Development of a New Curcumin-Loaded Dental Varnish for Antimicrobial Photodynamic Therapy Application
Funding: This work was supported by UBC Faculty Start-Up Funds; UBC Advancing Multifunctional Dental Biomaterials (AMDB) Research Excellence Cluster; and Colgate Award for Research Excellence (CARE Award) (2020–4082462349).
ABSTRACT
The main objective of this study was to develop a natural curcumin-loaded varnish that responds to blue light in an antimicrobial photodynamic therapy-based approach with photoelimination of targeted cells. Fluoride-free curcumin-loaded varnish (CUR-V) consisting of gum rosin (80 wt%) and ethanol (20 wt%) was loaded with 0, 0.25, and 10.0 wt% curcumin and then analyzed with FTIR. Subsequent investigation included applying ~19 mW/cm2 blue light for 15, 30, or 60 min (in order of increasing energy dose) to the varnishes and measuring the change in varnish color and the production of reactive oxygen species (singlet oxygen and superoxide anion), as well as performing CFU count and XTT assays of 24-h mono-species biofilms of Streptococcus mutans and Candida albicans. Lastly, a 72-h dual-species biofilm of both species was assessed with a CFU count assay. As the light energy applied to the 0.25% CUR-V and 0.00% CUR-V samples increased, there was a reduced and quantifiable difference in color between these two varnish groups (p < 0.001). In addition, singlet oxygen production was most dependent on curcumin loading in the varnish, while superoxide anion production appeared more dependent on blue light energy (p < 0.001 for both). In subsequent investigations of mono- and dual-species biofilms, the ability to induce an agonistic antimicrobial response as a function of curcumin loading in the natural varnish and blue light energy was demonstrated. Inherently, as curcumin loading and blue light energy increased, the photoelimination of both species investigated increased. As expected, the impact of aPDT on the 72-h biofilm was less than that observed for the 24-h biofilm; however, the more mature dual-species biofilm still observed a photoelimination effect with greater curcumin concentration. This study provides early evidence toward the development of a new natural curcumin-loaded fluoride-free varnish that, when combined with blue light irradiation, shows significant potential to effectively provide a targeted antimicrobial response against cariogenic species. To date, there is no similar approach that can serve as a natural, fluoride-free over-the-counter alternative to assist in caries prevention and treatment.
1 Introduction
Oral health remains a global concern, with not only strong causative links to dental diseases but also to the general health of an individual. Unfortunately, the number of people unable to access oral treatment continues to increase, with more than 50% of the population worldwide suffering from the consequences of no oral health care coverage [1-3]. The direct cost associated with dental diseases is estimated at more than 350 billion USD globally, although almost 95% of this expenditure is concentrated in high-income regions of North America, Western Europe, East Asia, and High-income Asia Pacific [1-3]. As a result, considering the huge discrepancy between access to professional dental care and the high prevalence of dental caries in low- and middle-income countries, new and less costly strategies are needed to address the needs of underserved populations [1, 4, 5]. Among all oral diseases, the most prevalent condition continues to be dental caries in permanent/deciduous teeth, affecting approximately 3 billion people globally [6, 7].
Among the recommendations for public health intervention to manage dental caries, fluoride supplementation has been indicated as a low-cost strategy [4, 8-10]. Worldwide, this strategy has included community fluoride administration in water systems, tooth brushing with fluoride-containing toothpaste (1000–1500 ppm) after age 6, and regular application of high-concentration topical fluoride in the form of a varnish [4, 11]. Unfortunately, while a proven effective strategy [8, 9], the high fluoride concentration (22,600 ppm) in fluoride-containing varnishes and associated risks of systemic toxicity have limited its application to an in-office treatment by oral care providers 2–4 times a year [12]. Therefore, new efficacious, safe, and over-the-counter low-cost alternatives are still needed to reduce and manage caries incidence. A varnish which may be applied by the consumer directly to their own teeth on a semi-regular basis responds to photodynamic therapy (PDT) to manage caries-responsible microorganisms and readily rinses away after PDT is the strategy of interest here.
In the last decade, there has been an increase in the interest in PDT in the field of Dentistry, particularly in antimicrobial approaches [13-18]. Antimicrobial photodynamic therapy (aPDT) involves the use of a photosensitizer (PS), the presence of oxygen, and a light source of PS-matching wavelength to activate the PS. The resultant photochemical reaction produces reactive oxygen species (ROS) that cause damage to targeted cells and cell death [19, 20]. By requiring a minimum energy, wavelength, and PS concentration, PDT allows for more tuning of an antimicrobial response than many non-light-based therapies. An ideal PS should have a suitable half-life and selectivity for the target microorganisms, be biocompatible and soluble in water for easy administration in the oral environment and be readily available [20]. Curcumin, an orange natural compound from turmeric (Curcuma longa), has been shown to have anti-cancer, anti-inflammatory, antioxidant, and antimicrobial properties [21-28]. Recently, the aPDT potential of using curcumin with blue light irradiation has been explored against cariogenic pathogens in planktonic [22, 29, 30] and biofilm models [16, 22, 29-34], demonstrating the potential of using curcumin as a potent PS. However, most studies have utilized solutions of curcumin, with very few exploring curcumin-loaded dental care materials. This study will be the first to explore a curcumin-loaded and fluoride-free natural varnish in combination with blue light toward aPDT.
