Recombinant perlecan domain V covalently immobilized on silk biomaterials via plasma immersion ion implantation supports the formation of functional endothelium
Abstract
Strategies to promote rapid formation of functional endothelium are required to maintain blood fluidity and regulate smooth muscle cell proliferation in synthetic vascular conduits. In this work, we explored the biofunctionalization of silk biomaterials with recombinantly expressed domain V of human perlecan (rDV) to promote endothelial cell interactions and the formation of functional endothelium. Perlecan is essential in vascular development and homeostasis and rDV has been shown to uniquely support endothelial cell, while inhibiting smooth muscle cell and platelet interactions, both key contributors of vascular graft failure. rDV was covalently immobilized on silk using plasma immersion ion implantation (PIII), a simple one-step surface treatment process which enables strong immobilization in the absence of chemical cross-linkers. rDV immobilization on surface-modified silk was assessed for amount, orientation, and bio-functionality in terms of endothelial cell interactions and functional endothelial layer formation. rDV immobilized on PIII-treated silk (rDV-PIII-silk) supported rapid endothelial cell adhesion, spreading, and proliferation to form functional endothelium, as evidenced by the expression of vinculin and VE-cadherin markers. Taken together, the results provide evidence for the potential of rDV-PIII-silk as a biomimetic vascular graft material.
1 INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide, associated with 31% of all deaths in 2016 (17.7 m people).1 Atherosclerosis (thickening and hardening of blood vessels because of plaque buildup) is the dominant cause of cardiovascular disease.2 Vascular bypass surgery is a common intervention for reinstating blood flow in occluded coronary and peripheral vessels with ~400,000 coronary artery bypass grafting procedures performed each year,3 making it the most common cardiac surgery worldwide.4 However, there is currently a critical unmet need for synthetic conduits for ~20% of bypass patients who do not have a suitable autologous graft available to transplant. Current synthetic grafts, including those made of expanded PTFE (ePTFE, GoreTex) or polyethylene terephthalate (PET, Dacron)5, 6 are not suitable for applications in vessels <6 mm in diameter (coronary and peripheral vessel grafting applications) where they fail because of thrombosis or neo-intimal hyperplasia. These failure mechanisms and poor graft patency are driven by the lack of healthy endothelium on synthetic grafts, which in healthy blood vessels plays a critical role in maintaining blood fluidity and regulating smooth muscle cell proliferation. A healthy, quiescent endothelium maintains blood fluidity via synthesis of anti-coagulant substances including heparin-like molecules such as thrombomodulin and tissue factor pathway inhibitor, and inhibits neo-intimal hyperplasia by promoting a quiescent contractile phenotype over a proliferative phenotype in smooth muscle cells.7 Therefore, strategies to promote rapid and functional endothelialization of vascular conduits are widely explored.
Perlecan (heparan sulfate proteoglycan, HSPG2) is a key component of the vascular basement membrane and plays vital roles in cardiovascular development and homeostasis, including vasculogenesis, angiogenesis, wound healing, vascular cell, and growth factor modulation.8-10 It consists of a protein core of ~460 kDa, divided into five distinct regions and contains four sites for glycosaminoglycan (GAG) chains decoration (three in Domain I and one in Domain V) and depending on the GAG chain substitution, the molecular weight can reach up to 700 kDa (Figure 1B).11 Perlecan has been demonstrated to support endothelial cell, while inhibiting smooth muscle cell and platelet interactions, making it uniquely suitable to vascular graft bio-functionalization.8, 12, 13 In fact, bio-functionalization of ePTFE grafts with perlecan was shown to improve their endothelialization and functional outcomes in a sheep carotid interposition model.14 However, as perlecan is difficult to isolate or synthesize in large quantity and modulation of vascular cell interactions is attributed to both GAG chains and the α2β1 cell integrin binding site in the C-terminal domain V, this region of perlecan serves as a promising target for recombinant expression and vascular applications.11 A recombinant product, encompassing domain V of human perlecan (3626L to 4391 S) referred as rDV, was expressed in HEK-293 cells as a proteoglycan decorated with either heparan sulfate (HS) or chondroitin sulfate (CS).15, 16 rDV has been shown to impart similar desirable vascular properties as full length perlecan, promoting endothelial cell interactions8, 15, 16 and angiogenesis,17 while also inhibiting smooth muscle cell8 and platelet interactions.10, 13
Bombyx mori (B. mori) silk fibroin is a cytocompatible natural polymer that has been explored in a range of cardiovascular applications including vascular grafts,18, 19 revascularization of implanted scaffolds20-22 and for cardiac tissue engineering.23 Silk fibroin has been engineered into vascular conduits with mechanical properties similar to native vessels18, 19 and higher patency rates compared to commonly used synthetic materials such as PTFE and PET in preclinical testing.19, 24, 25 However, as B. mori silk fibroin does not contain any cell binding motifs, bio-functionalization presents a promising method of further improving graft performance and supporting rapid and functional graft endothelialization. A review of silk bio-functionalization for vascular graft applications can be found in Fukayama et al.26
Silk fibroin has a limited number of modifiable chemical functional groups applicable for biomedical applications, limiting standard bio-functionalization chemistries as reviewed in Cordelle and Mantero.27 Other strategies such as physisorption results in weak secondary bonds that are easily displaced in blood-contacting applications.28 Recently, plasma immersion ion implantation (PIII) has been used to bio-functionalize silk with biological molecules, including rDV.29, 30 PIII is a physical plasma surface modification method that uses energetic ion bombardment under impulses of highly negative potential bias to attract positively charged ions in the plasma phase toward polymeric substrates. This ion implantation process results in a nano-scale carbonized layer containing unpaired electrons. The unpaired electrons, commonly referred to as radicals, are implanted deep into the polymeric structure with depths in the range of 10–100 nm. Regulated by thermally activated process, the radicals migrate to the surface and undergo chemical reactions with atmospheric species, such as oxygen, and proximate macromolecules, even after a year post-treatment.31, 32 The radical-driven chemical reactions also establish covalent bonds between amino acid side chains of biomolecules and PIII-treated polymeric surfaces, facilitating stable covalent bio-functionalization.33 This technique has been well established to confer covalent immobilization of biomolecules on synthetic polymers and unlike classic covalent immobilization chemistries such as 1-ethyl-3-(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS), PIII does not rely on specific functional groups in the polymer chain (Figure 1A).
