Mesoporous Bioactive Glass-Based Composite Cryogel for Noncompressible Hemorrhage
Funding: This work was supported by Research on the construction of a question bank of practical skills examination resources for the qualification examination of practicing assistant physician of Chinese Medicine (2019XJMP03), General project of Chongqing Three Gorges Medical College (XJ2023002903), The technology project of Chongqing level rural revitalization counterpart support (2023TIAD-ZXX0010), Science and technology research project of Chongqing Education Commission (KJQN202402712, KJQN202302703).
ABSTRACT
Uncontrollable bleeding is one of the important causes of death in war, road traffic injuries, surgical accidents, and other accidents. Using hemostatic materials to control bleeding quickly and effectively can improve the survival rate of patients, especially for incompressible visceral bleeding. Traditional inorganic materials and natural polymers alone still have limitations, such as non-degradability and ineffective control of bleeding through wounds. In this work, we designed a cryogel sponge combined Mesoporous bioactive glass (MBG) with Gelatin Methacryloyl (GelMA), which has enhanced mechanical strength and improved in vitro coagulation properties. And MBG@GelMA cryogel could absorb water more than 5 times in 5 min, while it also demonstrates significant improvement in mechanical strength from 4 kap to 12 kpa. Additionally, MBG@GelMA cryogel showed excellent biocompatibility and hemostatic performance. The multi-stage pore structure and hydrophilicity of frozen gel and MBG help to concentrate blood quickly and activate endogenous coagulation pathway through the release of calcium ions to promote coagulation. The findings of this study demonstrate that the MBG@GelMA composite cryogel possesses outstanding properties in terms of hemostasis, portability, and ease of use, suggesting its remarkable potential as a promptly applicable hemostatic material in both civil and military settings.
1 Introduction
Bleeding control remains a major challenge, especially for bleeding from deep incompressible wounds caused by gunshot wounds and sharp objects [1]. According to statistics, bleeding deaths account for 30%–40% of injury deaths, and 33%–56% of them occur before patients are sent to the hospital [2]. Therefore, the use of hemostatic materials to control bleeding early before going to the hospital can effectively reduce mortality. However, traditional methods of hemostasis such as gauze, tourniquet, and pressure hemostasis are not effective for extreme bleeding [3]. In addition, porous zeolite, kaolin, gelatin sponge, and hydrogel hemostatic agents are unsuitable for such wounds. Therefore, it is necessary to develop a hemostatic sponge with high strength, rapid blood-sucking, and good biocompatibility at the same time, which can be naturally degraded in the body to avoid secondary injury caused by removal operation.
Gelatin is a hydrolysate of collagen, which contains many arginine-glycine-aspartate (RGD) sequences that promote cell adhesion [4]. Due to the advantages of low antigenicity, good biocompatibility, good biodegradability, and low cost, gelatin has received widespread attention as a hemostatic material. GelMA microneedles were developed for synchronous loading of Yunnan baiyao and VEGF, showing good hemostasis and cutaneous wound healing in rat liver bleeding models [5]. Self-assembled microgels based on GelMA also exhibited good mechanics, allowing wound closure and promoting tissue regeneration [6]. Bioactive glass, due to its ability to deposit hydroxyapatite in body fluids and form chemical bonds with bone formation, is widely used in bone tissue engineering [7]. Recent studies have also demonstrated that bioactive glass could be utilized for the repair of soft tissues, such as wound healing, nerve regeneration, and cardiac tissue repair [8]. Mesoporous bioactive glass (MBG) is a class of nanoparticles with regular shape and mesoporous structure prepared by alkaline sol–gel method [9-11]. While MBG demonstrates impressive water absorption capacity and the capability to stimulate the coagulation cascade, it was easy to escape to the distal blood vessel to form blood clots due to the nanoscale particle size, along with the tendency for excessive ion release, imposing certain limitations on its utilization in the hemostasis domain [12, 13]. Cryogel loaded with bioactive glass of different forms has a large pore structure, shape memory function, and rapid water absorption, enabling it to regain its shape by absorbing blood and exert pressure on injuries to halt bleeding. Thus, the composite cryogel represents a highly suitable alternative for the treatment of incompressible wounds.