While several sources of light may be used with aPDT, light units containing blue light wavelength (450–550 nm) light-emitting diodes (LEDs) are widely used in dental offices as a tool for photopolymerization and thus are readily accessible for PDT within the dental field [20]. In addition, as low-powered blue LED devices are available in pharmacies and cosmetic stores for tooth bleaching, the accessibility of our proposed alternative—a natural fluoride-free varnish for aPDT application—to all communities is high [35, 36]. In this way, considering limitations of the commercially available high concentration fluoride varnish [37-39] and the antimicrobial and photocatalytic potential of curcumin [16, 29-34], an innovative fluoride-free curcumin-containing dental varnish to be associated with blue light for aPDT has been proposed in the present investigation. As a fundamental study seeking to provide new evidence of this varnish for an aPDT approach, the response of two cariogenic species (Streptococcus mutans [S. mutans] and Candida albicans [C. albicans]) to different energy levels of blue light treatment and curcumin concentrations loaded in the varnish was investigated. The metabolism of sugars by acidogenic/aciduric microorganisms, such as S. mutans, results in an acidic microenvironment within the biofilm that further aids in the selective growth of these microorganisms and promotes continuous dissolution of tooth enamel [40]. Meanwhile, C. albicans has been shown to not only enhance biofilm assembly, but to also support S. mutans viability within the extracellular polymeric substance (EPS)-based matrix [40-42] and aid in collagenolytic processes seen with dental caries [42-44]. Altogether, S. mutans and C. albicans are two oral species commonly investigated in a dual-species model for dental caries due to their synergisms [45]. In this study, both mono-species and dual-species models are used. It was hypothesized that the greater the curcumin concentration added to the varnish and the greater the blue light energy applied, the stronger the antibacterial response as measured by mono- and dual-species biofilm CFU count and XTT assays. The overarching aim of this study was to develop an accessible and effective natural dental varnish capable of treating an oral cariogenic biofilm with intentional application of blue light. More specifically, this innovative material aims to controllably reduce the concentration of pathogenic microorganisms in the oral cavity with a non-toxic strategy that is also accessible to resource-limited, vulnerable, and remote populations with poor access to preventive care provided by dental professionals.
2 Materials and Methods
2.1 Varnish Fabrication
In a glass beaker, 20 mL of anhydrous ethanol was gently warmed to ~60°C on a Corning hot plate stirrer (Model PC-351, Corning Incorporated, Corning, NY, USA) before adding gum rosin powder (CAS 8050-09-7; Sigma-Aldrich, St. Louis, MO, USA) and mixed using a Cole-Parmer mixer set to an impeller speed of 225 rpm (Model 50,006–01, Cole-Parmer Co, Vernon Hills, Il, USA) until all powder was dissolved. The final weight ratio of gum rosin: ethanol in all varnishes was 80:20/wt. The varnish (“CUR-V”) was stored at room temperature in sealed amber glass vials until needed for further characterization.
In this study, a key independent variable was curcumin (CUR) concentration in the varnish and was set at three levels of 0.00%/w (“0.00% CUR-V”), 0.25%/w (“0.25% CUR-V”), and 10.0%/w (“10.0% CUR-V”). The solubility of curcumin in the ethanol phase of this varnish was integral to the curcumin levels studied. The lower level of 0.25%/w curcumin was selected based on the amount of ethanol in the varnish formulation and on our preliminary investigations of curcumin solubility in ethanol. A 0.25%/w curcumin content in the varnish matches the maximum solubility of curcumin in ethanol and was selected as our low concentration formulation. Meanwhile, the 10%/w (high concentration formulation) was chosen based on preliminary varnish mixing observations aiming to increase curcumin concentration beyond the ethanol solubility-based threshold but still have powder dispersion for the content that is not solubilized. At each concentration, curcumin was readily hand-mixed into the varnish within the amber vials using a metal spatula for ~10 min at room temperature. The unloaded varnish (i.e., 0.00 CUR-V) served as an experimental control in all characterization. Curcumin powder of > 95% purity was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
2.2 FTIR Spectroscopy of Varnish
All varnish samples were assessed using Fourier transform infrared (FTIR) spectroscopy equipped with an Attenuated Total Reflectance (ATR) accessory (Spectrum Two, Perkin Elmer, Waltham, MA, USA). Samples were placed in direct contact with the ATR element (diamond crystal) and a constant gauge pressure was applied with the help of the accessory knob before starting the sample scan. Spectra were collected over a range of 400–4000 cm−1 at a resolution of 4.0 cm−1 and with 32 scans (n = 3).