Currently, silk and chitosan34, 35 are the only two natural polymers that have been PIII-treated, with silk being the only natural polymer on which covalent immobilization of a biomolecule has been demonstrated.29, 30 In particular, when rDV was immobilized on PIII-treated silk, rDV-PIII-silk supported excellent blood interactions when rDV was present, but PIII-silk was thrombogenic in the absence of rDV.30 PIII surface modification was also shown to result in minor changes to bulk chemical, mechanical, degradation properties, and immune response to silk showing PIII-treatment to be a simple way of modifying the surface of silk biomaterials without re-optimizing graft manufacturing conditions.36 Therefore, the work to date has demonstrated covalent rDV immobilization on silk biomaterials and functionality of the immobilized rDV in terms of maintaining blood fluidity.30 The present study explores the effect of rDV biofunctionalization of silk biomaterials in promoting endothelial cell interactions and the formation of a functional endothelial layer on rDV-PIII-silk, a key criterion in developing the next generation of small-diameter vascular grafts.
2 MATERIALS AND METHODS
2.1 Cells and reagents
All reagents were purchased from Sigma-Aldrich unless stated otherwise. Rabbit polyclonal anti-domain V antibody (anti-endorepellin, α-DV)37 was provided by Prof. Renato Iozzo. Human umbilical vein endothelial cells (HUVECs, Lonza) were maintained in EGM™-2 (Endothelial Cell Growth Medium-2 BulletKit, Lonza) and used at passage 5–9. The media was replaced every 3–4 days and cells were passaged every 7 days.
2.2 Expression and purification of recombinantly produced domain V
Recombinant human perlecan domain V expressed by transfected human embryonic kidney-293 (HEK-293) cells was collected and purified as previously described in Jung et al.15 Briefly, domain V DNA (2446 bp, exons 79–97) was amplified from human coronary artery endothelial cell (HCAEC) mRNA, cloned into CEFLsec vector with a BM40 signal peptide and transfected into HEK-293 cells using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific). Stably transfected HEK-293 cells were selected using Geneticin and cultured in Dulbecco's Modified Eagle Medium (DMEM) high glucose with 10% (v/v) fetal bovine serum (Bovogen Biologicals) and 1% (v/v) penicillin–streptomycin. Conditioned media was routinely collected every 3–4 days and passaged once a week. Purification of rDV was done via anion exchange chromatography on diethylaminoethyl (DEAE) matrix, as described in Whitelock et al.38 DEAE-purified rDV is a mixed population of rDV proteoglycan decorated either a single chondroitin sulfate (CS) or a single heparan sulfate (HS) glycosaminoglycan (GAG) chain.16 The concentration of rDV was measured using Pierce™ Coomassie (Bradford) protein assay (Thermo Fisher Scientific) while the post-translational modification with CS and HS was in a ratio of approximately 2:1 as determined by Western blot using rDV and digested conducted with either heparinase III (Hep III) (0.01 U/mL) (Iduron) and/or chondroitinase ABC (C'ABC; 0.05 U/mL; Amsbio) in PBS (pH 7.2, Astral Scientific) at 37°C for 16 h.16
2.3 Preparation of regenerated silk solution
Bombyx mori (B. mori) silk cocoons (Tajima Shoji Co. Ltd.) were processed into regenerated silk fibroin solution as previously described in Rockwood et al.39 Briefly, silk cocoons were cut into pieces and boiled in 0.02 M sodium bicarbonate solution (5 g/2 L) for 30 min to remove sericin. Washed and dried silk fibroin fibers were dissolved in 9.3 M lithium bromide solution at 25% (w/v) for 2–4 h at 60°C. Silk solution was dialyzed against MilliQ water using 3.5 MWCO dialysis tubing (SnakeSkin™, Life Technologies) for 3 days to remove lithium bromide from the silk solution. The remaining silk solution was centrifuged twice at 8700 rpm for 15 min at 4°C to remove debris. Concentration of silk solution was determined by weighing a known volume of solution post-drying overnight at 60°C. This process resulted in a silk solution with concentrations ranging from 7% to 9% (w/v). Silk solution was stored at 4°C.
