Assessment of the Effects of Nano TiO2 and HA Reinforcement Ratio on Mechanical, Morphological, and Thermal Properties of PLA Matrix Bio Composites
Funding: This work was supported by the Karabük University Scientific Research Foundation (FYL-2020-2135).
ABSTRACT
Although bone tissue has the ability to regenerate itself, this regeneration capacity may be limited in large defects or pathological conditions. The development of biomaterials and tissue scaffolds is of critical importance in supporting bone regeneration. In this context, polymer nanocomposites, which are increasingly gaining interest in bone tissue engineering, benefit from both the flexibility of the polymer and the mechanical strength of inorganic components by dispersing nano-sized fillers in the polymer matrix. This study is important in terms of the multifaceted characterization of the hybrid composite material formed with optimized reinforcement ratios of polylactic acid (PLA), hydroxyapatite (HA), and titanium dioxide (TiO2) components. The originality of the study stems from the comprehensive examination of the mechanical, morphological, thermal, and biological properties of the material in question and the determination of the optimum reinforcement range in light of data obtained from the studies of different researchers in the literature. This multiparameter and holistic approach contributes to the expansion of the potential application areas of the material and the development of a more in-depth understanding of the field of materials science. This study aims to investigate the thermal, mechanical, and morphological effects of HA and TiO2 reinforcement and reinforcement ratio on nano PLA matrix material. To synthesize these composites, 10% nano HA and different ratios of nano TiO2 (1%, 2%, and 3%) were added to the nano PLA matrix material. Specimens were prepared by using the casting particle removal method. For the biocompatibility test of the samples, all composite samples were immersed for 1–4 weeks in simulated body fluid (SBF). For the investigation of mechanical, morphological, and thermal properties, SEM, EDS, XRD, DTA, DCS, TG analyses, and compression tests were performed. As a result, it was observed that the apatite layer on the sample surfaces gradually thickened as the residence time in the SBF increased, and the HA and TiO2 reinforcement to the matrix material supported the formation of the apatite layer. Also, the highest mass loss was seen in PLA/HA samples. The decomposition temperature of the composites decreased with the addition of HA and TiO2 to the PLA matrix material. In addition, it has been observed that increasing the TiO2 reinforcement ratio improves the mechanical properties of the composite and increases its strength.
1 Introduction
In today's medical world, the treatment of bone defects resulting from trauma, tumor resection, or degenerative diseases is an important clinical problem, and traditional methods may be inadequate in the treatment of these problems. Bone regeneration is a natural process that has the capacity to renew itself. However, in large defects, this natural process may be inadequate. At this point, biomaterials and tissue engineering approaches that support bone regeneration gain great importance. Bone tissue engineering is one of the most exciting areas of modern medicine. Tissue engineering aims to accelerate bone healing by bringing together components such as scaffolds, growth factors, and stem cells that provide a suitable environment for cell proliferation and differentiation.
The design of an ideal bone tissue engineering scaffold poses a great challenge due to the complex structure of bone tissue, consisting of both organic (type I collagen) and inorganic (hydroxyapatite crystals) components. In this context, biodegradable polymers stand out as promising scaffold materials for bone tissue engineering. These polymers offer significant advantages due to their properties of controlled degradation in the body, the ability to form three-dimensional structures, and the ability to be modified with various bioactive agents. Because the development of biodegradable polymers promises solutions to existing problems, their use in tissue engineering and medicine has increased in recent years [1, 2].
The use of biodegradable polymers alone cannot fully meet the mechanical properties of bone tissue. Therefore, the development of polymer nanocomposites, especially in recent years, has made significant progress in the field of bone tissue engineering. Research about polymer nanocomposites is being developed on the separation and purification of biomolecules such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), and their use in the treatment of joint, muscle, and bone injuries, genetic, and molecular biology [3, 4]. Polymer matrices reinforced with nano-sized inorganic components exhibit improved mechanical properties and bioactivity. These composite structures can better mimic the hierarchical organization of the natural bone matrix. In addition, these materials can show high selectivity and affinity towards biomolecules due to their high surface-to-volume ratio and modifiable surface properties. Biodegradable polymers can be self-degraded without any additional cleaning, allowing applicability [5]. Biodegradable plastics degrade in nature; they are produced from natural polymers such as starch, cellulose, and protein. Biodegradable polymers have been a subject of interest in recent years due to the disadvantages of conventional polymers, such as containing petroleum and not being lost in nature for many years [6]. Biodegradable polymers are classified as natural and synthetic polymers according to their source. Natural biodegradable polymers include alginate, polysaccharides, starch, chitin/chitosan, or proteins (soy, fibrin, silk) and natural fibrils used as reinforcers/supporters [7]. Examples of synthetic biodegradable polymers are polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), polyurethane, and polyesteramide. Synthetic polymers are materials whose mechanical, physical, thermal, and degradation properties can be predicted, and their response under all conditions can be predicted [8].
