Mineral matrix deposition of MC3T3-E1 pre-osteoblastic cells exposed to silicocarnotite and nagelschmidtite bioceramics: In vitro comparison to hydroxyapatite
Abstract
This work presents the effect of the silicocarnotite (SC) and nagelschmidtite (Nagel) phases on in vitro osteogenesis. The known hydroxyapatite of biological origin (BHAp) was used as a standard of osteoconductive characteristics. The evaluation was carried out in conventional and osteogenic media for comparative purposes to assess the osteogenic ability of the bioceramics. First, the effect of the material on cell viability at 24 h, 7 and 14 days of incubation was evaluated. In addition, cell morphology and attachment on dense bioceramic surfaces were observed by fluorescence microscopy. Specifically, alkaline phosphatase (ALP) activity was evaluated as an osteogenic marker of the early stages of bone cell differentiation. Mineralized extracellular matrix was observed by calcium phosphate deposits and extracellular vesicle formation. Furthermore, cell phenotype determination was confirmed by scanning electron microscope. The results provided relevant information on the cell attachment, proliferation, and osteogenic differentiation processes after 7 and 14 days of incubation. Finally, it was demonstrated that SC and Nagel phases promote cell proliferation and differentiation, while the Nagel phase exhibited a superior osteoconductive behavior and could promote MC3T3-E1 cell differentiation to a higher extent than SC and BHAp, which was reflected in a higher number of deposits in a shorter period for both conventional and osteogenic media.
1 INTRODUCTION
Large bone defects resulting from various conditions, such as accidents, trauma, aging, and bone tumors, contribute to a decline in health and overall quality of life.1, 2 Typically, these situations necessitate surgical intervention and a considerable period for patient recovery, especially in cases of extensive bone loss. However, the application of autografts or allografts, considered the “gold standards,” is constrained by donor size or availability.3 Consequently, numerous studies are dedicated to the development of alternative strategies to facilitate bone tissue regeneration.4 Over time, natural and synthetic biomaterials have been crafted to either partially or entirely replace any bone defect, with the aim of preserving or enhancing the quality of life for potential patients. Biomaterials have undergone several evolutionary stages, often classified into three or four generations depending on the author.1 Presently, scientists are concentrating on the development of materials capable of stimulating desired cellular responses at the molecular level.2 This involves utilizing bioactive and bioresorbable materials that can expedite the patient recovery process without the need for additional surgical interventions or donor-site morbidity. Accelerating the regeneration process serves multiple critical purposes, including: (1) restoring the patient's quality of life as effectively and promptly as possible, (2) reducing public health spending per patient, considering the rising demand on hospitals, and (3) minimizing the time lost in economic productivity for surgically operated patients.
Among the most widely used biomaterials for bone repair is the well-known hydroxyapatite (HAp), a calcium phosphate bioceramic, recognized for being biocompatible, bioactive, osteoconductive, and similar to the mineral fraction of natural bone.5 However, the main drawbacks of HAp are its low mechanical resistance, poor biodegradability, and lack of rapid stimulus induction after implantation.5, 6 The addition of ions to HAp such as silicates, magnesium, sodium, and carbonates, among others, has shown an improvement in its mechanical and biological performance, leading to the development of ceramics with improved characteristics.5-8 On the other hand, the origin of HAp can be relevant to its biological behavior, this is the case of bovine derived HAp (BHAp), which has been frequently reported and used as a bone graft due to its biocompatibility and similarities with natural bone.9
According to several authors, the presence of ions in the physiological environment released by bioceramic materials can stimulate cellular responses improving the processes of mineralization, calcification, and bone metabolism.10, 11
Inspired by the chemical composition of bioactive glasses, crystalline bioceramics belonging to the calcium silicophosphate (CSP) family, have been reported to induce stimuli such as osteogenesis and angiogenesis.12-25 These characteristics make CSPs stand out as potential candidates for bone repair and wound healing applications.12-25 Since the CSP family presents a wide range of structures and chemical compositions, its physicochemical properties, such as bioactivity, mechanical resistance, degradation, and cellular response, can be tailored depending on the required biological application.12-27 Together with these materials, silicocarnotite (SC) and nagelschmidtite (Nagel) have shown promising characteristics that enhance apatite formation, cell infiltration, and osteogenic differentiation.13, 14, 25, 28-31 The SC phase presents an orthorhombic structure, Pnma space group, and lattice parameters a = 6.737 Å, b = 15.5080 Å, and c = 10.132 Å. SC with the general formula (Ca5−x(PO4)2+x(SiO4)1−x; x ≤ 0.3) is considered a highly substituted polymorph of the HAp, Ca10(PO4)6−x(SiO4)x(OH)2−x.32 When x = 2, it results in the stoichiometry of Ca5(PO4)2SiO4. The similarities between the structures SC and HAp have allowed the synthesis of SC from a precursor of silicon-substituted apatite and using aqueous precipitation or mechano-chemical methods.32-35 In both cases, the HAp structure is destabilized due to the formation of OH− vacancies in its hexagonal channel leading to SC formation through the incorporation of SiO44− groups, which in turn partially replace the PO43− groups.32, 34, 35 Regarding the biological properties of SC ceramics, in vitro studies have shown improvement and acceleration of the osteogenic differentiation process of bone cells compared to HAp.7, 30, 36
On the other hand, the Nagel phase with the general formula (Ca7−xNax(PO4)2+x(SiO4)2−x; 0 ≤ x ≤ 2) presents a hexagonal crystallographic structure, space group P61, and lattice parameters that vary depending on its stoichiometry. The composition Ca5Na2(PO4)4 (x = 2), presents a = b = 10.6336 Å, c = 21.6422 Å, while the composition Ca7(PO4)2(SiO4)2 (x = 0) exhibits a = b = 10.775 Å, and c = 21.4166 Å.37 These two stoichiometries have also been called “end-members” of the Nagel structure.37 Nagel ceramics have shown a significant enhancement of apatite mineralization, in vitro degradation, and stimulation of bone-related osteoblast and periodontal ligament cell gene expression.12, 23 Other studies have also shown the ability of Nagel phases to promote diabetic wound healing, and to regenerate soft tissue to inhibit inflammatory responses and bacterial infections.13, 38 The ionic release of SC and Nagel bioceramics plays a fundamental role in the regulation of osteogenesis and angiogenesis signals, leading these materials as potential implant candidates for bone regeneration.13, 14, 19, 21, 25, 28-31, 38, 39 The crystallography of HAp, SC, and Nagel phases plays an important role in these ionic release phenomena, and a comparison of these structures is presented in Figure 1A–C, respectively.