2.4 Preparation of biofunctionalized silk films
For ELISA and cell adhesion studies, 15 μL of 2% (w/v) silk solution was cast onto flat μ-slide 18-well chambered coverslips (ibidi). For surface zeta potential, cell spreading, proliferation, and immunocytochemistry studies, silk films were prepared by dip coating 2% (w/v) silk solution onto the cell-culture treated side of 22 × 60 mm Thermanox coverslips (ProSciTech). All silk formats were dried overnight at room temperature (RT) and water annealed the following day, as previously described in Hu et al.40 to induce film stability in an aqueous environment via β-sheet formation. Silk films on coverslips used for surface zeta potential analysis were cut into 20 × 10 mm rectangular films, while silk films for cell studies were cut into 5 mm diameter samples using a biopsy punch. Biofunctionalization of silk films with rDV was done by either passive adsorption, EDC/NHS wet chemical crosslinking, or nitrogen PIII dry physical crosslinking. For passive adsorption, silk films were incubated with rDV with (mixed population of rDV molecules decorated with CS or HS GAG chain) or without GAG chains (pre-digested prior to incubation as described in Section 2.2; 10 μg/mL in PBS, pH 2, 4, 7.2, 9, or 12, 15 μL for flat μ-slide, 60 μL for 96-well plates) for either 2 h at RT or overnight at 4°C and rinsed twice with PBS. For EDC/NHS surface activation, silk films were first incubated in 2-(N-morpholino) ethane sulfonic acid (MES) buffered saline (0.1 M MES, 0.9% (w/v) sodium chloride, pH 6.0) for 30 min at RT. Carboxyl (COOH) groups in silk films were activated with 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC–HCl, Life Technologies)/N-hydroxysuccinimide (NHS, Life Technologies) solution (0.5 mg/mL of EDC and 0.7 mg/mL of NHS in MES buffered saline, pH 6.0) for 30 min at RT, generating amine-reactive NHS-esters on the silk film surface. Activated silk surfaces were rinsed thrice with MES buffered saline and biofunctionalized with rDV as previously described for passive adsorption, but using MES buffered saline instead. For dry surface activation with PIII, silk samples were mounted onto a metal substrate holder, with a metallic mesh placed 5 cm in front of the sample holder.29 The substrate holder was placed into the vacuum chamber and evacuated to a base pressure of 10−5 Torr, with nitrogen gas introduced with a flow rate of ~72 standard cubic centimeters per minute (sccm) generating a working pressure of 2 × 10−3 Torr. The source of ions for PIII treatment was generated from inductively coupled RF plasma (13.56 MHz), with a forward power of 100 W and a reverse power of 12 W. Acceleration of plasma ions was achieved with 20 kV bias pulses of 20 μs duration to the substrate holder and mesh at a frequency of 50 Hz for 800 s. Sample holder was earthed between pulses. The PIII treatment endowed the silk film with surface-embedded radicals. Samples were aged for ~1 week at RT and room relative humidity in the dark before use. Biofunctionalization with rDV on PIII-treated silk was then conducted as described for passive adsorption.
2.5 Surface zeta potential of surface-modified silk films
Silk coated on Thermanox coverslips were cut into rectangular films, as described in Section 2.4, and mounted onto a SURPASS 3 electrokinetic analyzer (Anton Paar) by double-sided tape. Streaming current was measured starting from pH 9 by adjusting the pH of the 1 mM KCl running solution using 0.1 M potassium hydroxide (KOH) and measurements were incrementally taken to pH 3 with the addition of 0.25 M hydrochloric acid (HCl). Surface zeta potential was then calculated using the SURPASS 3 software.
2.6 Analysis of rDV amount and orientation on biofunctionalized silk surfaces
Silk samples were biofunctionalized with rDV, as described in Section 2.4. Wells were blocked with 0.1% (w/v) casein in PBS (blocking solution) to prevent nonspecific binding for 1 h at RT. After washing twice PBS-T (PBS, 0.1% (w/v) Tween 20), either polyclonal antibody α-DV or monoclonal antibodies E6 (Santa Cruz Biotechnology) or A74 (Abcam) primary antibodies was incubated onto the surface at a concentration of 1 μg/mL for polyclonal or 2 μg/mL for monoclonal antibodies in blocking solution for either 2 h at RT or overnight at 4°C. Samples were washed twice with PBS-T, then biotinylated goat anti-mouse or anti-rabbit IgG antibody (GE Healthcare) of final concentration 1 μg/mL in blocking solution incubated for 1 h at RT was added. After washing twice with PBS-T, Streptavidin-horseradish peroxidase (SA-HRP, GE Healthcare) at a 1:500 dilution with blocking solution was added and incubated for 30 min at RT. After washing four times with PBS-T, 2,2′-azino-di-(3-rhylbenzthiazoline sulfonic acid) (ABTS) color substrate reagent with 1:1000 dilution of H2O2 (Riedel-de-Haen) was added and incubated at RT until appropriate color developed. ABTS solution was transferred into a 384-well plate and was read at 405 nm absorbance using a Synergy HTX plate reader (Biotek).
2.7 Endothelial cell interaction and functionality assays
For cell culture studies, blocked tissue culture plastic (bTCP) was prepared by coating tissue culture plastic (TCP) with denatured bovine serum albumin (dBSA; 1% BSA in PBS, boiled at 80–90°C for 10 min, transferred to ice) for 30 min at 37°C and rinsed twice with PBS.
For cell adhesion studies, 5 × 103 HUVECs/well in EBM-2 basal medium (Lonza) were seeded onto TCP (positive control), bTCP (negative control), and surface-modified silk with or without rDV biofunctionalization for 1 h at 37°C, 5% CO2. Wells were washed four times in PBS and quantified using the CyQuant Cell Proliferation Assay (Life Technologies) according to manufacturer's instructions.
For cell spreading studies, 5 mm diameter samples were placed in ProxiPlate-96 shallow well micro-plate (PerkinElmer). 1 × 104 HUVECs/well in EBM-2 basal medium were seeded onto TCP (positive control), bTCP (negative control), and surface-modified silk with or without rDV biofunctionalization for 3 h at 37°C, 5% CO2. Wells were washed thrice in PBS and fixed in 4% (w/v) paraformaldehyde (PFA) in PBS for 30 min at 37°C and rinsed thrice with PBS.