2.3 Quantifying Color Difference Using the CIELab Color System
Samples of the CUR-V varnish with 0, 0.25, and 10.0 wt% curcumin were evaluated for the potential photobleaching effect upon light treatment. The experimental varnishes were applied to the 6 mm circular hole within a polyacetate film strip and sandwiched between two intact strips of film. A flat-edged spatula was then used to flatten the sample and ensure sample height was kept consistent (height of 1 mm). The CUR-V samples were then placed on a white background and treated with blue light using the BioLight, a custom in-house LED device designed for studies on PDT [46, 47], delivering 19 mW/cm2 light (royal blue LED array, λmax = 440–460 nm) applied in accordance with the treatments listed in Table 1. It is important to note that the light irradiance (in mW/cm2) associated with commercial blue light devices is at least an order of magnitude greater than that provided by our in-house benchtop light device used for in vitro characterization. As a result, to reach the same light energy or dose, much less time will be necessary to apply the commercial blue light.
| Light treatment time (min) | Maximum light energy (J/cm2) |
|---|---|
| 0 | 0 |
| 15 | 17.4 |
| 30 | 34.9 |
| 60 | 69.7 |
The difference in color between each light-treated varnish and the untreated (0 min) varnish at a given curcumin concentration was also calculated using Equation (1) (∆Eab identified as ∆E0 min in this latter case).
2.4 Reactive Oxygen Species (ROS) Detection With a Colourimetric Assay
A thin (~0.5 mm thick) coating of each CUR-V was first applied to the bottom of a 24-well plate using a sterile microbrush. To quantify the two prevalent ROS species and their production from the CUR-V as a result of different light treatments (Table 1), two different colourimetric assays were performed [49, 50]. For both assays, a 20 mM solution of sodium phosphate buffer (NaPB) was first prepared by adding the requisite amounts of sodium phosphate monobasic monohydrate and sodium phosphate dibasic heptahydrate powder to ultrapure water. For singlet oxygen determination, 500 μL of NaPB solution containing 50 μM p-nitrosodimethylaniline (RNO) and 50 μM imidazole was added (named “solution A”). Here, RNO bleaching at 440 nm using imidazole as a selective acceptor of singlet oxygen allows for colourimetric analysis (absorbance at 440 nm decreases). Meanwhile, for superoxide anion determination, 500 μL of NaPB solution containing nitroblue tetrazolium (NBT) was added to each well (named “solution B”). Here, when the NBT is reduced as a result of superoxide anion presence, its absorbance at 560 nm increases. In either ROS determination, a representative well for each varnish containing only the NaPB served as the subtractive background. The percentage change in either ROS is relative to the non-irradiated (i.e., dark) solutions, respectively. This test was performed in triplicate and in two independent runs (n = 6). The required reagents, acetonitrile, imidazole, and RNO were purchased from Fisher Scientific (Hampton, NH, USA), while NBT, sodium phosphate monobasic monohydrate, and sodium phosphate dibasic heptahydrate were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.5 Bacterial Strain and Growth Conditions
Stock cultures of standard strains of S. mutans and C. albicans from the American Type Culture Collection (ATCC 33535 and ATCC 90028, respectively; Rockville, MD, USA) were maintained at −80°C, reactivated onto CDC Anaerobe 5% Sheep Blood Agar plates (BBL, Becton, Dickinson and Company, Sparks, MD, USA) and incubated at 37°C (5% CO2) (Isotemp CO2 incubator, Thermo Fisher Scientific, Marietta, OH, USA) for 48 h. Next, the species were individually reactivated by transferring single colonies (10–12 for S. mutans and 5–8 for C. albicans) into 5 mL of culture medium and kept overnight in the incubator (5% CO2, 37°C). In preparation for the 24-h mono-species assays, single colonies of S. mutans were transferred into brain heart infusion broth culture medium (“BHI”; BD BBL, Becton, Dickinson and Company, Sparks, MD, USA), while those of C. albicans were transferred into Trypticasein Soy Broth (“TSB”; Becton, Dickinson and Company, Sparks, MD, USA) with 0.5% yeast extract (“YE”, Difco, FL, USA) and 1% glucose (Sigma Aldrich, St. Louis, MO, USA) added. Meanwhile, for the 72-h dual-species assay, the same number of single colonies of each species were added to respective conical tubes, each containing 5 mL of TSB with 0.5% YE and 1% glucose. After storing the pre-inoculums in the incubator (5% CO2, 37°C) overnight (24 h for S. mutans and 20 h for C. albicans) the inoculum was then prepared from an absorbance of 0.08–0.10 read at an optical density (OD) of 600 nm for S. mutans and 0.25 at an OD of 540 nm for C. albicans with the aid of an Epoch microplate reader (BioTek, Winnoski, VT, USA); this optical density corresponded to 1.5 × 108 colony forming units (CFU)/mL.