PLA, a corn-based polymer, is the most promising biodegradable polymer and has been marketed as an alternative to traditional synthetic polymers [9, 10]. PLA-based automotive parts emit less carbon dioxide (CO2) compared to other petroleum-based thermoplastics [11]. Apart from that, PLA has high strength, thermal plasticity, and biocompatibility [12]. While this polymer provides good strength and easy processability in most equipment, it is considered expensive [13]. In addition to all positive features, the fact that contact with oxygen increases the brittleness of the material limits the applications of PLA polymer [13]. Therefore, in addition to improving the mechanical and thermal properties of PLA, making it biocompatible and porous is important in terms of increasing its usability in bone tissue applications.
The use of titanium dioxide (TiO2) and hydroxyapatite (HA) together with biodegradable polymers can create synergistic effects by combining the advantages of these components. The antimicrobial properties of TiO2 and the osteoconductive qualities of HA can provide an ideal environment for bone tissue regeneration. Such polymer nanocomposites have the potential to optimize critical properties for bone tissue engineering, such as mechanical strength, biocompatibility, controlled degradation rate, and bioactivity.
TiO2 nanoparticles are one of the frequently preferred inorganic components in polymer composites due to their excellent biocompatibility, antimicrobial properties, and surface activity. The photocatalytic properties of TiO2 can reduce bacterial colonization on implant surfaces and thus reduce the risk of infection, which is one of the important causes of implant failure. In addition, it is being used as a filler in biodegradable polymer matrix composites due to its high adhesion to composite surfaces [14].
HA is the most widely used calcium phosphate compound in the biomedical field and is represented as Ca10(PO4)6(OH)2; also, it stands out due to its similarity to the natural bone mineral phase. Hydroxyapatite is the main component of bone mineral structure and has osteoconductive properties; that is, it supports the attachment, proliferation, and differentiation of bone cells. HA, which is one of the elements found in the inorganic parts of bone and teeth, is highly preferred in various applications in the field of medicine due to its high biocompatibility, bioactivity, similarity to bone minerals, and the rate of degradation in the body [15]. For example, it is used as a filling material after deformations in the bone, in dental implants, and in coatings in dentistry [16, 17]. It has been reported in the literature that the use of synthetic hydroxyapatite (SHA) nanoparticles has a positive effect on buffering the acidic environment of the implantation site and reduces the osteoconductivity of the nanocomposite [18]. Increasing the HA content can lead to an increase in the crystallinity of the nanocomposites. It has been emphasized in the literature that some newly developed nanocomposites resemble trabecular bone properties, making them suitable candidates for bone repair applications [19]. The addition of nano-hydroxyapatite to polymer matrices improves the mechanical properties of scaffold structures while promoting bone-like apatite formation and cell differentiation. When combined with TiO2, it enhances the mechanical strength and bioactivity of implants, strengthening bone–implant integration. Scaffold structures produced from these materials support bone tissue formation by allowing cell migration, vascularization, and nutrient transport thanks to their porous structures. In addition, these biomaterials can be combined with nanotechnology to create more effective regenerative treatment options.
Many studies have been carried out in bone tissue engineering. The polymeric scaffolds to be created must have various distinctive features. They should be osteoconductive, osteoinductive, and osteogenic in nature, together with features such as being biocompatible, non-toxic, having a controllable degradation process, and being porous [20].
Tanodekaew et al. tried to obtain bone tissue samples with a porous structure using PLA/HA composite materials, and they showed that osteoconductivity was positively affected by the increase in HA and pore size [21].
Toniatto et al. studied the development of PLA/TiO2 nanocomposites with high antibacterial properties by the electrospinning method [22]. Pattamaphon et al. investigated the application of anatase-formed TiO2 as a photocatalyst for the PLA matrix by the solution casting method [23]. Luo et al. studied the preparation of PLA/TiO2 photodegradable materials that can be used as environmentally friendly materials [24]. Mai et al. fabricated composites based on PLA, thermoplastic starch (TPS), and TiO2 nanoparticles (TiO2NP) and investigated the effects of TiO2 on the interface compatibility of TPS and PLA [25].
In Erel and Evlen's study, PLA matrix HA and three different ratios of TiO2 (0.5%, 1%, and 1.5%) reinforced nanocomposite scaffolds were produced by the infiltration method. As a result of the study, it was concluded that the mechanical properties of the composite material increased as the TiO2 reinforcement ratio increased [26].
In conclusion, polymer nanocomposites, when combined with bioactive nanomaterials such as TiO2 and hydroxyapatite, offer promising solutions in bone tissue engineering. The continuous development and optimization of these biomaterials open new horizons in the treatment of bone defects in fields such as orthopedics, dentistry, and maxillofacial surgery.
In this study, it was aimed to investigate the effects of reinforcement ratio on the mechanical, morphological, and thermal properties of polymer matrix nano-reinforced biodegradable polymer composites. For this purpose, bio-composite materials with PLA matrix were designed. HA and different ratios of TiO2 were used as reinforcement materials. Compression tests for the determination of the mechanical properties, differential thermal analysis (DTA) and differential scanning calorimeter (DSC) analyses for the determination of the thermal properties, scanning electron microscope (SEM) and energy dispersive spectrometry (EDS) analyses for the investigation of the morphological properties of the nanocomposite samples prepared by the casting particle removal method were performed.