PFA-fixed samples were permeabilized with 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 2 mM HEPES, and 0.5% (v/v) Triton X-100, pH 7.2 for 5 min at 4°C and then blocked in 1% (w/v) BSA in PBS containing 0.05% (w/v) Tween-20 (PBS-T) for 1 h at room temperature. Samples were rinsed with PBS-T and stained using rhodamine phalloidin (1:200, Life Technologies) for 1 h at 37°C, rinsed with PBS-T, and further stained with TO-PRO3 Iodide nuclear stain (1:1000, Life Technologies) for 30 min at 37°C. Samples were rinsed with PBS-T, stored in PBS prior to imaging. Cell spreading samples were imaged with a TCS SP2 confocal microscope (Leica) using a 10× objective at the center field of view and 40× objective at the center top and center bottom field of view. Images were then quantified as cell area from images taken at 10× objective (n = 3–4) using Image J (National Institutes of Health, USA) by overlaying a 3 × 3 equally spaced grid across each image and measuring the area of the cell closest to each grid intersection. Up to nine cells were measured per image to ensure a similar number of cells were quantified for each condition. The number of cells was quantified by counting the number of nuclei from confocal images taken at 10× objective (n = 4) using Image J.
For cell proliferation studies, 5 mm diameter samples were placed in ProxiPlate-96 shallow well micro-plate. 1 × 104 HUVECs in EGM™-2 supplemented media (Lonza) were seeded onto TCP (positive control), bTCP (negative control), and surface-modified silk with or without rDV biofunctionalization overnight at 37°C, 5% CO2. The following day, samples were washed twice with PBS and transferred to a standard 96 nonbinding well plate (Grenier Bio-One) and quantified at 1, 3, and 5 days post-seeding using the Alamar Blue Cell Viability Reagent (Life Technologies) according to manufacturer's instructions. To further analyze cell-to-cell interactions on silk samples, HUVECs were cultured for 10 days in EGM™-2 supplemented media, washed thrice in PBS, and fixed in 4% (w/v) PFA in PBS as described above.
For actin–vinculin colocalization and endothelialization studies, fixed cells were permeabilized and blocked as described above then probed for either anti-vinculin antibody (clone VIN-11-5, 5 μg/mL) or anti-VE Cadherin antibody (1 μg/mL, Abcam) in blocking solution overnight at 4°C and rinsed twice with PBS-T. Goat anti-mouse Ig (H&L) conjugated with 488 nm Alexa Fluor (5 μg/mL, Thermo Fisher Scientific) for anti-vinculin and goat anti-rabbit Ig (H&L) conjugated with 488 nm Alexa Fluor (4 μg/mL, Thermo Fisher Scientific) for anti-VE-cadherin antibody, was incubated for 90 min or 30 min, respectively, at room temperature, rinsed twice with PBS-T and further stained with rhodamine-phalloidin and TO-PRO3 Iodide or Hoeschst-33342 (1 μg/mL, Thermo Fisher Scientific) as described above. Samples were rinsed thrice with PBS-T and stored in PBS prior to imaging. Vinculin-stained samples were imaged using an SP8 confocal microscope (Leica) using a 63× objective at the center top and center bottom field of view using the same imaging settings. Actin-vinculin colocalization was analyzed by Image J using the “Just Another Colocalization Plugin” (JaCOP). The Thresholded Mander's Colocalization Coefficient was obtained as M1 and M2, where M1 is defined as the ratio of the “summed intensities of pixels from channel 1 (actin) for which the intensity in channel 2 (vinculin) is above zero” to the “total intensity in channel 1,” and vice versa for M2.41 Endothelialization studies were imaged with a TCS SP2 confocal microscope (Leica) using 40× objective at the center top and center bottom field of view using the same imaging settings. Cell–cell junction analysis was quantified by Image J by counting cells with a defined VE-cadherin positive stained ring around the cell, then expressed as a percentage of the total number of cells, quantified using the nuclei stain.
2.8 Statistical analysis
Data were expressed as mean ± standard deviation (SD). Logarithmic transformation of non-Gaussian datasets was applied to better fit statistical analysis models, as defined by linear fit to QQ plots. Significant statistical differences were determined by either by nested t-test, nested one-way analysis of variance (ANOVA), one-way, or two-way ANOVA, with Dunnett's multiple comparison post-test using GraphPad Prism 9 software. Accepted statistical difference was at p < .05 and indicated in the figures as *p < .05, **p < .01, ***p < .001, and ****p < .0001.