2.6 24-Hour Mono-Species Response Assays
2.6.1 Colony Forming Unit (CFU) Count Assay
Similar to the ROS protocol (Section 2.4) a thin coating of each CUR-V was first applied to the bottom of a 24-well plate using a microbrush. Separate 24-well plates were prepared for each light treatment and microorganism (see Table 1; 8 plates prepared in total per run). To each sample and test control (varnish-free) well, 0.5 mL of the diluted species inoculum at 1 × 106 CFU/mL was added, and the plates were stored at 37°C and 5% CO2 (Isotemp CO2 incubator, Thermo Fisher Scientific, Marietta, OH, USA). The biofilms were kept undisturbed for 24 h to allow biofilm formation at 37°C under microaerophilic conditions. After 24 h, each well was washed with 1 mL of sterile phosphate buffered saline (PBS), 0.5 mL of fresh PBS was added, and the 24-well plate was placed under the custom in-house light device (λ = 440–460 nm, 19 mW/cm2) as per the light treatments listed in Table 1.
After treatment, an additional 0.5 mL of fresh PBS was added to each well, and the surface of the varnish (or well in the case of the varnish-free test control) was sonicated for 30 s at amplitude 20 (Model Q55, QSonica sonicators, Newton, CT, USA). Serial dilutions, by factors of 10 until a final serial dilution of 10−7, were performed in sterile PBS and plated onto CDC Anaerobe 5% Sheep Blood Agar plates for the S. mutans 24-h mono-species assay and Difco BD Sabouraud Dextrose agar (“SD” plates; Becton, Dickinson and Company, Sparks, MD, USA) for the C. albicans 24-h mono-species assay. Plates were incubated for 48 h, and colonies were counted to obtain the bacterial viability measured in CFU/mL. All tests were performed in triplicate and in three independent runs (i.e., n = 9).
2.6.2 XTT Assay
A CyQuant XTT Cell Viability Assay kit was used to detect cell viability. First, a thin coating of each CUR-V was added to the bottom of wells in a 96-well plate using a sterile microbrush. Here, separate 96-well plates were prepared for each light treatment and microorganism (see Table 1; 8 plates prepared in total per run). To the first three rows, 0.1 mL of 1 × 106 CFU/mL of either S. mutans (in BHI) or C. albicans (in TSB) was added to three varnish-free and all CUR-V (at 0, 0.25, and 10.0 wt% curcumin) wells. Meanwhile, to the bottom three rows of the same plate, 0.1 mL of media-only (cell-free) was added to three varnish-free and CUR-V wells. All plates were then incubated at 37°C and 5% CO2 (Isotemp CO2 incubator, Thermo Fisher Scientific, Marietta, OH, USA) for 24 h before light treatment (Table 1). Finally, following kit protocol, 70 μL of the XTT working solution was added to each sample and control well, and the plates were incubated again at 37°C and 5% CO2 for 3 h before reading OD at 450 nm (OD450). The data from this assay was corrected for the OD450 of the cell-free wells. All tests were performed in triplicate and in three independent runs (n = 9).
2.7 72-h Dual-Species Response Assay
2.7.1 Colony Forming Unit (CFU) Count Assay
Similar to the ROS protocol (Section 2.4), a thin coating of each CUR-V was first applied to the bottom of a 24-well plate using a microbrush. Separate 24-well plates were prepared for each light treatment only in the dual-species assay, as the inoculum of both species was added to each well (see Table 1; 2 plates prepared in total per run). To each sample and test control (no varnish) well, 1 mL of the diluted S. mutans inoculum at 2 × 106 CFU/mL and 1 mL of the diluted C. albicans inoculum at 1 × 106 CFU/mL were added, and the plates were stored at 37°C and 5% CO2 (Isotemp CO2 incubator, Thermo Fisher Scientific, Marietta, OH, USA). The biofilms were stored for 72 h to encourage more mature biofilm formation at 37°C under microaerophilic conditions; TSB was refreshed every 24 h. After 72 h, each well was washed with 1 mL of sterile phosphate buffered saline (PBS), 0.5 mL of fresh PBS was added, and the 24-well plate was placed under the custom in-house light device (λ = 440–460 nm, 19 mW/cm2) for 0 and 15 min only.
An additional 0.5 mL of fresh PBS was added to each well after treatment, and the surface of the varnish (or well in the case of the varnish-free test control) was sonicated for 30 s at amplitude 20 (Model Q55, QSonica sonicators, Newton, CT, USA). The 0.5 mL of collected cells in PBS was then serially diluted by factors of 10 until a final serial dilution of 10−7 using sterile PBS, and 10 μL drops from each dilution were then plated on both Difco BD Mitis Salivarius agar (“MS” plates; Becton, Dickinson and Company, Sparks, MD, USA) and SD agar plates for the 72-h dual-species assay. The dilutions in this dual-species assay contain both S. mutans and C. albicans and, as a result, specific agar plates were chosen to be selective toward one of the two species grown—MS plates for S. mutans and SD plates for C. albicans. Plates were incubated for 48 h, and colonies were counted to obtain the bacterial viability measured in CFU/mL. All tests were performed in triplicates and in three independent runs (n = 9).