2 Materials and Methods
In the study, nano PLA (molecular weight 2 × 104 g/mol, melting temperature 165°C, 150 mesh, Nanography Nano Technology, Turkey) was used as the polymer matrix material. Nano HA (99.9% purity, 60 nm grain size, Nanography Nano Technology, Turkey) and nano titanium dioxide (TiO2NP) (99.995% purity, 17 nm grain size, Nanography Nano Technology, Turkey) were used as the reinforcement phase. To produce biocomposites, PLA was first brought into liquid form by using dichloromethane (DCM) (CH2Cl2, Merck, Balmumcu Chemistry, Turkey).
In this study, solvent cast particle removal method was used as the bone tissue production method. The solvent casting method is used to produce porous tissue scaffolds. With this technique, extremely porous scaffolds of up to 93% are obtained. In this method, polymer dissolved in organic solvents is mixed with porogenic granules such as sugar and inorganic salt and poured into the mold for production.
In this study, composite samples were produced by adding HA (10%) and TiO2 at three different rates (1%, 2%, and 3%) to PLA matrix material, and NaCl was used as a porogen. The weight ratios of the materials used in sample preparation are given in Table 1. The optimum ratios of the matrix and reinforcement materials used in the study were determined based on the studies in the literature [27].
| DCM (g) | PLA (g) | HA (g) | TiO2 (g) | NaCI (g) | |
|---|---|---|---|---|---|
| PLA | 40 | 15 | 1.5 | — | 50 |
| PLA/10%HA | 40 | 13.5 | 1.5 | — | 50 |
| PLA/10%HA/1%TiO2 | 40 | 13.35 | 1.5 | 0.15 | 50 |
| PLA/10%HA/2%TiO2 | 40 | 13.2 | 1.5 | 0.3 | 50 |
| PLA/10%HA/3%TiO2 | 40 | 13.05 | 1.5 | 0.45 | 50 |
It is stated in the literature that while the optimum ratio of nano additives reinforced into the polymer matrix positively affects the properties of the materials, when this limit is exceeded, it causes agglomeration and has a negative effect [28]. However, in the authors' previous scientific studies, they applied 0.5%, 1%, and 1.5% TiO2 reinforcement to the PLA matrix material and emphasized the increase in the TiO2 ratio since the 1% increases the mechanical strength of the biocomposite [28]. In this direction, 1%, 2%, and 3% TiO2 reinforcement ratios were selected by considering the authors' previous studies and the explanations in the literature.
The solution was prepared at room temperature in the amounts specified in Table 1. Pure PLA powder was added to the specified amount of dichloromethane by weighing it on a precision balance. It was mixed in a magnetic stirrer for about 90 min at 200 rpm until the powder material was completely dissolved. Then, HA was added and mixed for approximately 30 min. Finally, the added TiO2 was mixed for another 30 min at 200 rpm in a magnetic stirrer. The thoroughly mixed materials are poured into the mold (Figure 1) previously prepared for the infiltration process, in which 250–600 μm salt (NaCl) is added. After pouring the mixture into the mold, the mold is ready for infiltration. Argon gas, an inert gas, was used for the infiltration process. In the oven with a maximum temperature of 36°C, a total of three infiltration processes were carried out by applying 5 bar pressure for 3 min every hour. The infiltrated molds were left to dry in an oven at 36°C for 10–12 days. To remove the sodium chloride (NaCl) crystals, the samples were mixed in hot water for 2 days in a magnetic stirrer with 200 rpm. After this process, the samples were left to dry again for 2 days at 36°C in an oven.
In order to determine the apatite formation capabilities and biocompatibility of the samples produced by the infiltration method, bioactivity tests were applied for 1, 2, 3, and 4 weeks. Simulated body fluid (SBF) solution developed by Kokubo was used for the bioactivity tests [29]. The pH value of the liquid was adjusted to 7.4, which corresponds to the value of human plasma. Morphological examination was carried out before SBF for three samples from each sample group with different parameters. Bioactivity tests were applied to three samples from each of the parameters PLA, PLA/HA, PLA/HA/1%TiO2, PLA/HA/2%TiO2, PLA/HA/3%TiO2, a total of 60 samples. The samples were immersed in SBF and kept constant at 36.5°C. At the end of each week, the samples removed from the SBF were cleaned with distilled water and dried in an oven at 36°C for 1 day. When the immersion times of all samples were finished, weight changes were determined.
The surface morphologies and apatite formations of the samples were examined by SEM before immersion in SBF and 4 weeks after immersion in SBF. Image analyses for all samples were made using a Carl Zeiss Ultra Plus Gemini Fesem brand device. X-ray diffraction (XRD) analysis was performed to examine the phase structures on the sample surface. XRD analysis was performed with a Rigaku Ultima IV-X-Ray Diffraction Spectrometer instrument at an angular range of 2θ (10°–90°) at a speed of 2°/min.
In order to determine the thermal properties of the produced composite samples, thermal analyses were made with the Hitachi Sta 7300 model Thermo-Gravimetric Differential Scanning Analyzer device. Analyses were carried out in a nitrogen environment at a heating rate of 15°C/min and a temperature range of 25°C–750°C.