In previous studies, the mechanisms of apatite mineralization from the BHAp, SC, and Nagel phases were proposed.29, 31, 40 In the case of the SC, an epitaxial growth of the layer was identified, which follows the crystal structure of the SC with a composition closer to HAp. This is related to the polymorphism between both structures.29, 32 On the other hand, the Nagel phase showed a behavior comparable to that of bioactive glasses (e.g., 45S5), with a more accelerated bone-like apatite growth than SC and BHAp.29, 31, 40 In this case, the apatite layer grown on Nagel reached 10 μm of thickness after 21 days of immersion in Hank's solution.31 Even though apatite mineralization mechanisms are only one indicator of the ability of bioceramics to interact with the physiological environment, they provide an insight into the release and degradation that will directly affect the biological performance of these materials. Although the biological responses to bioactive ions released from inorganic biomaterials have been widely studied, the underlying relationship between the bioactive behavior, ionic release, cell interaction, and the effect of extracellular matrix deposition as a result of cell maturation due to the biomineralization process, are not yet fully understood.12, 41-45
The in vitro study of cell and tissue biomineralization is important to understand the effect of biomaterials on bone regeneration processes, considering that mineralization of the extracellular matrix is essential in the bone formation process.43, 46-51 The MC3T3-E1 cell line is one of the most implemented in vitro models to monitor the differentiation processes from pre-osteoblasts to osteoblasts in contact with a biomaterial.43, 44, 50-53
Building upon these considerations, the current study seeks to explore the impact of SC and Nagel phases on fostering the proliferation and differentiation of an immature bone cell line, pre-osteoblastic MC3T3-E1, in comparison to conventional osteoconductive materials (BHAp) and an osteogenic medium. The objective is to ascertain whether these CSP phases could serve as viable alternatives in developing new strategies for bone tissue regeneration.
2 MATERIALS AND METHODS
2.1 Material fabrication and characterization
Dense ceramics comprising BHAp, SC, and Nagel phases were successfully fabricated using a methodology established in prior studies54, 55 using HAp (BHAp) and bioactive glass (BG) as precursors. BHAp powders were obtained from bovine bones after cleaning, milling, and sintering processes employing a methodology detailed in our previous report.55 The bioactive glass, with a composition of 45S5, was sourced from Vitryxx® (Schott, Germany). For the SC and Nagel dense ceramic samples fabrication, a high-energy ball mill (8000D Mixer/Mill®, SPEX, USA) and yttria-stabilized zirconia (YSZ) balls with a diameter of 10 mm were used. The BHAp/BG ratios were set at 85/15 and 70/30 vol % for SC and Nagel, respectively.
Each dense ceramic sample, weighing 0.25 g, was uniaxially pressed at 35 kg/cm2 to form disk-shaped green samples using a die with a diameter of 10 mm. Subsequently, the green bodies underwent sintering at 1220°C for 4 h, with a heating rate of 5°C/min and a cooling rate of 10°C/min in a chamber furnace (Thermolyne 46100, Thermo Fisher Scientific). This process yielded dense ceramics measuring approximately 9.5 mm in diameter and 3 mm in thickness.
Before the in vitro tests, the dense ceramics were characterized by r-ray diffraction (XRD) to evaluate the structure associated with each phase (Table 1) and a scanning electron microscope (SEM) was used to evaluate phase morphology and cell adhesion to the sample surface. Samples were sputter-coated with a palladium–gold target and micrographs were recorded using a secondary electron (SE) detector at different magnifications. Furthermore, the chemical composition of the surface of sintered dense ceramics was analyzed using an Energy Dispersive Spectroscopy Analyzer (EDS, Bruker) coupled to the SEM, and the results are summarized in Table 2. All dense ceramic samples used in the in vitro tests were sterilized at 160°C for 2 h in a convection oven.
| Phase | Theoretical value of lattice parameters (Å) | Experimental value of lattice parameters (Å) | Lattice parameters strain (%) | χ2 | F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | a | b | c | ∆a/a | ∆b/b | ∆c/c | |||
HAp Ca10(PO4)6(OH)2 |
9.432 | 9.432 | 6.881 | 9.420 | 9.420 | 6.888 | 0.128 | 0.128 | −0.104 | 1.56 | 0.048 |
SC Ca5(PO4)2SiO4 |
6.737 | 15.508 | 10.132 | 6.705 | 15.401 | 10.106 | 0.471 | 0.687 | 0.259 | 1.97 | 0.057 |
Nagel Ca5Na2(PO4)4 Ca7Si2P2O16 |
10.636 | 10.636 | 21.642 | 10.679 | 10.679 | 21.630 | −0.409 | −0.409 | 0.059 | 1.89 | 0.052 |
| 10.775 | 10.775 | 21.417 | |||||||||
- Abbreviations: BHAp, bovine derived hydroxyapatite; Nagel, nagelschmidtite; SC, silicocarnotite.