2.8 Data Analysis
All statistical analysis was conducted using IBM SPSS Statistics for Windows, Version 28.0 software package (IBM Corp., Armonk, NY, USA; source: https://www.ibm.com/products/spss-statistics). The data was analyzed with a multi-factor univariate general linear model and post hoc Tukey test (α = 0.05). Data was presented as the mean ± one standard deviation. Statistical significance was accepted as p < 0.05.
3 Results
ATR-FTIR spectra of the CUR-V varnishes are shown in Figure 1 with key peaks indicative of ethanol, gum rosin, and curcumin identified. The key curcumin peaks were only evident at a 10.0 wt% loading of curcumin in the varnish.
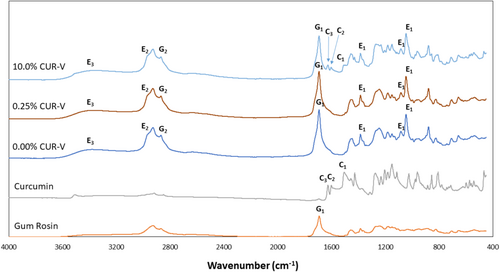
Peaks at 1384, 1093, and 1042 cm−1 for the varnish are indicative of the CO, 2972 cm−1 of CH, and 3364 cm−1 of OH of the ethanol [51]. Meanwhile, the peaks at 1690 and 2868 cm−1 represent CO and sp2CH, respectively, for gum rosin [52]. Lastly, a peak at 1513 cm−1 is attributed to the benzene ring bending vibration, at 1587 cm−1 to CC aromatic stretching, and at 1625 cm−1 to CO stretching for curcumin [53].
The deep orange color of the curcumin was readily conveyed in the varnish upon loading, as expected. Interestingly, for both the unloaded (0.00%) and 0.25% CUR-V varnishes, an increase in irradiation time with blue light energy at 19 mW/cm2 resulted in detectable photobleaching. This was not as strongly apparent for the 10.0% CUR-V samples.
The color difference between the unloaded (0.00%) varnish and the 0.25% CUR-V was dependent on light treatment time (p < 0.001; Table 2). The color difference between these two varnishes decreased as light treatment time (and corresponding blue light energy) increased. Applying 30 and 60 min of blue light photobleached the 0.25% CUR-V such that its color became closer to the curcumin-free varnish (p values for ΔE0%are 0.007 and p < 0.001, respectively). The color of the 10.0% CUR-V samples was out of the range of the VITA EasyShade V system, and the difference as a function of light treatment time could not be detected.
| Light treatment time (minutes) | ∆E(0% CUR-V) | |
|---|---|---|
| 0.25% CUR-V | 10.0% CUR-V | |
| 0 | 23.17 ± 0.43A | ND |
| 15 | 17.65 ± 4.02AB | ND |
| 30 | 14.41 ± 5.19B | ND |
| 60 | 6.14 ± 2.95C | ND |
- Note: Means that are statistically different (p < 0.05) are assigned different capital letters.
- Abbreviation: ND, not detected.
As shown in Table 3 the color difference between the untreated (0 min) and treated samples was dependent on both the light treatment time (p < 0.001) and curcumin concentration (p = 0.025). For the 0.00% CUR-V varnish, the color difference from the untreated samples was greatest at 15 min of light treatment (p < 0.001 for both), while for the 0.25% CUR-V varnish, this difference was greatest at 60 min of light treatment (p < 0.005 for both). Meanwhile, the color difference between untreated and 15 min of treatment was greater for the 0.00% CUR-V varnish than for the 0.25% CUR-V varnish (p < 0.001), with the opposite true for the color difference between untreated and either 30 or 60 min of treatment (p = 0.033 and p < 0.001, respectively).
| Light treatment time (minutes) | ∆E(0min) | ||
|---|---|---|---|
| 0.00% CUR-V | 0.25% CUR-V | 10.0% CUR-V | |
| 15 | 36.64 ± 4.65A | 24.25 ± 4.06B,C | ND |
| 30 | 19.26 ± 1.83C | 25.22 ± 2.97B | ND |
| 60 | 18.84 ± 2.02C | 32.83 ± 2.77A | ND |
- Note: Means that are detectably different (p < 0.05) are assigned different letters.
- Abbreviation: ND, not detected.