For the strength analyses of the produced PLA matrix HA and TiO2 reinforced nanocomposites, the samples prepared in TS EN ISO 844 standard (50 mm height and 12 mm diameter) were tested with a Zwick/Poell Z6oo brand compression test device at 1.5 mm/min. Compressive strength measurements were made at 50% deformation with head speed. Compression tests were applied to five samples from all sample groups that were kept in SBF for 4 weeks and not kept in SBF. The obtained stress and strain values were evaluated by averaging.
3 Results
3.1 Bioactivity Test Results
In order to determine the bioactivity behavior and biocompatibility of the composite material groups, the bioactivity test was carried out. Dry weights of each composite group were measured before the bioactivity test. After 4 weeks of bioactivity testing, the mass changes of each sample group were measured. The dry weights of the samples before the bioactivity test and after the 4-week bioactivity test are given in Figure 2.
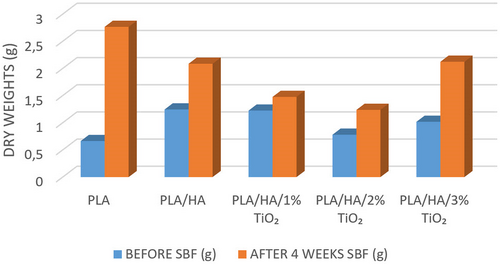
Accordingly, when Figure 2 is examined, the highest dry weight value was determined in PLA/HA samples before the bioactivity test. As a result of the 4-week in vitro test, it is seen that there is an increase in dry weights of all sample groups in general. As a result of the 4-week bioactivity test, the highest increase in dry weights occurred in PLA material (413%) and 3% TiO2 reinforced composite samples (209%), while the least weight increase occurred in 1% TiO2 reinforced samples (121%). This change in weight increase is due to the apatite layer formed on the surface as a result of the samples being kept in SBF. Apatite layers formed because of immersing the samples in SBF can also be seen in the SEM and EDS results in Figure 3.

In Figure 3, SEM images and EDS results of all samples before bioactivity and after 4 weeks of bioactivity are given. When the surface SEM images of the composite samples are examined, it is understood that the surface of the sample immersed in SBF is covered with a layer of apatite, and calcium phosphate precipitation occurs. After calcium phosphate precipitation, an amorphous calcium phosphate phase is formed. The formed amorphous phase turns into a structure like a bunch of grapes (it is circled in red in the SEM images in Figure 3), and then the tuber structure starts to fill with water. In the following stage, it develops and turns into large-grained grape bunches. These grape cluster-like structures are carbonated apatite (HA) structures [30]. As a result of the EDS taken from the surface of the samples, the change in the percentage ratios of the Ca (calcium) and P (phosphate) phases (Table 2), supports the increase in the apatite layer on the sample surfaces with the increasing of immersion time in SBF.
| %Ca by weight | % Ca/P ratio by weight | |||
|---|---|---|---|---|
| Before SBF | After the 4-week SBF test | Before SBF | After the 4-week SBF test | |
| PLA | 0.99 | 17.07 | 0.027 | 4.1 |
| PLA/HA | 24.44 | 19.76 | 1.55 | 1.38 |
| PLA/HA/1%TiO2 | 0.11 | 20.24 | 0.009 | 1.172 |
| PLA/HA/2%TiO2 | 1.5 | 0.35 | 0.115 | 0.97 |
| PLA/HA/3%TiO2 | 6.86 | 23.72 | 0.31 | 1.27 |
When Table 2 is examined, it is seen that there is an increase in both %Ca and %Ca/P ratios at the end of the 4-week bioactivity test in all sample groups. While the %Ca ratio of pure nano PLA material was 0.99, it increased to 17.07 after SBF; while the % Ca/P ratio before SBF was 0.027, it increased to 4.1 after SBF. Similarly, in all other PLA matrix nanocomposite scaffolds, there is an increase in wt% Ca and %Ca/P ratios before and after SBF.
However, when the results are examined in general, it is seen that the increase in both %Ca and %Ca/P ratio is less than PLA and PLA/HA nanocomposites as the reinforcement ratio of TiO2, which is the reinforcement material, increases. This is thought to be due to the fact that TiO2 is a bioinert material. When bioinert materials are used as alloying elements, they establish a mechanical bond with other alloying elements and improve the mechanical properties of the composite material group to which they are reinforced. But they are not a bioactive material [31-34]. Therefore, they can negatively affect bioactivity. Some studies in the literature emphasized that TiO2, a ceramic with bioinert properties, should be doped as little as possible into the porous tissue scaffolds. In a study, the in vitro bioactivities of 5% and 7% nano TiO2 doped scaffolds were evaluated by keeping them in SBF for 6 days. It was observed that the doped scaffolds containing 5% TiO2 had better bioactivity, and the bioactivity of the samples decreased when the TiO2 ratio was increased to 7% [35].
