younger population.
Our study also has limitations. The dropout rate in the African Americans was 50%, especially in the highest dose group, and in whites it was 25%, leaving relatively small sample sizes for each dose group. Because it was conducted in healthy young whites and African Americans, these findings may not apply to other ethnic groups or those with medical problems. The highest vitamin D dosage used in our study was 2400 IU/d and it is not possible to predict the dose-response curve beyond this dose.
In summary, the increase in serum 25OHD after vitamin D doses of 400, 800, 1600, and 2400 IU/d in the group aged 25 to 45 years is similar to that in the group aged 57 to 87 years, and similar in white and African American women.
These studies allow us to make general recommendations for the RDA for a population of younger women. A dose of vitamin 400 IU/d increases serum 25OHD above 20 ng/mL in 97.5% of young white women. Recently, the IOM suggested that the RDA for people in the age group 19 to 50 years should be 600 IU/d.1 Vitamin D 1200 IU/d would exceed a serum 25OHD of 20 ng/mL in 97.5% of African Americans; however, there is more uncertainty because of smaller numbers in the groups.
Disclosures
JCG received calcium tablets from Bayer Pharmaceuticals at no cost. PJ and LS state that they have no conflicts of interest.
Acknowledgments
This study was supported by a grant from the Department of Defense (DOD) (W81XWH-07-1-201). The Department of Defense was not involved in the study design, protocol development except for review of the safety aspects of the trial; it was not involved in data collection, data analysis, data management or interpretation, or preparation of the manuscript. We thank all VITADAS participants and the Bone Metabolism Unit Research staff for their hard work and contribution to the study and Corinna Suiter for performing the assays. We thank Jane Meza, PhD, Professor and Chair, Department of Biostatistics Director, Center for Collaboration on Research, Design and Analysis at College of Public Health University of Nebraska Medical Center for statistical advice. We thank members of the Data Safety and Monitoring Board (Dr Dan Bikle, Dr Steve Harris, and Dr John Adams) for their scientific input.
Authors' roles: Study design: JCG and LMS. Study conduct: JCG. Data collection: JCG and LMS. Data analysis: LMS and JCG. Data interpretation: JCG, LMS, and PSJ. Drafting manuscript: JCG, LMS, and PSJ. Revising manuscript content: JCG, LMS, and PSJ. Approving final version of manuscript: JCG, LMS, and PSJ. JCG and LMS take responsibility for the integrity of the data analysis.