3 RESULTS AND DISCUSSION
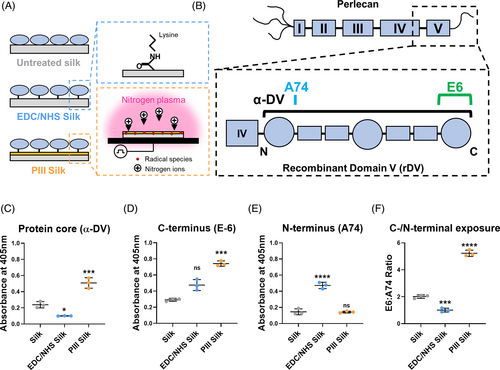
3.1 Surface modification altered the rDV amount and orientation on silk biomaterials
The function of proteins is intricately linked to their structure, which can be compromised when proteins are immobilized on a biomaterial surface. Therefore, to investigate the presentation of rDV on silk biomaterials, rDV was immobilized on the silk material surface via passive adsorption (silk), carbodiimide chemistry (EDC/NHS-silk), or PIII treatment (PIII-silk; Figure 1A). The amount and orientation of rDV were determined by ELISA assays using a well-established panel of antibodies for perlecan.15 Here a polyclonal antibody targeting multiple epitopes along the protein core (α-DV)37 and thus indicative of rDV amount, or monoclonal antibodies targeting a single epitope in the N-terminal (clone A74)15 and C-terminal (clone E6) regions of rDV indicating rDV orientation on the surface (Figure 1B) were utilized. rDV was successfully immobilized on silk biomaterials via all three techniques (Figure 1C), but with significantly more rDV immobilized on PIII-silk relative to silk (p < .001) or EDC/NHS-silk.10 This data is in agreement with previous work showing EDC/NHS-based10 and PIII-based30 rDV immobilization on silk using surface plasmon resonance, validating the use of the polyclonal antibody to quantify rDV amount on the silk surface. This is in line with previous work, which has demonstrated that more rDV is immobilized on PIII-silk relative to silk and that this immobilization is covalent.30 Analysis of the C-terminal (Figure 1D) and N-terminal (Figure 1E) epitopes revealed an interesting dichotomy, where relative to silk, PIII-silk demonstrated a 2.5-fold higher C-terminal (p < .001), but no difference in N-terminal signal, while EDC/NHS-silk had an opposite effect with a 3.2-fold higher N-terminal (p < .0001) signal, but no difference in C-terminal signal. As the absolute antibody reactivity on these surfaces may be related to the amount of immobilized rDV, the data were also analyzed as the ratio of E6 (C-terminal) to A74 (N-terminal) absorbance signals to study rDV orientation. The E6:A74 ratio (Figure 1F) was significantly lower on EDC/NHS-silk (p < .001) and significantly higher on PIII-silk (p < .0001) relative to silk, suggesting that both covalent immobilization methods oriented rDV differently on silk biomaterial surfaces compared to passive adsorption. One previous study reported conformational differences when tropoelastin was immobilized on PIII-treated PTFE, attributed to changes in surface wettability leading to preferential binding of the C-terminal region of tropoelastin onto the PIII-treated surface compared to the untreated PTFE.42 C-terminal region of rDV contains biologically important features, including the GAG attachment and cell adhesion sites, and its increased exposure on PIII-silk may have important implications for biological interactions with these materials.
3.2 rDV immobilized on PIII-silk supports endothelial cell interactions
To test the ability of immobilized rDV to support endothelial cell interactions, HUVECs were seeded onto rDV-biofunctionalized silk biomaterials and examined at early timepoints post-seeding to assess cell adhesion and spreading as shown in Figure 2. HUVEC adhesion (Figure 2A) was compared on TCP (positive control), bTCP (negative control), and surface-modified silk with or without rDV at 1 h post-seeding in the absence of serum proteins. Cell adhesion on silk and EDC/NHS-silk in the absence of rDV was low, comparable to the negative control, while cell adhesion on PIII-silk was significantly higher than on silk (p < .0001) and on the positive control (p < .05). Following rDV-biofunctionalization, a 1.9-fold higher endothelial cell adhesion was detected on rDV-biofunctionalized silk (rDV-silk) compared to silk (p < .05), while immobilization on EDC/NHS-silk (rDV-EDC/NHS-silk) and PIII-silk (rDV-PIII-silk) did not show any difference compared to their unfunctionalized counterparts. HUVEC adhesion on rDV-PIII-silk showed no difference compared to PIII-silk (p > .05) but was 4.4-fold higher than silk (p < .0001) and 2.3-fold higher than rDV-silk (p < .0001).
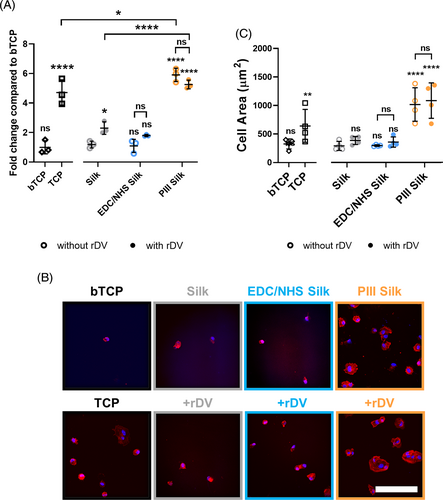
To investigate how endothelial cells spread on rDV-biofunctionalized silk, HUVECs were seeded in a similar manner to cell adhesion and incubated for 3 h in the absence of serum proteins. Samples were then fixed and stained for F-actin and nuclei to visualize the cell morphology. Representative confocal images of endothelial cells on different surfaces are shown in Figure 2B. Consistent with cell adhesion at 1 h, confocal images of cell spreading at 3 h showed few cells adhered on silk and EDC/NHS-silk in the absence of rDV, similar to the observations on the negative control. Cells on these surfaces were small and round with little evidence of cell spreading. In contrast, PIII-silk supported more cell adhesion with adhered cells appearing larger than on silk, showing defined spread morphology with polymerized actin fibers, like the morphology on positive control. Biofunctionalization with rDV did not appear to have a major effect on cell morphology on rDV-silk or rDV-EDC/NHS-silk, while cells adhered on rDV-PIII-silk were comparable to PIII-silk. Quantification of cell spreading on different rDV-biofunctionalized silk biomaterials was performed by measuring the cell area and is presented as mean cell spreading on different surfaces (Figure 2C). Quantitative analysis confirmed qualitative observations with no significant differences found between silk conditions with or without rDV-biofunctionalization. Cell spreading on rDV-EDC/NHS-silk was not significantly different from its bare counterpart or silk and was comparable with the negative control. In contrast, rDV-PIII-silk showed significantly higher cell spreading compared to silk (p < .0001) and rDV-silk (p < .001), comparable to the positive control.