Another feature expected from tissue scaffolds is that they have a controllable porous structure that will allow the diffusion of nutrients and oxygen necessary for cell survival and cell migration. Proper porosity and homogeneity of pore distribution are important variables in increasing the specific surface area for cell attachment and tissue development. Their provision facilitates the uniform distribution of cells, adequate transport of nutrients, and removal of cellular waste products [36]. Tissue scaffolds with a porous structure, thanks to the spaces they can grow and develop, perform the fusion activity of tissue and material faster than dense structures, and support bone regeneration more. However, very small pore sizes cause the pores to be closed by cells, which limits cell migration. Conversely, too large pore sizes negatively affect cell adhesion [37]. For the formation of bone tissue, a pore diameter of at least 100 μm and a porosity of about 90% are required [38, 39].
Table 3 gives the % porosity calculations made before the bioactivity test and after 4 weeks of bioactivity. When Table 3 is examined, it can be seen that before the bioactivity test, the relative density increased with HA supplementation to the nano PLA material. Relative density decreased with the addition of TiO2 and increasing the ratio. On the other hand, the opposite situation occurred in the % porosity value; the % porosity decreased with HA supplementation to the PLA matrix material, and the % porosity increased again with the increase of the TiO2 reinforcement ratio. As a result of the 4-week bioactivity test, the relative density of the PLA matrix material increased with HA supplementation, and the relative density first decreased and then increased again with TiO2 supplementation. At % porosity values, the porosity decreased with HA supplementation, and the porosity increased with 1%TiO2 reinforcement, and then when the supplementation rate of TiO2 increased, porosity decreased again. In general, % porosity has increased in all sample groups of 4 weeks SBF analysis and reached approximately 90% levels. So, according to the literature, it can be said that all sample groups immersed in SBF for 4 weeks in this study are suitable for the formation of bone tissue [38, 39]. Also, this situation can be explained through EDS mapping analyses.
| Before SBF test | After the 4-week SBF test | |||||
|---|---|---|---|---|---|---|
| Theoretical density (g/cm3) | Relative density | % Porosity | Theoretical density (g/cm3) | Relative density | % Porosity | |
| PLA | 1.73 | 13.64 | 86 | 1.73 | 10.35 | 90 |
| PLA-HA | 1.87 | 61.47 | 39 | 1.87 | 12.82 | 87 |
| PLA-HA-1%TiO2 | 1.9 | 24.47 | 76 | 1.9 | 4.75 | 95 |
| PLA-HA-2%TiO2 | 1.92 | 17.81 | 82 | 1.92 | 9.69 | 90 |
| PLA-HA-3%TiO2 | 1.94 | 2.00 | 98 | 1.94 | 13.17 | 87 |
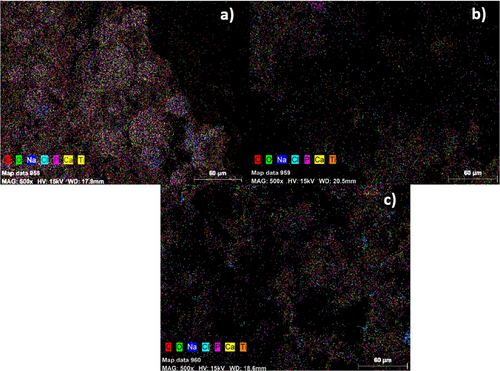
In Figure 4, EDS (MAP) images of 1%, 2%, and 3% TiO2-reinforced samples after a 4-week SBF test are shown. As a result of the 4 weeks SBF analysis, it is estimated that the decrease in porosity with the increase of TiO2 supplementation is due to agglomerations occurring in the porous regions of the sample. According to the EDS (Mapping) results given in Figure 4, it is seen that agglomeration increases as the TiO2 ratio increases. It has been stated in the literature that as the TiO2 ratio increases (> 2%), agglomerations increase in the nanocomposite structure [40].
The surfaces of the samples were examined by XRD analysis before the SBF test and after the 4-week SBF test. XRD results of all samples are given in Figure 5. In addition, Card numbers of the phases seen as a result of XRD analysis are given on the graphs.
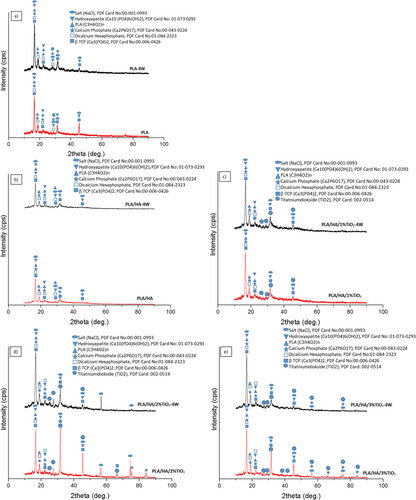
As a result of the examinations, PLA, HA, beta-tricalcium phosphate (β-TCP), dicalcium hexaphosphate, calcium phosphate, hydroxyapatite, and NaCl peaks were observed in all samples, while TiO2 peaks were observed in the samples reinforced with TiO2. When the results of the bioactive sample are examined, the HA and HA-like phases are encountered. The reason for this is the precipitation of apatite calcium phosphate that occurs during the waiting period in SBF [30]. Also, it is seen that the peaks of HA phases are higher in samples kept in SBF.