| Sample | Na | Ca | Mg | P | Si | O |
|---|---|---|---|---|---|---|
| Theoretical values (wt %) | ||||||
| Silicocarnotite Ca5(PO4)2SiO4 | 41.539 | 12.841 | 5.822 | 39.798 | ||
| Si-rich nagelschmidtite Ca7Si2P2O16 | 42.854 | 9.463 | 8.580 | 39.103 | ||
| Na-rich nagelschmidtite Ca5Na2P4O16 | 7.342 | 31.998 | 19.783 | 40.876 | ||
| EDS measurements (wt %) | ||||||
| BHAp | 1.136 ± 0.138 | 37.057 ± 0.961 | 0.649 ± 0.049 | 17.971 ± 0.065 | 43.030 ± 0.808 | |
| SC | 3.388 ± 0.058 | 36.217 ± 0.087 | 0.195 ± 0.023 | 17.412 ± 0.050 | 2.148 ± 0.020 | 40.608 ± 0.117 |
| Nagel | 6.259 ± 0.072 | 33.689 ± 0.182 | 0.462 ± 0.011 | 15.111 ± 0.003 | 4.899 ± 0.174 | 39.521 ± 0.281 |
- Abbreviations: BHAp, bovine derived hydroxyapatite; Nagel, nagelschmidtite; SC, silicocarnotite.
2.2 In vitro
2.2.1 Incubation conditions of MC3T3-E1 mouse pre-osteoblasts (α-MEM, osteogenic medium)
MC3T3-E1 mouse pre-osteoblastic cells (Sigma–Aldrich, Germany) were cultured in alpha medium (α-MEM) (Dulbecco, Germany) supplemented with 10% of fetal bovine serum (FBS, Sigma–Aldrich, Germany), 1 vol % of penicillin/streptomycin (Sigma–Aldrich, Germany) and 1 vol % of L- Glutamine (Life Technology, Germany), and kept at 37°C in a humidified atmosphere with 5% CO2. Cells were cultured in 75 cm2 flasks (Nunc, Denmark) and grown for 2 days to confluence. Cells were then harvested using Trypsin and phosphate-buffered saline and counted using a hemocytometer (Roth, Germany). The final cell concentration of 104 cells/mL was obtained from the dilution of the initial medium. During exposure to the bioceramic samples, the medium was supplemented with osteogenic factors including 50 μg/mL ascorbic acid, 10 mmol beta-glycerol-phosphate, and 10 nM dexamethasone medium and replaced every third day.
2.2.2 Cell viability assessment and LIVE/DEAD (viability/cytotoxicity) staining assay
Cell viability was measured through the enzymatic conversion of tetrazolium salt (WST-8 assay kit, Sigma–Aldrich, Germany). After the selected time point, the medium was removed from the well, and cells were washed with PBS. Subsequently, α-MEM medium with WST-8 in a concentration of 1 vol % is added to each well plate and kept at 37°C for 1.5 h. Afterward, 100 μL of supernatant was transferred to a 98-well plate. As an indicator of metabolically active cells, the absorbance was measured at a wavelength of 450 nm using a microplate reader active cell (PHOmo, China). The results were normalized to the control using polystyrene wells with cells.
DAPI and calcein staining
To obtain more information on cell viability and cell attachment to the sample surface, calcein AM (Invitrogen™, USA) was used, and nuclei were stained with (DAPI) 4′,6-Diamidine-2′-phenylindole dihydrochloride, Invitrogen™, USA). Once the time point of interest was reached, the cultured media was removed, and the cells were washed twice with PBS. A PBS solution with a calcein concentration of 4 μL/mL was then added to each well and kept at 37°C for 45 min in the dark. After completion of calcein staining, cells were fixed to the surface using a fixation solution containing 3.7% (w/v) paraformaldehyde for 15 min; subsequently, a PBS solution with 1 μL/mL of DAPI was added to each well for 10 min. Finally, the evaluation of the samples was carried out in a fluorescence microscope (Zeiss Axio Scope A1, Germany) using green and blue filters, and images were processed to generate the merged images.
2.2.3 pH monitoring
To evaluate possible changes in pH values as a result of ionic release from the samples, media pH was monitored during incubation period. Every third day, before media replacement, the supernatant from three wells seeded with the same bioceramic was collected and pH measurements were taken by triplicate using a previously calibrated pH-meter (Hanna pH 211 microprocessor pH meter, Hanna Instruments, Inc., USA).
2.2.4 Alkaline phosphatase activity analysis
The specific alkaline phosphatase (ALP) enzyme activity was monitored using a colorimetric assay in which the absorbance of p-nitrophenyl-phosphate (pNpp) is modified by its conversion to p-nitrophenol (pNp). After the incubation period, cells are lysed and centrifuged for 5 min at 2000 rpm. Then, 250 μL of the supernatant was taken and transferred to a cuvette, where 100 μL of a solution containing 0.1-M Tris, 2-mM MgCl2, and 9-mM p-NPP were added. Samples were incubated for 2 h in the dark, and the reaction was stopped using 650 μL of 1 M-NaOH solution. Afterward, samples were evaluated using a spectrophotometer at 405 and 690 nm. The specific ALP activity was calculated as the amount of nanomolar pNPP that was converted to p-nitrophenol by the ALP, produced by cells per minute and milligram of total protein. ALP expression was normalized to the total protein content of cells seeded on the sample using the Bradford test (AppliChem GmbH, Germany). A 25 μL of cell lysates was mixed with 975 μL Bradford protein assay kit and incubated for 10 min in the dark. After incubation, absorbance was measured at 595 nm using a UV–vis spectrophotometer.