In this study, we propose a high-performance visceral hemostatic sponge that can be used for incompressible wound bleeding control with excellent mechanical properties and biocompatibility. The material consists of methacrylate gelatin (GelMA) and MBG, Ammonium persulphate (APS), and tetramethyl ethylenediamine (TEMED) heat initiation crosslinking GelMA at low temperature, freeze-dried to obtain a shape memory cryogel. Among them, MBG activates an endogenous coagulation pathway, and GelMA has good biocompatibility and degradability as a matrix.
2 Results
2.1 Preparation of MBG and MBG@GelMA
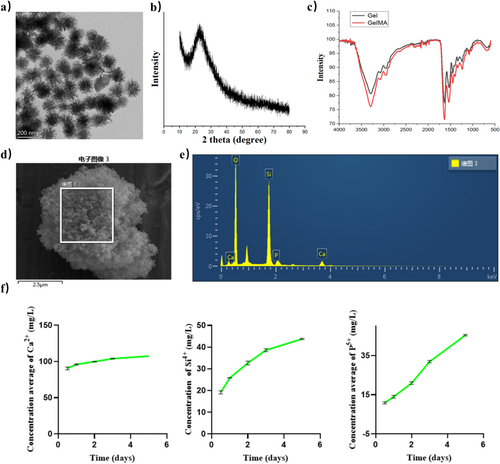
MBG based on the 58S formula was prepared. Clear mesoporous channels and particle size distribution of about 200 nm could be observed from transmission electron microscopy (Figure 1a) [14]. From the XRD pattern, it could be observed that there was a diffuse steamed bun peak at 20°–30°, which was the typical amorphous structure of bioactive glass (Figure 1b). Compared with Gel, the FTIR spectra of GelMA showed a significant increase in absorption intensity at 1640, 1541, and 1240 cm, which was related to CO stretching (amide I), NH bending (amide II), and CN stretching plus NH bending (amide III), which proved the successful synthesis of GelMA (Figure 1c) [15]. Next, the prepared bioactive glass powder was scanned for the element content (Figure 1d,e). Ca, P, Si, and O were the main elements in the bioactive glass network. The continuous release of calcium ions in bioactive glass raised to above 120 mg/mL in SBF (Figure 1f). At the same time, the continuous release of silicon and phosphorus ions was also observed, indicating the continuous degradation of the bioactive glass network.
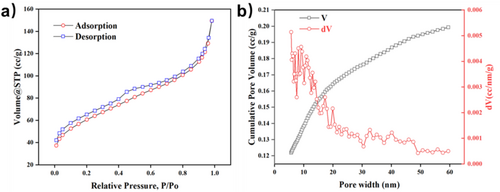
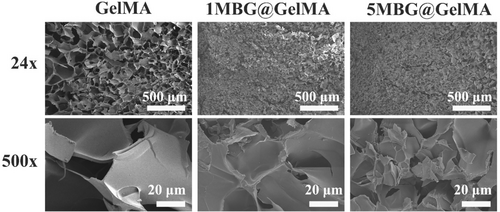
MBG behaves as an ultra-high specific surface area that has rapid water absorption and amplifies the coagulation cascade by the release of calcium ions (coagulation factor IV) [10, 11]. The isotherms of MBG possess a type IV curve through N2 adsorption–desorption (Figure 2a) with a specific surface area of 252.704 m2 g−1 and total pore volume of 0.199 cc g−1 according to the DFT method. The pore diameter of MBG was mainly distributed in 0–20 nm and average pore diameter was 6.26 nm, proving successful fabrication of MBG (Figure 2b). Cryogel sponges loaded with MBG showed a distinct difference, and due to the introduction of MBG as a physical crosslinking point, the pore size of the cryogel shrunk and became dense overall (Figure 3).