Taken together, these results demonstrate that rDV immobilized via passive adsorption or PIII treatment supported endothelial cell adhesion. This is consistent with previous reports demonstrating rDV-supporting endothelial interactions, including where human coronary artery endothelial cell (HCAEC) adhesion on rDV-TCP was significantly higher than the negative control (bTCP).16 Another study also showed rDV immobilized on silk or EDC/NHS-silk resulted in higher HCAEC adhesion and spreading compared to the untreated silk.10 These results were attributed to the interaction between the endothelial cell and the α2β1 integrin binding site at the C-terminal region of rDV. Interestingly, in contrast to the previous study, the present results found no significant difference between rDV-EDC/NHS-silk compared to the unfunctionalized counterpart. This is likely due to the difference in immobilization pH used (pH 7.2 here vs. pH 6.0 in the previous study) which may influence the orientation of rDV and exposure of the integrin binding site.
3.3 Buffer pH affects rDV immobilization and endothelial cell interactions
Proteins contain both positive and negative regions and fold into secondary and tertiary structures and attraction/repulsion mechanism with the surface can lead to differences in protein orientation and folding subsequently affecting protein function.43 These electrostatic interactions can influence protein immobilization on biomaterial surfaces and can potentially be modulated by the pH of the reaction buffer. PIII treatment of silk has been shown to alter the material surface chemistry36 and in turn alter the amount and presentation of rDV on the surface of PIII-silk compared to silk and EDC/NHS-silk (Figure 1C,D). This is likely attributed to the change in surface chemistry influencing the electrostatic interactions between the silk and rDV,44 but this phenomenon has not been demonstrated. To investigate the effect of pH on rDV immobilization on silk biomaterials, we first determined the surface charge of untreated and PIII-treated silk films at different pH values (Figure 3A). Surface zeta potential of silk reached a positive plateau at pH values lower than ~3 and a negative plateau from ~pH 6 onwards, with a linearly decreasing region between (pH 3–6). Following PIII-treatment, PIII-silk showed a positive plateau beginning at a similar pH to silk, but the negative plateau started from pH ~7.5 onwards with a shallower linear decrease compared to silk, as observed between pH 3–7.5, suggesting that the surface charge of the two materials is different between ~pH 4 and 8. The isoelectric point was determined by the intersection of the slope with the x-axis and was between 4 and 4.5 for both silk and PIII-silk (p > .05; Figure 3B). The isoelectric point of silk materials is known to vary with the fabrication technique,45 but the zeta potential curves and the isoelectric point of both silk and PIII-silk were consistent with previous reports on silk films which ranged from 3.5 to 5.45-47 While there are no reports of the effect of the PIII-treatment on the surface zeta potential, previous reports of oxygen plasma-treated silk films show a positive increase in zeta potential after the isoelectric point (pH > ~4),47 consistent with the current findings. The difference in surface charge in this region is likely due to the introduction of new surface functional groups following PIII treatment29 or diffusion of radical species with an unpaired electron to the surface over time.33 Based on the rDV amino acid sequence, the theoretical isoelectric point of the rDV protein core is 6.01 calculated based on a method in Kozlowski et al.48 which is in agreement with the isoelectric point of the full-length perlecan protein core estimated at 6.049 and perlecan domain V LG3 region (C-terminal-most region) which was estimated to be around 5.03.50 However, the actual charge of rDV is expected to be more negative due to the presence of the negatively charged GAG chains.
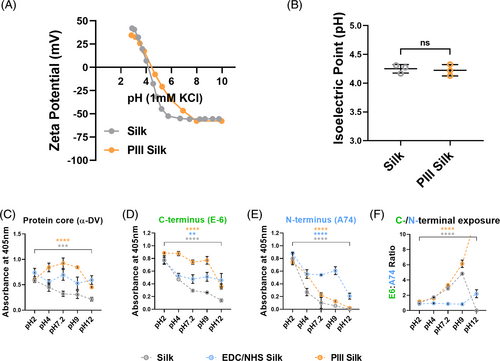
Next, the pH of the rDV solution was altered prior to surface immobilization to study the amount and orientation of rDV on surface-modified silks (Figure 3C–F). rDV solutions were prepared at different pH values (pH 2, 4, 7.2, 9, and 12) and immobilized on surface-modified silks (equilibrated at the same pH) and analyzed using a polyclonal antibody targeting the protein core (α-DV; Figure 3C) or monoclonal antibodies against the C-terminal (clone E6) (Figure 3D) or N-terminal (clone A74; Figure 3E) regions of rDV. When rDV was immobilized onto silk biomaterials at different pH values, there was an overall statistical effect of immobilization pH on α-DV absorbance values in silk (p < .001) and PIII-silk (p < .0001) but not on EDC/NHS-silk (p > .05; Figure 3C). For rDV-silk, the α-DV signal was the highest at pH 2.0 and steadily decreased with increasing immobilization pH. In contrast, the α-DV antibody signal for rDV-PIII-silk was consistently high at pH 4, 7.2, and 9, with a significant decrease at pH 2.0 and 12.0 (p < .0001) compared to rDV immobilization at pH 7.2 (physiological pH). Typically, the highest protein immobilization is achieved at pH close to the protein's isoelectric point, as demonstrated for antibodies51 and BSA.52 This is attributed to the zero net charge at the isoelectric point which allows proteins to be adsorbed in a compact state and form a tightly packed configuration on the surface.52 This was the case for PIII-silk in the current work, but not for other conditions. This may be due to the rapid covalent crosslinking of rDV on PIII-silk, but not on silk where the absorbance was highest at pH 2. However, at extreme pH values, repulsion between charged groups can lead to protein unfolding53 resulting in exposure of additional epitopes.