2.2.5 Staining for bone mineralization (Osteo-Image mineralization assay)
The biomineralization process during cell culture was assessed by forming mineralized nodules composed of biological HAp. For this, the specific assay OsteoImage™ fluorescence kit (Lonza, Germany), in which the reagent binds to the biological HAp portion of the bone-like nodules deposited by cells, was used.56 After the incubation period, culture media was removed, and cells were washed twice with PBS. Then, a medium solution with 4 μL/mL of the red stain Vybrant™ (Molecular probes, The Netherlands) was added to each well and incubated for 45 min to evaluate the cell's cytoplasm. Once the Vybrant™ staining was completed, cells were fixed to the surface using a fixing solution containing 3.7% (w/v) paraformaldehyde solution for 15 min; after that, a PBS solution with 1 μL/mL of DAPI was added to each well for 10 min. Then, samples were rinsed twice with a wash buffer from the OsteoImage™ kit, and subsequently, 300 μL of staining reagent were added to each well and incubated at room temperature, protected from light, for 30 min. Finally, samples were washed three times with PBS and then analyzed using a fluorescence microscope (Zeiss Axio Scope A1, Germany) using the green filter for the calcium deposits, red filter for the cytoplasm, blue filter for nuclei, and image were processed to generate the merged images. Additionally, images were processed by the ImageJ software, using three images for each treatment. The analysis was carried out by processing the green fluorescent channel for each image denoting the area with the fire filter. In this way, the same number of areas of the image was selected for densitometric analysis of pixels that fluoresce in green only, generating the representative mean fluorescence intensity data for each treatment.
2.2.6 Cell phenotype determination by SEM
For the morphological assessment, cells were fixed using a solution containing 3% (v/v) glutaraldehyde and 3% (v/v) paraformaldehyde (Sigma–Aldrich, Germany) in 0.2 mol/L sodium cacodylate buffer (pH 7.4). The samples were dehydrated in a graded ethanol series from 30 to 99.8 vol % (30, 50, 75, 80, 85, 90, 95, and 99.8) for 1 h each and stored in 99.8 vol % ethanol to be critical-point dried (EM-CPD300, Leica, Germany). Samples were sputtered with gold to avoid charging effects and analyzed by SEM (Auriga, Zeiss, Germany), using a SE detector and 1.5 kV of acceleration voltage.
2.2.7 Statistical analysis
Statistical analysis was performed using one-way or two-way analysis of variance (ANOVA) with post-hoc Bonferroni for multiple comparisons of means using the GraphPad® Prism software. Samples were compared against the controls and the reference sample BHAp. Differences were considered significant with *p < .05, **p < .01, and ***p < .001.
3 RESULTS AND DISCUSSION
3.1 XRD and SEM characterization of HAp, SC, and Nagel bioceramics
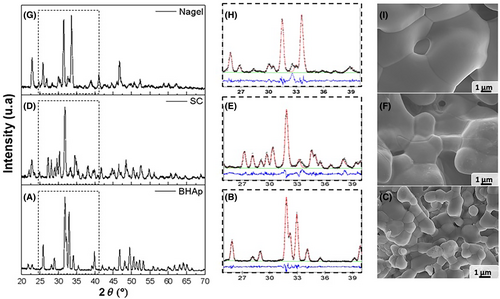
The phase fraction and crystallographic parameters of each bioceramic were calculated from a Rietveld refinement analysis of the XRD patterns using GSAS® and reported in Table 1. Figure 1 shows the XRD patterns and microstructure images associated with each sample: Figure 1A,D-BHAp, Figure 1B,E-SC, and Figure 1C,F-Nagel.
The crystal structure identified and analyzed in each case is consistent with the values reported elsewhere,54 where single-phase ceramics were determined in all cases. According to Table 1, all phases exhibited deformations in their lattice parameters, due to the substitutions generated by the fabrication process and the implementation of mixtures of HAp and bioactive glass as raw precursors.
As expected, the HAp phase was identified in the BHAp sample with deformations in the lattice parameters a and c, related to the contents of Na+ and Mg2+ that replace Ca2+ in the structure, due to its biological origin.54 The SC phase obtained in the SC sample exhibited a contraction of the lattice parameters a, b, and c, which would be related to substitutions in the atomic positions of Ca2+ by Na+ or Mg2+ and Si4+ for P5+, as a result of a non-stoichiometric phase and can present a wide range of solid solutions with CaO, SiO2, and P2O5. Finally, an intermediate structure to the end members of Nagel phase, with a composition of Ca, Na, P, Si, and O, was identified in Nagel sample, showing the lattice parameters a, expanded b and contracted c, suggesting partial substitutions of P5+ at the atomic position of Si4+. In the Nagel phase, a solid solution range described by Ca7xNax(PO4)2+x(SiO4)2−x, where the x moles are replaced by (PO4)3− at the expense of (SiO4)4− and the charges are compensated by the replacement of Ca2+ by Na+.54
In all single-phase compositions, contractions of lattice parameters are possibly related to substitutions in the structure to form nonstoichiometric phases. In Table 2, the chemical composition of the samples is shown. The composition of the SC and Nagel samples exhibit different chemical species than those expected theoretically, such as the incorporation of Na and the partial substitution of P for Si. For example, the theoretical composition of the Silicocarnotite phase has 12.8 wt % of P as well as 4.9 wt % of Si and the ceramic sample SC presents 17.4 wt % P and 2.15 wt % Si of experimental composition, which suggests the substitution of P in atomic positions of Si. In the same way, there is a substitution of Na for Ca. Theoretically the Ca wt% is 41.5 and experimentally it is 36.2, therefore, the 3.4 wt wt% detected of Na compensates for the cationic charge required in the crystal structure.