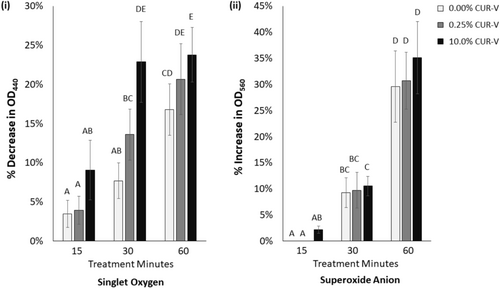
ROS production as a function of curcumin concentration and light treatment time is shown in Figure 2. Singlet oxygen had a detectable dependence on both the curcumin concentration in the varnish and light treatment time (p < 0.001 for both). Following 30 min of blue light treatment, the 10.0% CUR-V produced more singlet oxygen than either the 0.25% or the 0.00% CUR-V varnishes (p < 0.001 for both). In addition, 30 and 60 min of light treatment produced detectably more singlet oxygen from each experimental curcumin varnish than 15 min of treatment (p < 0.001 for each). No further change in singlet oxygen production was observed after 30 min of light treatment for the 10.0% CUR-V, while the 0.25% CUR-V continued to produce more singlet oxygen as light treatment increased from 30 to 60 min. Meanwhile, superoxide anion production only showed a strong dependence on light treatment time (p < 0.001). Similar to singlet oxygen production, light treatments of 30 and 60 min of each varnish, including control, resulted in more superoxide anion production than 15 min of treatment (p < 0.030 for each). However, contrary to singlet oxygen, the superoxide anion production increased further for each varnish following 60 min of light treatment when compared to 30 min (p < 0.001).
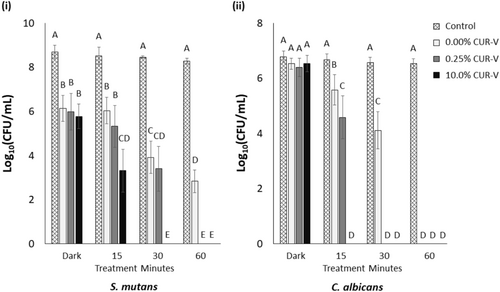
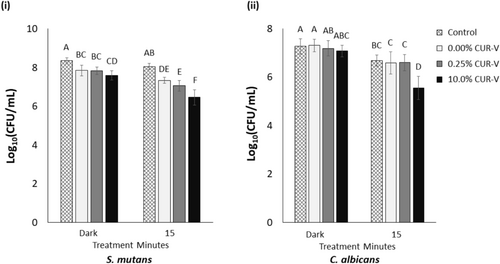
The CFU count of 24-h mono-species biofilms as a function of curcumin concentration and light treatment time is reported in Figure 3. These two independent variables were found to significantly impact CFU count on mono-species biofilms of S. mutans and C. albicans (p < 0.001 for each). For both species, it was noted that as the light treatment time (and energy) increased, the CFU count decreased (p ≤ 0.033 for each energy level). In addition, the higher the curcumin content, the less light treatment time (and energy) was required to result in a full CFU count reduction for either mono-species biofilm (p < 0.001). Furthermore, from the 24-h mono-species biofilm CFU assay, it appears that less light treatment time is required to reduce the CFU count of C. albicans compared to that of S. mutans.
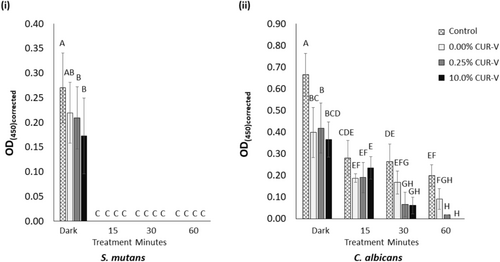
In a subsequent XTT assay of the 24-h mono-species biofilms, OD450 (representative of the metabolic activity of the cells) was reported (Figure 4). Here, the metabolic activity of S. mutans was most impacted by light treatment (p < 0.001) and curcumin concentration (p = 0.042). These two independent variables also detectably impacted the metabolic activity of C. albicans (p < 0.001 for both). Contrary to the CFU assay results for the 24-h mono-species biofilms of both species, 15 min of light treatment was also detrimental to the metabolic activity of the experimental control (i.e., varnish-free samples) when compared to the untreated (dark) control (p < 0.001 for both species). For S. mutans, 15 min of light treatment was also sufficient to fully reduce the metabolic activity of each species in their 24-h biofilm compared to the untreated samples (p < 0.001). Meanwhile, the reduction in metabolic activity of the C. albicans gradually increased with increasing light treatment of their 24-h biofilm. It took 30 and 60 min of light treatment of the C. albicans 24-h biofilm for the curcumin-loaded varnishes to result in a detectable reduction in metabolic activity compared to the control of the matching light treatment (p = 0.030 and p < 0.001, respectively).
The CFU assay results related to the 72-h dual-species biofilm of S. mutans and C. albicans are presented in Figure 5. The findings demonstrated that curcumin concentration and light treatment time each significantly impacted the CFU count for both species (p < 0.001). The CFU count following a 15-min light treatment of the 72-h dual-species biofilm grown on each varnish was notably reduced compared to the dark samples for both species (p < 0.001 for 0.00% CUR-V and 10.0% CUR-V, p ≤ 0.011 for 0.25% CUR-V). Meanwhile, the CFU count of both species following 15 min of light treatment was notably lower for 10.0% CUR-V than either 0.00% CUR-V or 0.25% CUR-V (p < 0.001 for all).