When the exposure of individual C-terminal (Figure 3D) or N-terminal (Figure 3E) epitopes was considered, it was interesting to note a similar overall trend regardless of the immobilization method, showing the highest C-terminus and N-terminus exposure at pH 2, which steadily decreased with increasing pH. When rDV orientation was examined via the ratio of C-terminal and N-terminal epitope signal (Figure 3F), the very low/no signal at pH 12 distorted E6:A74 ratio and was, therefore, omitted from the analysis. There was an overall significant difference in E6:A74 ratio with immobilization pH on both silk and PIII-silk (p < .0001) but not for EDC/NHS-silk (p > .05). The ratio was ~1 at pH 2 for all conditions but increased with increasing pH on silk and PIII-silk, suggesting more exposure of C-terminal regions with increased pH of the immobilization buffer. It is interesting to note that this effect was observed on PIII-silk, which does not rely on specific amino acids to immobilize rDV but was not observed on EDC/NHS immobilization which relies on the availability of lysines on rDV (Figure S1). This could be attributed to the specificity of the carbodiimide chemistry which targets a relatively low number of sites, limiting the possible orientations of rDV on activated silk surfaces. Previous work has demonstrated that the choice of rDV immobilization on silk can affect its presentation and subsequent cell adhesion,10 but that work did not look at PIII immobilization or the ratio of C-terminal and N-terminal epitopes available on the surface, an effect that may have implications for the choice of immobilization technique for different applications.
Overall, these results demonstrate that PIII-silk was able to immobilize more rDV, while retaining similar rDV orientation to silk, making PIII-treatment a promising covalent immobilization process. PIII-silk also supported manipulation of rDV orientation via modulating rDV solution pH, an effect that was not observed with EDC/NHS-crosslinking. This finding warrants exploration of other conditions previously shown to alter molecular presentation on the material surface, including ionic strength52 and voltage.54
The observed differences in rDV presentation on the silk surfaces may have functional implications as rDV presentation on the surface affecting the availability and access to functional domains such as the α2β1 integrin binding site, VEGF receptor binding site, as well as GAG chains involved in growth factor biding and signaling and inhibition of platelet binding.55 Therefore, the vascular cell interactions with rDV immobilized at different pHs on different surface-modified silk were investigated. HUVECs were seeded onto TCP (positive control), bTCP (negative control), and surface-modified silk with or without rDV for 1 h in the absence of serum proteins with cell adhesion quantified using the CyQuant Cell Proliferation assay as shown in Figure 4A. The immobilization pH only had an overall significant effect on endothelial cell adhesion on rDV-silk (p < .01), but not on rDV-EDC/NHS-silk and rDV-PIII-silk (p > .05). HUVEC adhesion on rDV-silk was significantly higher than on silk regardless of the immobilization pH used (p < .05–p < .001), with the exception of pH 9. It was interesting to note that cell adhesion on rDV-silk was higher on all pH values compared to pH 9 (p < .05–p < .01), a condition that showed a high C-terminal epitope exposure ratio (Figure 3C). While immobilization pH did not significantly influence HUVEC adhesion on rDV-EDC/NHS-silk, there was still an improvement in HUVEC adhesion relative to silk at pH 2 and pH 12 (p < .05) compared to untreated silk. Like EDC/NHS-silk, PIII-silk showed no effect of immobilization pH on cell adhesion in the presence of rDV but compared to silk remained significantly higher regardless of the presence of rDV or immobilization pH used (p < .0001). This suggests that PIII-silk supports high levels of HUVEC adhesion regardless of rDV or rDV immobilization pH, while HUVEC adhesion on silk generally improved when rDV was immobilized on the surface. From these results, it appears that silk and EDC/NHS-silk promoted HUVEC adhesion when at the extreme immobilization pHs. In contrast, PIII-silk promoted HUVEC adhesion regardless of rDV the immobilization pH. Overall, the observations coupled with higher rDV immobilization and improved endothelial cell adhesion at low pH suggest acidic immobilization conditions may be advantageous for rDV immobilization on silk for vascular applications.