SEM images exhibit the characteristic microstructure of each phase, showing a grain size between 1 and 2 μm for BHAp, 2 and 4 μm for SC, and larger than 4 μm for Nagel. Likewise, the ceramics are dense, with some pores distributed on their surfaces. Moreover, the porosity of the ceramic samples was determined by density measurement using the Archimedes method, obtaining the following values: BHAp 4.42%, SC 3.92%, and Nagel 5.76%. The results suggest that the ceramic samples are highly dense.
3.2 Cell viability
Cell viability was measured after being exposed to SC, Nagel, and BHAp for 24 h, as shown in Figure 2. For SC and Nagel ceramics, viability levels are very close to those observed in the control (Figure 2A). In contrast, for BHAp, deficient viability levels were detected, indicating a cytotoxic effect of this material. As a complementary measurement, fluorescence micrographs are shown in Figure 2B. The green staining observed in the images is associated with the cytoplasm of metabolically active cells, while the blue staining is with the cell nucleus.
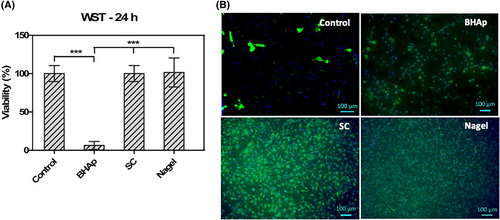
In the cases of the control, SC, and Nagel samples, the membrane and nuclei integrity are well-preserved, and the cells are spread all along the entire surface in an interconnected monolayer. On the other hand, the number of cells on the BHAp surface was significantly decreased compared with the control, the remaining cells exhibited a rounded morphology usually associated with a lack of adhesion to the surface and a cytotoxic behavior of the material.
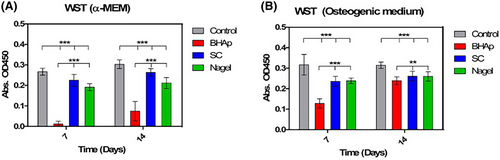
Cell viability and proliferation were also analyzed after 7 and 14 days using two different culture media and carried out by WST-8 kit to evaluate the effect of the ionic release of each phase on the cell behavior. The results are presented in Figure 3. Again, the viability of cells seeded on BHAp samples and cultured in α-MEM is considerably low even after 14 days of immersion. For the control, SC, and Nagel samples, cells have reached the confluence after 7 days, as noticed by the plateau in the proliferation curve, resulting in similar values detected at 14 days in both cell culture media. For the cells cultured on BHAp ceramics using the supplemented medium with osteogenic factors, a different behavior was observed. In this case, a significant increase in cell viability was observed after 14 days in which the values were comparable with the absorbances measured for the SC and Nagel ceramics.
These changes in the viability of cells exposed to BHAp ceramics can be associated with the variations in the pH values as shown in Figure 4A,B. For the α-medium, the pH behavior is very similar between the control, SC, and Nagel samples.
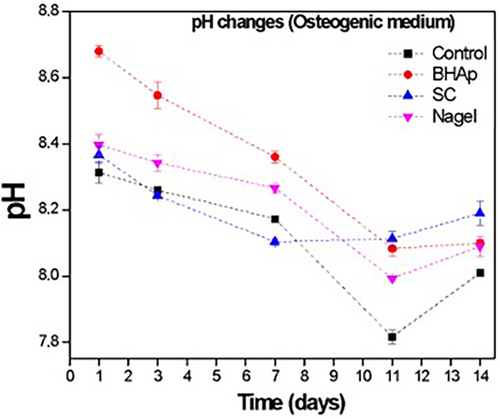
In contrast, for the BHAp the values from 1 to 7 days were considerably higher in comparison with the control, SC, and Nagel samples. After 1 and 3 days of immersion, cells seeded with BHAp ceramics are exposed to an alkaline media with pH values of 8.7 and 8.65, respectively. After 7 days, the medium reaches a pH value within the range considered neutral in a physiological environment (between 7 and 8.5) that remains stable until 14 days, during which time cell viability increases considerably.
On the other hand, the pH values measured in the supernatant of cells seeded in a medium with osteogenic factors such as ascorbic acid and dexamethasone showed an initial behavior comparable to the one observed in the conventional alpha medium after 1 day. However, after 3 days, a continuous decrease in the pH value is observed for all the samples until 7 days. In the case of BHAp ceramics, the slope on the pH curve is higher compared with the SC and Nagel samples, reaching a value of 8.1 after 11 days of immersion, which is closer to the physiological pH of 7.4. In this case, cell viability measured by the WST-8 assay increases reaching values close to those of Nagel and SC ceramics.