Pure HA, Ca10(PO4)6(OH)2 contains 39.68% Ca, and 18.45% P by weight as theoretical composition. While the Ca/P ratio is 2.151 by weight, it is 1.667 in moles. The Ca/P ratios of dense hydroxyapatites vary according to the ratio of β-TCP and HA in them. If the Ca/P ratio is 1.67, only the HA phase can be seen in X-ray diffractometry. However, if the Ca/P ratio is lower than 1.67, phases such as β-TCP, TTCP (tetracalcium phosphate—Ca4P2O9 and Ca4(PO4)2) are formed together with the hydroxyapatite phase. If this ratio is greater than 1.67, the formation of the CaO phase is observed together with HA [41, 42]. The XRD results given in Figure 4 support this situation.
The samples, produced by the solvent-casting particle removal method, provided a hollow structure. Salt (NaCl) was added to the bottom of the mold, and the prepared mixture was poured on it. The samples, which were mixed thoroughly with each other by pressure, were exposed to heat in the furnace at the same time. The hollow structure was obtained by dissolving the salt in the samples removed from the mold in water. Finally, the samples were dried and ready. However, NaCl particles remaining on the samples formed sodium (Na) and chloride (CI) peaks in EDS and XRD analyses. Therefore, the NaCl phase was found in all parameters. The presence of the NaCl phase in the XRD results indicates that although particle removal was performed, 100% dissolution could not be achieved during dissolution.
3.2 DTA/TG/DSC Thermal Analysis Results
In this study, thermal DTA/TG and DSC analyses were performed before SBF tests of the PLA matrix reinforced with HA and TiO2. TGA and DTA results of the before SBF tests for PLA, PLA/HA, PLA/HA/1%TiO2, PLA/HA/2%TiO2, and PLA/HA/3%TiO2 samples are shown in Figure 6.
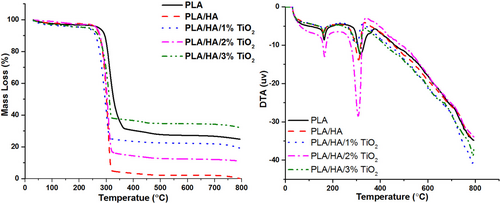
According to the TGA results given in Figure 6, it is understood that the mass loss of the composite increases when nano HA is added to the PLA matrix material. In a previous study, it was emphasized that as the HA reinforcement ratio increased to 10% and 20%, the mass loss of the composite increased [43]. This is associated with fiber breakage due to agglomeration of nano-HA particles or surface erosion as a result of their local positioning [44, 45]. When nano TiO2 was added to PLA/HA nanocomposite at increasing rates, mass loss decreased. While the least mass loss was obtained in the composite structure reinforced with 3% nano TiO2, the highest mass loss was obtained in PLA/HA composite samples. Decomposition temperatures of matrix material and nanocomposites are given in Table 4.
| Specimen type | First decomposition (°C) | Second decomposition (°C) |
|---|---|---|
| PLA | 131.5 | 330 |
| PLA/HA | 128.9 | 310 |
| PLA/HA/%1TiO2 | 196.3 | 316 |
| PLA/HA/%2TiO2 | 176.4 | 320 |
| PLA/HA/%3TiO2 | 165.9 | 322 |
In the DT analysis, an endothermic reaction occurred by showing two different peaks in the nanocomposite samples. The first of these two decomposition points is the melting point (Tm), and the second is the decomposition temperature (Td). When Table 4 and Figure 6 are examined together, while the degradation temperature of the PLA matrix material was in the range of 131.5°C–330°C, the PLA/HA nanocomposites were in the range of 128.9°C–310°C. This shows that the thermal stability of the matrix material decreases when nano HA is added to the PLA matrix material. When 1% TiO2 is added to the PLA/HA composite structure, the degradation temperature range of the new composite decreases again, and the thermal degradation temperature of the PLA/HA/1%TiO2 composite falls to the range of 196.3°C–316°C. In other words, 1% TiO2 reinforcement reduced the thermal stability of the composite again. When the TiO2 reinforcement ratio is increased to 2% and 3%, the melting temperature (Tm) of PLA/HA/2%TiO2 and PLA/HA/3%TiO2 composites decreases (176.4°C and 165.9°C, respectively), while their decomposition temperatures (Td) increase (320°C and 322°C, respectively). This shows that the thermal stability of composite materials increases when the TiO2 reinforcement ratio is increased (2% and 3%, respectively). It has been observed that the material group with the highest thermal stability belongs to the PLA matrix material; the thermal stability of HA and TiO2 reinforced to the matrix material first decreases and then increases with the increase of the TiO2 reinforcement ratio.
Thanks to the high thermal stability and surface area of TiO2, it not only increases the mechanical properties of the matrix material to which it is reinforced but also provides higher thermal stability to the polymer to which it is added. The effect of nanoparticles on the thermal stability of polymers can be attributed to the protective effect of the nanoparticles and/or the retarding effect of the nanoparticles on the movement of free radicals and volatile degradation products [46-52].