2.2 Characterization of MBG@GelMA Cryogel
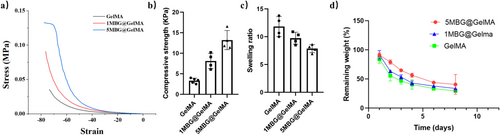
Frequent movement of the body may cause the hemostatic sponge to break or fall off, delaying wound healing. Therefore, a cryogel sponge with good mechanical strength and closure of the bleeding port will be more valuable. The introduction of MBG could significantly improve the mechanical strength of the cryogel sponge (Figure 4a). Increased strength after water absorption can block the hemostatic port and is not easy to tear up. The compressive strength of the composite cryogel sponge after complete water absorption increased from less than 4 kPa to above 12 kPa (Figure 4b). The rapid water absorption ability of cryogel sponges directly determines the effectiveness of hemostasis. Here, 5 min was selected as a water absorption time, and the sponges in each group could quickly absorb more than 5 times of self-quality (Figure 4c). Finally, similar quality maintenance curves were observed for different groups of MBG@GelMA cryogel with prolonged immersion time in PBS (Figure 4d). After 5 days, the mass loss of all groups of stents remained about 50%, and the sponge structure could be observed to be intact. However, slightly faster mass loss could be observed in the 5MBG@GelMA group, which might be related to ion release from MBG.
2.3 Cytocompatibility and Hemostatic Properties of MBG@GelMA Cryogel
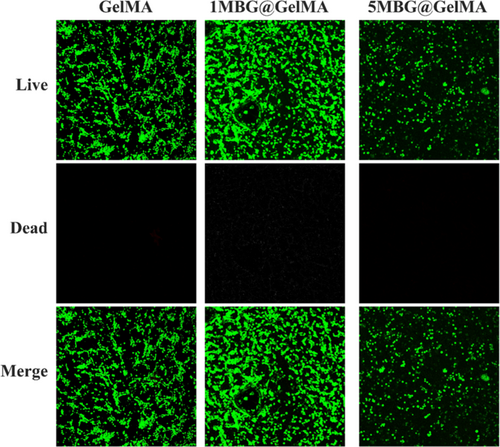
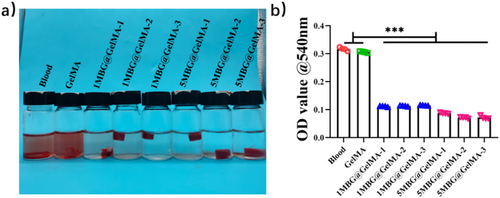
Good cytocompatibility is a prerequisite for hemostasis in vivo. In the contact culture mode, Bone Marrow Mesenchymal Stromal Cells (BMSCs) grew well in both GelMA and 1MBG@GelMA groups. Due to the ultra-high specific surface area and rapid release of calcium ions from MBG, there was a high concentration of ions in the local microenvironment. This resulted in certain cytotoxicity during initial exposure to 5MBG@GelMA (Figure 5). To evaluate the effect of cryogel sponge in promoting coagulation directly, recalcified anticoagulant sheep blood was directly added dropwise to the sponge; after the incubation was completed, deionized water was added to make the uncoagulated blood cells burst. While using deionized water as a control, it could be observed that GelMA sponge hardly caused coagulation similarly to the control group. Sponges loaded with MBG could significantly promote the formation of blood clots through the calcium-activated endogenous coagulation pathway [16] (Figure 6a). With loading the content of MBG increasing from 0% (GelMA) to 5% (5MBG@GelMA) gradually, the blood clotting effect was significantly improved with lower hemolytic absorbance and BCI (Figure 6b).