In contrast to the behavior of BHAp in α-MEM, cell viability measured by the WST-8 assay in the osteogenic medium at 14 days doubles the value at 7 days, reaching values close to those observed for Nagel and SC ceramics. The differences in the cell viability of the samples could be directly associated with the ionic release of each phase and therefore the modification of the pH, resulting in a positive or negative change in the environment in which the cells are found. It is possible that because SC and Nagel form a SiOH rich layer as a result of the interaction with physiological media such as cellular media (α-MEM media and osteogenic media), the accelerated precipitation of several cations released from bioceramics (i.e., Ca2+, Na+) into the SiOH layer favored the pH control.29, 31, 57
In addition, the osteogenic medium serves as a pH regulator and allows a better performance of the BHAp phase. Therefore, the SC and Nagel phases did not present a cytotoxic effect, while promoting cell proliferation and adhesion.
3.3 Enzymatic alkaline phosphatase (spec. ALP) detection
Figure 5 shows the ALP expression of cells exposed to bioceramics after 14 days with α-MEM and osteogenic medium. The spec. ALP enzymatic activity, normalized to DNA concentration, is significantly higher in cells seeded on BHAp surfaces with both cell culture media. ALP activity has been reported to be an early differentiation biomarker in osteoblastic cells and its expression starts immediately after the proliferation period. The correlation of the proliferation curves and ALP measurements suggests that the cells seeded on the BHAp surface are at an early stage of differentiation. Moreover, the ALP activity of cells seeded on SC and Nagel ceramics exhibits values comparable to those found in the controls in both media but is significantly lower compared with BHAp. An increase in ALP is observed in BHAp incubated in the osteogenic medium, which is related to increased cell viability due to the presence of beta-glycerophosphate as part of the supplemented medium.
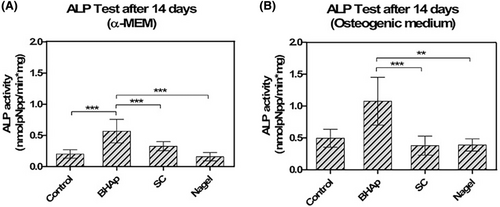
The assessment of additional biomarkers of osteoblast differentiation is discussed below to better understand and elucidate the differentiation stages of cells exposed to each bioceramic. Osteoblast differentiation is a complex process and in the particular case of in vitro assays, this occurs on the order of weeks.44 Various cell culture models have been developed and are believed to reasonably represent the progression of events that occur in vivo.44 For example, the murine pre-osteoblastic cell line MC3T3-E1, when in contact with ascorbic acid and an organic phosphate source (osteogenic medium), will differentiate and mineralize within 21 days.44 The differentiation process of MC3T3-E1 can be broadly divided into three different stages that are defined by: (1) proliferation, (2) matrix maturation, and (3) mineralization.44, 53 Several non-collagenous proteins, including the enzyme alkaline phosphatase (ALP), bone sialoprotein (BSP), osteopontin (OPN), and osteocalcin (OSC), have been identified as being expressed at high levels for discrete periods during in vitro differentiation.44, 53 Depending on how differentiation proceeds, the activity levels of the enzyme ALP increase, mainly in the early stages. The result of the differentiation process is the mineral calcium phosphate deposits (biological HAp), which are believed to occur through two possible mechanisms according to: (1) the formation of vesicles that bud from the plasma membrane and (2) the nucleation of calcium and phosphate ions mediated by the non-collagen proteins present in the ECM, conduction to the biomineralization process.46, 58, 59
In the early stages of differentiation, ALP levels increase producing high amounts of inorganic phosphates in the extracellular medium; however, when this exceeds the limit value that generates a positive effect, this inhibits the mineralization process.46, 58, 59 In agreement with our previous study, the ionic concentrations quantified after the degradation test at 120 h with constant agitation in a TRIS+HCl solution exhibited the highest concentration of P for the BHAp sample (13.07 ± 2.38 ppm) compared with SC (11.234 ± 1.173 ppm) and Nagel (7.042 ± 0.070 ppm).29, 31, 40 These results would be related to the quantification of ALP, which was much higher in BHAp in both culture media, followed by SC in the α-MEM medium. Therefore, it would be expected that the cells in contact with SC the Nagel would be at a more mature stage where the cells have already surpassed the peaks of destruction and production of ALP.
3.4 Bone mineralization staining (OsteoImage™ mineralization assay) and cell distribution (DAPI and Vybrant™ staining)
An important biomarker of osteoblast differentiation is the formation of calcium phosphate deposits as an indicator of the mineralization process in an osteoblastic cell line. The fluorescence micrographs in Figures 6 and 7 show the cytoplasm of metabolically active cells (Vybrant red staining) and their nuclei (DAPI blue staining) creating a monolayer along the ceramic surface, as well as calcium phosphate deposits (OsteoImage™ green staining) formed after 7- and 14-days of incubation in conventional α-MEM and osteogenic medium (Figure 6). It is evident that most of the samples after 7 and 14 days, seeded with both conventional and osteogenic medium exhibit a confluent surface, except for BHAp samples incubated in the α-MEM medium.