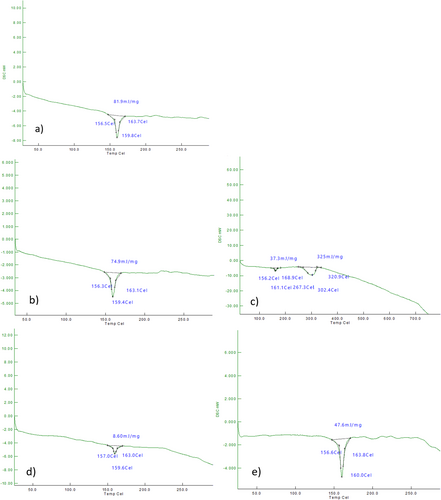
In Figure 7, DSC curves of before SBF tests for PLA matrix nano HA and TiO2 reinforced composite bone scaffold samples are given. Melting temperature (Tm), Polymer glass transition temperature (Tg), and enthalpy (ΔH) values of composite samples and PLA samples are seen from the curves. Using the data obtained from the DSC curves, the % crystallinity (%Xc) values were calculated (Table 5).
| Tm (°C) | Tg (°C) | ΔH (mJ/mg) | Xc (% Crystallinity of materials) | |
|---|---|---|---|---|
| PLA | 23.6 | 159.8 | 87.9 | 93.81 |
| PLA/HA | 21.1 | 159.4 | 74.6 | 79.61 |
| PLA/HA/1%TiO2 | 46 | 161.1 | 37.3 | 39.8 |
| PLA/HA/2%TiO2 | 42 | 159.6 | 8.6 | 9.17 |
| PLA/HA/3%TiO2 | 37.6 | 160 | 47.6 | 50.8 |
When the thermal properties (melting temperature Tm, glass transition temperature Tg, and crystallinity) of before SBF tests for composite samples in Table 5 are examined, it is seen that there is no significant change in Tm and Tg temperatures when HA and TiO2 are added to the PLA matrix material. This is because the TiO2 supplementation rate is low. Similar results are found in other studies with PLA matrix material [40, 53, 54]. When the enthalpy (ΔH) and % crystallinity values are examined, it is seen that there is a decrease in the ΔH and Xc values of the composite material groups with HA supplementation to the nano PLA matrix material, and this decrease becomes more pronounced with different ratios of TiO2 supplementation. Especially when 1% and 2% TiO2 are added, it is seen that there is a serious decrease in ΔH and Xc values. This was due to the low TiO2 doping ratio. When 3% TiO2 was supplemented, a significant increase in ΔH and Xc values occurred [48, 55].
3.3 Compression Test Results
To examine the effects of reinforcement phases and reinforcement ratio in the mechanical behavior of nano PLA matrix HA and TiO2 reinforced composites, a compression test with 50% deformation was applied for all nanocomposite samples before SBF and after the 4-week SBF tests. The stress values obtained from the compression test are given in Figure 8.
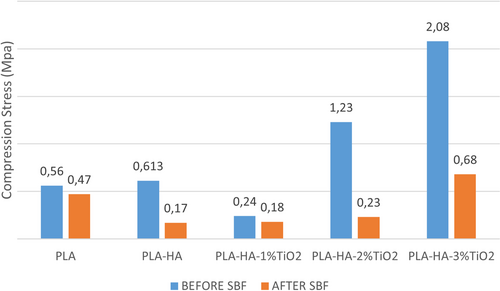
As a result of the compression test, the compressive stress of the PLA sample that was not kept in SBF was measured as 0.45 MPa. With the addition of HA to the pure PLA sample, the compressive stress increased by 136% and reached a value of 0.614 MPa. It is understood that the compressive strength of the new composite obtained by adding 1% TiO2 to the PLA/HA composite structure decreases, and the compressive strength of the composites increases quite rapidly when the TiO2 reinforcement ratio is increased. So much so that the compressive strength of the 3%TiO2 reinforced composite reached approximately 4.5 times (2.08 MPa) the compression strength of pure PLA material (0.45 MPa).
It is seen from the graph in Figure 8 that there is no significant change in the compressive strength of pure PLA material (0.47 MPa) after the 4-week bioactivity test. However, with HA reinforcement to the matrix material, the compressive strength of the composite decreased (0.17 MPa), and the strength values of the composites increased again with increasing TiO2 supplementation (1%, 2%, and 3%, respectively) (0.18, 0.23, and 0.68, respectively). This shows that the compressive stress increases with the increase in the TiO2 reinforcement ratio.
When the compressive stress graphs are examined, it is understood that the compressive stresses of the samples kept in SBF are lower than the stresses of the samples that were not kept in SBF. This is due to the fact that the samples are kept in distilled water to dissolve the porogen salt after production, and the matrix material PLA is a starch-based biodegradable material and is affected by moisture. In some previous studies, it was stated that the mechanical strength of the PLA polymer subjected to a bioactivity test decreased over time [26, 56, 57].
4 Results and Discussion
In this study, HA and TiO2 supplements were reinforced to PLA polymer. It is aimed to produce biodegradable nanocomposites reinforced with a PLA matrix HA and TiO2. It has been tried to obtain a hollow polymer material with biocompatibility, physical, and thermal properties suitable for use in medical technologies and medical fields. The solvent-casting particle removal method was preferred to obtain a porous structure as a production method.