4 Discussion
Dental caries remains the most prevalent and costly biofilm-dependent oral disease worldwide, with its prevalence notably dependent on health disparities such as income level, race, and medical status [3, 40, 45, 54]. The most effective nonrestorative treatment for controlling non-cavitated carious lesions is 5% sodium fluoride varnish [55]. However, owing to the high concentration of fluoride, this therapy must be performed by a dental professional. As a result, we sought to develop a fluoride-free varnish that can be an effective and non-toxic alternative, readily available in an over-the-counter formulation, and be utilized in combination with photodynamic therapy to reach the desired antimicrobial properties. Our fluoride-free varnish formulation is composed mainly of gum rosin and ethanol, loaded with a natural photosensitizer known as curcumin and intended for antimicrobial photodynamic therapy (aPDT). In this approach, blue light is applied to the curcumin-containing natural varnish and resultant ROS inactivate and/or kill the targeted microorganism(s). As, in theory, only cells with the requisite accumulation of photosensitizer and light exposure are killed during aPDT, this approach can be repeated several times and should not cause cumulative toxicity elsewhere in the body [56].
Curcumin, as the most significant component of turmeric powder (a common Indian spice), has been shown to exhibit several notable pharmacological effects, including anti-inflammatory [57, 58], anti-carcinogenic [59], and antimicrobial effects [28, 60, 61]. FTIR analysis confirmed the presence of curcumin and other anticipated chemistry of the varnish. A notable challenge for the use of curcumin as a photosensitizer in dental materials is aesthetic-based, as the powder alone has a very deep orange color. However, at low curcumin loading into the varnish (i.e., 0.25% CUR-V) increasing the amount of light energy resulted in a loss of the orange tint and a reduced quantified difference in color from the unloaded varnish. It is important to also note that this varnish is intended as a non-permanent, preventive material, and with support of its feasibility for use in aPDT, any color concern will only be for a short time. Furthermore, the orange color might be beneficial in some circumstances, as areas covered by the varnish would be easily visualized. Future studies will seek to further optimize curcumin loading while considering agonistic cell responses and other properties, including color as a function of curcumin degradation and photobleaching. That the unloaded (0.00% curcumin) also saw some photobleaching after application of blue light suggests that other varnish components also absorbed light; studies are ongoing on the potential role of other varnish components in PDT.
In aPDT there are two classes of ROS—one created as a result of electron transfer (type I reaction) and the second due to energy transfer (type II reaction) [6, 62]. In the type I reaction, resultant ROS include superoxide anion, hydrogen peroxide, and hydroxyl radicals. Meanwhile, in the type II reaction, the energy transfer to oxygen results in singlet oxygen formation [6, 62]. Photodynamic inactivation of microbiological species is dependent on several factors, including light wavelength and energy and interaction of the species with the photosensitizer. Thus, one key assay to confirm the feasibility of the curcumin-loaded varnish for aPDT is to measure ROS production in response to various factors; here, the two independent factors investigated were blue light energy (i.e., treatment time) and curcumin concentration. Interestingly, singlet oxygen production from the varnishes showed a much stronger dependence on curcumin concentration than superoxide anion production. This correlates well with our prior study, where ROS release from aqueous curcumin solution was measured and singlet oxygen production was similarly more dependent on aqueous curcumin concentration [50]. In contrast, this current study showed that superoxide anion production from the varnishes was more dependent on blue light energy and it increased as the energy did, independent of sample type. While organic photosensitizers are typically considered to participate more in type II PDT reactions [62], evidence of this in the literature is lacking when it comes to curcumin.
In our study, a combination of CFU and XTT assays was performed to better understand the response of two cariogenic microorganisms to curcumin-loaded varnishes and light treatment, resulting in three key observations. First, both assays reported a reduction in the S. mutans count and metabolic activity for the biofilm grown on the varnishes with no blue light treatment compared to the experimental control. While neither the 24-h nor 72-h biofilm CFU assay indicated a similar dark toxicity of the varnishes for C. albicans, the XTT assay does suggest some reduced metabolic activity in the dark for this species as well. The difference in S. mutans CFU count after 72-h dual-species biofilm growth for the varnishes from the control is much less apparent than after 24 h. Dark toxicity is also less evident with the 72-h dual-species biofilm. This dark toxicity was not observed in our previous study evaluating the response of the same species to aqueous curcumin solutions and blue light [50], thus suggesting that the dark toxicity observed here may be due more to the full chemistry and nature (e.g., viscosity, surface roughness) of the varnish. For example, it is well recognized that ethanol can improve the permeability of cell membranes/walls [63] and, at certain concentrations, lead to the denaturation and death of cells. In addition, it has been reported that cell sensitivity to ethanol is dependent on cell type, with some requiring greater ethanol content to experience a cytotoxic response [64]. It is therefore possible that any ethanol that eluted from the varnish into the culture media may have been adequate to effectively permeabilize the outer membrane of the S. mutans and increase entry (and subsequent action) of curcumin. The permeabilization of the C. albicans may have been less successful due to the different cell membrane and wall structures and, as a result, less curcumin was able to pass into the cells, resulting in no measurable dark toxicity. Furthermore, S. mutans cells are smaller (0.5–0.75 μm [65]) than C. albicans (5–6 μm [66]), and this difference in cell surface area also likely contributed to the noted dependence of assay results on cell type.