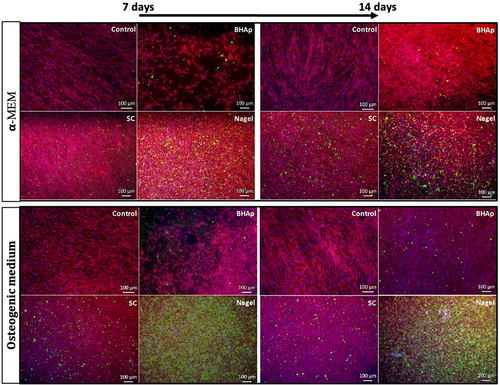
These observations are consistent with the results obtained for cell viability during the same incubation periods. As for the green-stained deposits, these are mostly observed in cells exposed to the Nagel phase. Considering that OsteoImage™ Fluorescent Staining Reagent specifically binds to the portion of bone-like HAp nodules deposited by cells, and only cells that have differentiated into osteoblasts have this ability, it is possible to suggest that both the SC and Nagel phases promote osteogenesis and accelerate the process of bone cell differentiation.
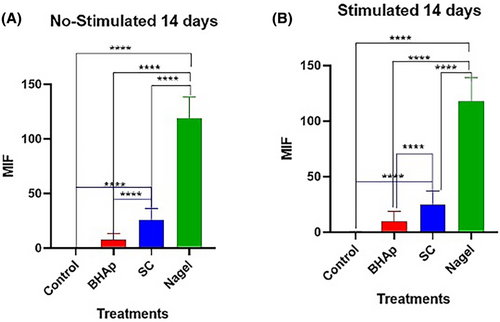
Comparing the number of nodules between SC and Nagel, after 7 and 14 days seeded in both, α-MEM and osteogenic medium, it is evident the presence of a greater number of these on the Nagel surface at both time periods, and it is also noticed that the mineralization process in these samples is independent of the type of culture medium. On the other hand, the cells seeded on the control well plates, using both media, did not exhibit the formation of calcium deposits at any of the explored incubation periods, evidencing the effect of the CSP phases on the accelerated differentiation process observed in the osteoblastic cell line. After conducting the corresponding densitometric analyses for the 7 and 14 days of exposure to the treatments, it was evident a highly significant difference (p ≤ .0001) between the cells treated with Nagel compared with all experimental groups (Figures 8 and 9). This indicates a high presence of calcium phosphate deposits, quantified by their mean fluorescence intensity (MFI) at 7 days, in both osteogenic and non-stimulated cell medium. Furthermore, no significant difference between the cells treated with BHAp and SC when compared with each other and with the control group (Figure 8) was observed from these plots.
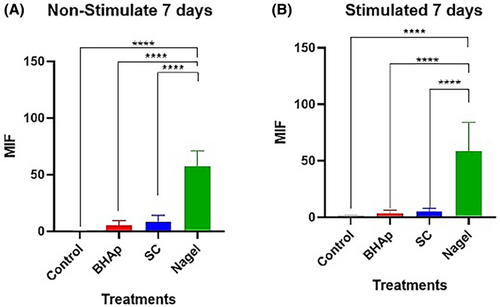
On the other hand, when cells treated for 14 days without stimulation were compared, they showed mean fluorescence intensities similar to those at 7 days. In this case, a highly significant difference (p ≤ .0001) was evident when comparing the Nagel-treated cell group versus control cells, BHAp-treated cells, and SC-treated cells. Meanwhile, when evaluating the stimulated cells at 14 days, a high MFI was identified for the cells treated with Nagel when compared with the treatments. Furthermore, a highly significant increase in MFI (p ≤ .0001) was observed for SC-treated cells compared with the BHAp-treated cells and the respective untreated control group (Figure 9). This strongly confirms the intrinsic potential of Nagel to induce the secretion of calcium phosphate deposits, both in the presence and absence of stimulation.
The pathways of matrix mineralization through non-collagenous proteins in pre-osteoblastic MC3T3-E1 cells have been reported as follows: extracellular OSC binds to Ca (i.e., calcium carbonate and phosphate) and HAp (Ca10(PO4)6(OH)2). Next, secreted OPN binds Ca and phosphate ions. Finally, BSP binds to type I collagen and participates in the nucleation process of biological HAp crystals. These non-collagenous proteins, located at the gap zones between collagen fibers, mediate mineral nucleation and promote their propagation within and along collagen fibers.59
All these mechanisms result in the formation of extracellular matrix deposition and subsequent biomineralization.44 On the other hand, bone apatite formation occurs through cooperative mechanisms, which may include matrix vesicles that bud from the plasma membrane and accumulate calcium (Ca2+) and phosphate (PO43−) ions extracellularly.59 Based on this fact, the mechanisms controlling the formation of the nodules observed on the SC and Nagel samples incubated at 7 and 14 days (in both media) could not be determined with the evidence presented in this work.
Based on the mineralization study of the Nagel phase presented in our previous work,31 the mechanism of apatite formation under acellular conditions points to a mimetic behavior during apatite biomineralization, emulating the ECM formation processes related to new bone formation in biological systems. Considering this, the formation kinetics and number of matrix nodules, observed at 7 and 14 days, could be related to a direct induction of the ionic release of Nagel on the biological mineralization process of osteoblasts.
Finally, the incubation periods and the results obtained agree with the expected responses according to Figure 3, where a plateau was detected in the proliferation of cells in contact with the SC and the Nagel after 7 days and a maximum peak of mineralized nodules after 14 days (Figures 7 and 8). It is important to highlight that the formation of these deposits was not observed in the osteogenic control, which according to Beck44 should exhibit mineralization after 21 days. Accordingly, the cells seeded on Nagel and SC ceramics show an advanced differentiation state compared with the osteogenic controls, suggesting the acceleration of this process due to the stimuli generated by the CSP phases.