- As a result of the bioactivity test, the highest dry weight increase was detected in the PLA sample, while the least dry weight increase was observed in the samples supplemented with 1% TiO2. In addition, the HA, tricalcium phosphate, CaO2 phases increased as the residence time in SBF increased. This shows that the composite structures formed are bioactive.
- When the surface SEM images of the composite samples are examined, it is understood that the surface of the sample waiting in SBF is covered with an apatite layer, and calcium phosphate precipitation occurs.
- At the end of the 4-week bioactivity test in all sample groups, it is seen that there is an increase in %Ca and %Ca/P ratios. However, when the reinforcement ratio of TiO2 increases, the increase in %Ca and %Ca/P ratio is less compared to PLA and PLA/HA nanocomposites.
- In general, the % porosity increased in all sample groups as a result of the 4-week SBF analysis.
- As a result of XRD analysis of the immersed in SBF samples, HA and HA-like phases were encountered.
- According to the TGA results, the mass loss of the composite increases when nano HA is added to the PLA matrix material. When nano TiO2 was added to the PLA/HA nanocomposite at increasing rates, mass loss decreased. While the least mass loss was obtained in the composite structure reinforced with 3% nano TiO2, the highest mass loss was obtained in PLA/HA composite samples.
- In DT analysis, an endothermic reaction was realized by showing two different peaks in nanocomposite samples. It has been observed that the material group with the highest thermal stability belongs to the PLA matrix material; the thermal stability of HA and TiO2 reinforced to the matrix material first decreases and then the stability increases with the increase of the TiO2 reinforcement ratio. Thanks to the high thermal stability and surface area of TiO2, it not only increases the mechanical properties of the matrix material to which it is reinforced but also provides higher thermal stability to the polymer to which it is added.
- When the enthalpy (ΔH) and % crystallinity values are examined, it is seen that there is a decrease in the ΔH and Xc values of the composite material groups with HA supplementation to the nano PLA matrix material, and this decrease becomes more pronounced with different ratios of TiO2 supplementation.
- As a result of the compression test, with the addition of HA to the pure PLA sample that was immersed in SBF, the compressive stress increased by 136%. When 1%TiO2 was reinforced to the PLA/HA composite structure, the new composite's compression stress decreased, and the compressive strength of the composites increased quite rapidly when the TiO2 reinforcement ratio was increased. So much so that the compressive strength of the 3%TiO2 reinforced composite reached approximately 4.5 times the compression strength of pure PLA material.
- After the 4-week bioactivity test, it was seen that there was no significant change in the compressive strength of the pure PLA material (0.47 MPa). However, with HA reinforcement to the matrix material, the compressive strength of the composite decreased (0.17 MPa), and the strength values of the composites increased again with increasing TiO2 supplementation (1%, 2%, and 3%, respectively) (0.175, 0.23, and 0.68, respectively). This shows that the compressive stress increases with the increase in the TiO2 reinforcement ratio.
- It is understood that the compressive stresses of the samples kept in SBF are lower than the stresses of the samples that were not kept in SBF. This is because the samples are kept in distilled water to dissolve the porogen salt after production, and the matrix material PLA is a starch-based biodegradable material and is affected by moisture.
5 Conclusion
In this study, the effects of HA and TiO2 reinforcement on the morphological, thermal, and mechanical properties of PLA matrix composites were investigated. As a result of the 4-week SBF analysis, it is estimated that the decrease in porosity with the increase of TiO2 supplementation is due to agglomerations and HA-like phases occurring in the porous regions of the sample. According to the EDS (Mapping) results, agglomeration increases as the TiO2 ratio increases. HA and HA-like phases increased with the increasing TiO2 ratio in the same regions, which can also be seen in XRD analyses. The addition of the reinforcement material decreased the crystallinity of PLA composites. This is an indication that the thermal stability increases as the TiO2 reinforcement ratio increases in PLA matrix composites. The addition of TiO2 to PLA polymer in increasing ratios improved the compressive strength of the composites. The PLA composite reinforced with 3 wt% TiO2 exhibited the best pore morphology and mechanical properties. These developments hold promise for future regenerative medicine applications, especially considering the aging population and increasing incidence of musculoskeletal diseases.
The main limitations of this study include the fact that only certain and low rates of HA and TiO2 reinforcement were examined. For future studies, it is recommended to develop new hybrid composites with higher reinforcement rates, different concentrations, and the addition of different bioceramic materials, to test different sample production methods (e.g., 3D printer technology, and plastic molding) and to conduct in vivo tests to evaluate the biocompatibility and mechanical properties of the material more comprehensively. Especially considering the potential for use in regenerative medicine applications, more specific studies can be conducted in the field of bone tissue engineering and biomaterials to investigate the potential of the material to be transferred to clinical application.
Acknowledgments
The authors thank the Karabük University Scientific Research Foundation for financial support as part of the FYL-2020-2135 numbered project. The authors also thank the Karabuk University Iron and Steel Institute for mechanical and morphological analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