3 Discussion
Hemorrhage deaths remain a global problem. Trauma caused by road traffic injuries and surgical accidents is responsible for 5.8 million deaths worldwide every year, while 30%–40% of trauma deaths are related to bleeding [17]. Bleeding that occurs on the trunk or at the junction of the trunk cannot be stopped by compressing blood vessels [18]. Emergency hemostatic material for deep traumatic hemorrhage has momentous clinical significance. Inorganic hemostats developed based on aluminosilicates, such as QuikClot and Combat Gauze, have become recommended products for the U.S. military battlefield because they can quickly absorb plasma, red blood cells, and coagulation factors, resulting in rapid hemostasis [16]. Inspired by inorganic hemostatic materials such as diatomite, kaolin, and zeolite, MBG with very ultra specific surface area was designed for hemostasis. The blood is concentrated through water absorption driven by the siphon effect of the mesopore structure, the negative potential of the MBG surface, and the release of calcium ions, which amplifies the coagulation cascade [19]. Due to the potential risk of micro-thrombosis formation, there is an urgent need to develop appropriate matrix to avoid MBG entering the capillaries. Simple MBG powder has been proved to have good hemostatic effect [20]. The cryogel method could greatly reduce the amount of crosslinker and thus avoid its cytotoxicity [11]. Cryogel based on quaternized chitosan and hydroxyethyl cellulose oxide had cell biocompatibility by not using small molecule aldehyde crosslinkers [10]. GelMA exhibits favorable biocompatibility, adjustable mechanical properties, and degradation rate, making it a popular choice in various tissue regeneration applications including bone [21], cartilage, skin [22], and nerves [23]. Upon contact with blood, the hemostatic sponge induces platelet adhesion, aggregation, and thrombosis, thus inhibiting blood flow from the wound. Furthermore, it initiates the release of clotting factors, promoting the formation of a stable polymerized fibrin network, ultimately leading to blood clot formation and successful hemostasis of the wound [24]. The special preparation method of cryogel enables a connected macroporous structure from a variety of natural proteins and polysaccharides, which can quickly absorb water through capillary action and hydrophilic interaction [25]. For noncompressible wound bleeding, cryogel is first injected and then the filling cavity is dilated, creating a physical barrier to prevent further blood loss. Mechanically enhanced cryogel by nanoparticles [26] and dual networks [27] can exhibit better hemostasis. Although the mechanical strength of the composite cryogel continued to rise with the loading of MBG increased, 1MBG@GelMA exhibited the best cytocompatibility, and 5MBG@GelMA showed considerable cytotoxicity due to the initial rapid ion release and high ion concentration, pH, and osmolarity. To retain a beneficial impact on tissue regeneration within the body and prevent the detrimental effects of secondary excision, 1MBG@GelMA was chosen as the final preferred formula for noncompressible hemorrhage.
4 Conclusion
This work presents the development of a novel composite cryogel by combining MBG and GelMA for treating the hemostasis problems associated with noncompressible hemorrhage. The composite cryogel exhibit an interconnected macroporous structure and ultrafast water absorption performance that aid in achieving hemostasis for noncompressible and coagulopathic hemorrhage. Notably, MBG was integrated into the composite cryogel as an active substance, which further improves the hemostatic performance for patients. The composite cryogel demonstrates good cell biocompatibility, along with its ability to exert pressure on the bleeding site and form a physical barrier, which enables rapid hemostasis. Moreover, the composite cryogel exhibits excellent hemostatic and biocompatibility, as well as high portability and usability, suggesting its potential as a new type of portable civil and military hemostatic material.