3.5 Morphology of adherent cells on the surface of the bioceramics HAp, SC, and Nagel
One of the characteristics of bioactive ceramics is osteoconductivity in which the interaction with the physiological environment and cells, induces their adherence and spread. Many processes are involved in this action, such as protein adsorption and sample surface topography, which facilitate or delay the adhesion mechanism. Also, cell morphology may be related to the differentiation process involving apatite deposits associated with the extracellular mineral matrix.
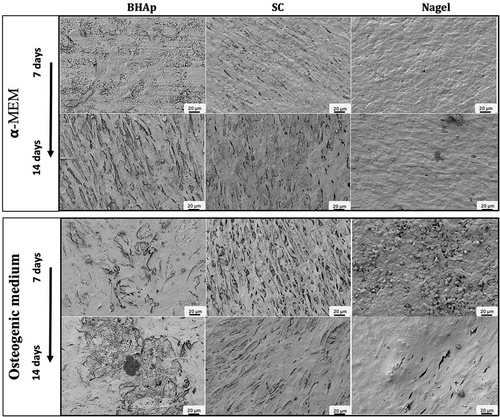
Figure 9 shows MC3T3-E1 pre-osteoblastic cells incubated for 7 and 14 days in α-MEM and osteogenic cell medium on the surface of BHAp, SC, and Nagel phases, exhibiting the effect of the phase composition and microstructure on cell morphology. SC and Nagel samples cultured in α-MEM medium at 7 days show a confluent surface, cells have an elongated morphology and form a monolayer (Figure 9). The adherent cells appear smooth and with few textured areas that seem as if they have adopted the roughness of the original surface of the samples. In the case of BHAp, there are few cells on the surface, and they barely manage to be in contact with each other. In contrast, the cells cultured with osteogenic medium exhibited confluent textured surfaces, which present an elongated growth (needle type), suggesting the formation of mineralized deposits (Figure 9). The increase in the cell viability on the BHAp bioceramics is the result of the action of osteogenic medium, giving a surface on which, a greater number of cells are joined in specific zones. One of the remarkable observations is the high elongation of the cells. Moreover, SEM analysis at 14 days showed numerous mineralized deposits on all the sample surfaces. This evidence suggests that, due to their shape and position on the cell membrane, they may contain biological HAp crystals.43, 50, 59
On the other hand, cells on SC at both 7 and 14 days showed highly elongated morphologies where filopodia covered with numerous extracellular mineralized deposits are defined. Although the cells on the Nagel phase show elongated filopodia, they do not have defined borders, a monolayer type, and spherical nodules distributed all over the surface are observed on the cells (Figure 9). This morphology has been reported in pre-osteoblasts MC3T3-E1 differentiated after 21 days.50, 51 Therefore, both extracellular matrix deposits and the morphology of the cells are strong evidence for the ability of SC and Nagel phases to induce osteogenic differentiation processes.43, 50, 59
However, there are yet unidentified proteins in the matrix that may play a regulatory role in the initiation of physiological processes such as cell differentiation and angiogenesis during the formation of mineralized tissues.50, 51 Recently, it has been reported that transforming growth factor beta receptor II interacting protein-1 (TRIP-1) was localized in the mineralized matrix of bone and dentin, and that TRIP-1 may activate TGF-β to promote the expression of a major regulator of VEGF,50 inducing in vitro and in vivo angiogenesis.50
Considering that in hard tissues, the calcified extracellular matrix acts as an osteoinductive matrix influencing the cell behavior and the physiological processes such as angiogenesis,50, 51 it is fundamental that bioceramics applied to bone regeneration induce and accelerate the biomineralization process.
The differentiation is associated with the formation of deposits of biological HAp crystals when the cells have reached a state of maturity.44, 52, 53 Among the techniques used for the detection of these mineral deposits or biomineralization in tissues are the classic histological methods such as Alizarin Red and Von Kossa staining, although these stains have proven to be insufficient for the precise evaluation of the mineral phase generated in cell cultures because the staining is nonspecific.56, 57, 60 In contrast, the OsteoImage™ mineralization assay used in this study is an in vitro test based on fluorescence; it is fast and allows evaluation of the mineralization of bone cells.53, 61-63 The OsteoImage™ assay can quantify in vitro mineralization by osteogenic stem cells, primary osteoblasts, and osteoblast-like cell lines.53, 56, 64 Moreover, it is based on the specific binding of the OsteoImage™ Fluorescent Staining Reagent to the HAp portion of the bone-like nodules deposited by cells.56
4 CONCLUSIONS
- SC and Nagel phases demonstrated the ability to promote both the proliferation and differentiation of the MC3T3-E1 cell line. Attached cells spread on the material surface, elongating their filopodia and maintaining cell connections, supporting the positive influence of SC and Nagel phases on these cellular processes.
- The Nagel phase exhibited approximately three times higher mineralized deposits than the SC phase, accelerating osteoblast maturation. When compared with HAp, the Nagel phase showed significantly higher mineralized deposits (above 90% compared to the HAp under the same conditions), emphasizing its potential for promoting tissue regeneration.
Further in-depth study is recommended to understand the relationship between biological mineralization processes and angiogenesis.
ACKNOWLEDGMENTS
The authors thank the following institutions: CONAHCYT, CINVESTAV—Querétaro (CENAPROT and LIDTRA), and the Institute of Biomaterials at FAU for financial support and for providing the facilities to carry out this research. Additionally, the authors thank Dr. Laura Ramos from the Institute of Biomaterials (FAU) and Dr. Miguel Ángel Hernández Vazquez at CINVESTAV-Querétaro, for their technical support in the SEM measurements and structural models' construction, respectively.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