A second key observation is that even a 15-min light treatment of the 72-h biofilm revealed dependence on the curcumin concentration loaded into the varnish, as measured by the CFU assay for the dual-species S. mutans and C. albicans biofilm. As expected, the 72-h dual-species biofilm was less responsive to aPDT than either 24-h mono-species biofilm, likely owing in part to the biofilm maturation time, amount of extracellular matrix formed, and the interaction between the two microbiological species [40, 67-69]. Optimization of the minimum amount of blue light energy necessary to best control cariogenic species activity in a biofilm of any level of maturity would be very beneficial. Future studies will seek to investigate biofilms consisting of more species than the two studied here, as well as further elucidate biofilm response to aPDT and curcumin-loaded varnish as a function of biofilm developmental stage and amount of soluble and insoluble extracellular matrix. Greater amounts of blue light energy will also be considered in the future, as will the impact of such treatment on the growth-supportive condition of culture media used in different assays. The impact of routine blue light exposure on the oral mucosa and eukaryotic cells will also need to be investigated (e.g., cytotoxicity assays). More investigation is needed on the fundamental mechanisms that govern PDT of PS-loaded biomaterials, such as the varnish developed in this study.
A third key observation involves the slightly different results of the XTT and CFU assays for 24-h mono-species biofilm following light treatment, which can be explained by test differences. For instance, there was no detected metabolic activity of S. mutans following any amount of light energy (i.e., 15 min or more) for any of the varnishes. However, S. mutans CFUs were still counted on the agar plates for each varnish, including those subjected to up to 30 min of light treatment. To explain this difference, it is important to remember that while the XTT assay protocol does not involve sonication, no fresh media is added following light treatment. In contrast, the CFU assay follows light treatment with dilutions in chilled PBS and plating on agar plates rich in fresh nutrients, but also involves a sonication step that may damage some species more than others [70]. Biofilms have a number of mechanisms by which they react to environmental stress, including downregulating metabolism and entering into a viable but non-culturable state [71, 72]. In addition, as the biofilm matures, there may be a very large number of cells but low overall metabolic activity (dependent on the growth phase) and/or staining by the XTT reagent may be poor [73]. Thus, direct comparison between the two assays—with different protocol steps and risk of inducing environmental stress—may be limited.
In our prior study, C. albicans required 15 min of light before a minimum inhibitory concentration (MIC) was reported in the range of the aqueous curcumin concentrations investigated, while an MIC was observed for S. mutans after only 1 min [50]. These earlier results correlate best with the XTT assay results for the varnish in this study. However, similar to XTT assays, no sonication step is involved in an MIC assay, and any stress to the cells is largely controlled and owing to the light treatment and/or curcumin concentration of our studies. The dependence of an aPDT-based cell response on singlet oxygen production is much more obvious in this varnish study, as all biofilm assays conducted in our latest study show a strong dependence on curcumin concentration.
The findings from this study support the original hypothesis that increased curcumin content in the varnish and greater blue light energy in aPDT increases the antimicrobial response. In addition, the new evidence connecting ROS production and antimicrobial response in aPDT is very promising for optimizing future material system design in this field.
5 Conclusion
In conclusion, this study provides the first evidence for the potential of a curcumin-loaded natural varnish in an aPDT-based approach for managing cariogenic species. The synergistic investigation of ROS production and antimicrobial response as a function of curcumin concentration and blue light energy supports the use of this new material system in aPDT. As both curcumin loading in the varnish and blue light energy increased, the ROS and antimicrobial response also increased. Meanwhile, after applying 60 min of blue light energy (~19 mW/cm2) there was evident photobleaching of the 0.25% CUR-V varnish. Overall, this approach offers considerable benefits to end users with the potential to be packaged in formulations available over-the-counter and utilized in all communities independent of any inherent health disparity.
Acknowledgments
The authors would like to thank Kathryn Nguyen and Sana Aghakeshmiri for their assistance in early pilot investigations of the proposed varnish system. We would also like to thank Dr. Angela Tether for assistance in the editing of this manuscript. The authors also acknowledge the opportunities for collaborations leveraged through the UBC Advancing Multifunctional Dental Biomaterials (AMDB) Research Excellence Cluster. The authors would like to acknowledge the Colgate Award for Research Excellence (2020-4082462349) and Faculty Start-up Funds for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available and may be provided upon reasonable request to the corresponding author.