5 Experimental Section
5.1 Fabrication and Characterization of MBG
MBG was prepared using the sol–gel method in combination with a microemulsion co-template technique [2]. In a brief, a certain amount of cetyltrimethylammonium bromide was dissolved in deionized water. Ethyl acetate was added after complete dissolution, and co-template microemulsion droplets were formed after stirring for 30 min. Subsequently, a certain molar concentration of ammonia is added to corrode the co-template by decomposing ethyl acetate and forming a mesoporous channel. Tetraethyl orthosilicate, triethyl phosphate, and calcium nitrate were added sequentially at intervals of 30 min while stirring the solution evenly for 4 h to fully react. The clear solution gradually became turbid to form a white precipitate. After the end of the reaction, it was aged for 24 h followed by centrifugation. After centrifugation, the white pellet was collected and washed three times alternately with absolute ethanol and deionized water. Then, the samples were dried in a 60°C oven for 24 h followed by calcination in air at 650°C for 3 h to obtain product. Field emission high power electron microscopy and X-ray spectroscopy were implemented to characterize the topography, monodisperse property, and crystal structure of bioactive glasses. The specific surface, pore volume, and the pore size distribution were detected by multipoint Brunauer–Emmett–Teller N2 absorption technique at 100°C for 4 h. MBG powder was immersed in a simulated body fluid (SBF) solution at a concentration of 1 mg/mL. And then it was placed in a constant temperature shaker (37°C, 120 rpm). The supernatant was obtained on day 0.5, 1, 2, 3, 5. After centrifugation, the supernatant was taken and tested for the concentration of different ions by a inductively coupled plasmaoptical emission spectrometer (ICP-OES, PerkinElmer PinAAcle 900 T, Germany).
5.2 Fabrication of GelMA and MBG@GelMA Cryogel
50 millimolar of morpholine ethanesulfonic acid buffer was prepared. 5 g of Type A gelatin was added to the buffer. 5 mL of methacrylic anhydride was added and reacted for three hours. Upon completion of the reaction, transfer the solution to a dialysis bag with 14,000 Da. After dialysis was completed, it was lyophilized to obtain GelMA. 5% wt of GelMA was dissolved in deionized water; MBG of 1 mg/mL and 5 mg/mL was added; and then APS and TEMED were added and stirred quickly for 10 min. The mixed solution was transferred to a mold and frozen at −20°C for 4 days, respectively. After lyophilization, they were labeled as GelMA, 1MBG@GelMA, and 1MBG@GelMA, respectively. The Fourier-Transform Infrared Spectroscopy (FTIR, Vertex 70, Bruker, German) was utilized to analyze the characteristic peaks of amide groups.
5.3 Characterization of MBG@GelMA Cryogel
The obtained cryogel was soaked in excess deionized water for 5 min, and the mass before and after rehydration was obtained to calculate the swelling ratio. Compression curve tests were conducted using dynamic thermomechanical instruments with a maximum pressure of 18 N [28]. The section with better linearity was uniformly selected to calculate the compressive strength in the 5%–20% deformation interval. After autoclaving, BMSCs were seeded in the cryogel and cultured for 24 h; live/dead kit and laser confocal were used to photograph the adhesion morphology of cells on the cryogel surface. The in vitro degradation behavior of bioactive glass composite frozen gel sponges was evaluated using PBS. MBG@GelMA cryogel was immersed in PBS at 0.2 g/1 mL after hydration adequately. The remaining mass was weighed at different time points after lyophilization completely.
5.4 Characterization of Hemostatic Performance of MBG@GelMA Cryogel In Vitro
One Milliliter of sheep anticoagulant whole blood was added dropwise to PBS and each group of cryogel, and then 10 mL PBS was added and incubated at 37°C for 30 min to observe coagulation [29]. Next, 100 μL of the upper PBS was aspirated and the absorbance was measured at 540 nm to quantify the amount of hemoglobin and unclotting erythrocytes.
5.5 Statistical Analysis
Statistical analyses were performed using the SPSS Statistical program and Graph Prism. Comparisons between the two groups used an independent sample t-test. p values ≤ 0.05 were considered as significant. Graphs were prepared in GraphPad Prism and Origin.
Acknowledgments
This work was financially supported by the science and technology research project of Chongqing Education Commission (KJQN202402712, KJQN202302703), the technology project of Chongqing level rural revitalization counterpart support (2023TIAD-ZXX0010), General project of Chongqing Three Gorges Medical College (XJ2023002903), and Research on the construction of question bank of practical skills examination resources for the qualification examination of practicing assistant physician of Chinese Medicine (2019XJMP03).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