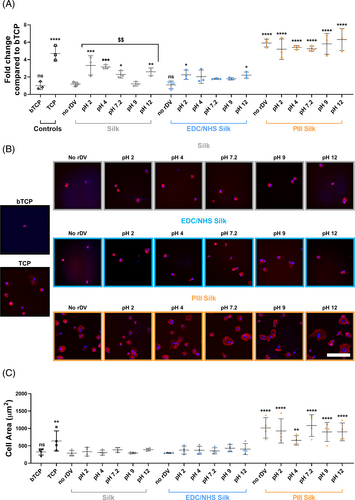
To investigate the effect of rDV immobilization pH on cell spreading, HUVECs were seeded in a similar manner to cell adhesion studies and incubated for 3 h in the absence of serum protein. Samples were fixed and stained for F-actin and nuclei to visualize the number of cells and the cell morphology on different surfaces. Representative confocal images are shown in Figure 4B and quantification of cell spreading on rDV-biofunctionalized silk biomaterials is shown in Figure 4C. Qualitative confocal image analysis of HUVECs in the presence of rDV (Figure 4B) showed an increase in the number of cells on the surface when rDV was bound at acidic pH compared to silk and rDV bound on silk at physiological or alkaline pHs. However, HUVECs adhered on rDV-silk remained round, evident by the lack of F-actin staining suggesting that rDV did not result in any changes to cell spreading when immobilized on silk surfaces. Similarly, rDV immobilized on EDC/NHS-silk did not result in any obvious difference in cell number or spreading regardless of rDV immobilization pH, but appeared to have more cells adhered compared to EDC/NHS-silk. In contrast, rDV immobilized on PIII-silk at different immobilization pHs had an obviously higher cell adhesion and spreading compared to both silk and EDC/NHS-silk conditions, with no obvious differences between different immobilization pHs and comparable to both the untreated PIII-silk and the TCP positive control. Following quantification of HUVEC spreading on rDV-biofunctionalized silk biomaterials (Figure 4C), no overall effect of immobilization pH was observed for any silk condition compared to the unfunctionalized counterparts (p > .05), consistent with qualitative observations. Furthermore, there was no significant difference in cell spreading on EDC/NHS-silk compared to silk, whereas PIII-silk consistently had a significantly higher cell area compared to silk, regardless of rDV immobilization and rDV immobilization pH (p < .01–p < .0001), comparable to the TCP positive control.
While ELISA analyzes (Figure 3C–F) showed differences in rDV presentation on silk biomaterials when immobilized under different pH conditions, this translated to relatively small differences in HUVEC interactions. Overall, immobilization of rDV in acidic pH conditions onto silk and EDC/NHS-silk appeared to be more favorable, supporting higher endothelial cell adhesion. However, this did not significantly influence HUVEC spreading on silk or EDC/NHS-silk. This is likely attributed to acidic pH being closer to the isoelectric point of silk which is commonly associated with peak protein adsorption. Therefore, taken together the results show that the amount and orientation of rDV could be modified by adjusting the immobilization pH, and even at high acidic pH (pH 2) and alkaline pH (pH 12), rDV was able to support endothelial cell attachment. rDV immobilized onto PIII-silk was consistently able to support higher cell adhesion and spreading compared to rDV immobilized on either silk or EDC/NHS-silk, regardless of pH.
3.4 rDV-biofunctionalized PIII-silk biomaterials supported formation of quiescent endothelium
To investigate the effect of rDV-biofunctionalized silk biomaterials on endothelial cells over a longer culture period, HUVEC proliferation assay was established by seeding cells onto bTCP (negative control), TCP (positive control), or surface modified silk with or without rDV in the presence of serum and growth factors. HUVECs cultured over a 5-day period were analyzed with the Alamar Blue cell viability reagent at 1, 3, and 5 days post-seeding (Figure 5A). Overall, the results showed that all silk biomaterials were able to support endothelial cell proliferation over the 5-day period, as indicated by an increase in metabolic activity (Alamar blue signal) which generally correlates with an increase in cell number, as observed with a similar increase in both DNA content and metabolic activity in human aortic endothelial cell for the first 7 days, prior to confluence.56 Silk biofunctionalized with rDV resulted in a significant increase in metabolic activity compared to silk on day 3 (p < .01) and 5 (p < .05). In contrast, rDV-biofunctionalization of EDC/NHS-silk or PIII-silk did not result in any significant difference in cell metabolic activity compared to their unfunctionalized counterparts at all timepoints. rDV-PIII-silk supported a significantly higher metabolic activity compared to rDV-silk, starting from 5.0-fold higher on day 1 (p < .01) to 2.7-fold higher by day 5 post-seeding (p < .0001). While obvious differences in endothelial cell metabolic activity (and hence presumably cell numbers) were observed on different silk biomaterials, the differences appeared to be related to the initial cell adhesion on the biomaterial surface, as metabolic activity normalized to day 1 of each condition (Figure S3) showed similar cell proliferation rates for each condition. This suggests that silk modification and rDV-biofunctionalization affects early cell interactions with the biomaterial, but not cell proliferation rates. The results presented are consistent with previous studies that also demonstrated that rDV and perlecan can support endothelial proliferation.10, 14
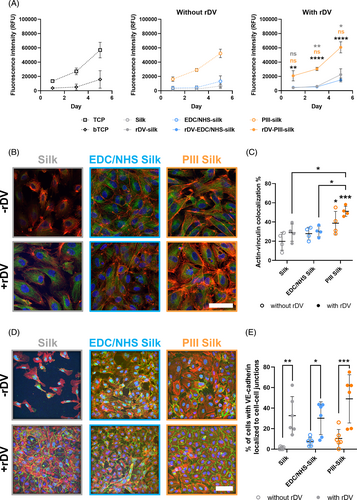
While PIII-silk and rDV-PIII-silk biomaterials were able to support endothelial cell adhesion, spreading, and proliferation, it was important to investigate the integrity of the endothelial layer on these biomaterials, as a lack of functional endothelium is associated with vascular graft failure. Vinculin is an important cytoskeletal protein in endothelial cells integrating focal adhesion with the actin cytoskeleton.57 It is required to maintain sprouting integrity during lumen expansion by protecting cell–cell contacts against mechanical stress58 and plays an important role in the restoration of endothelial cell monolayer integrity and endothelial permeability.57 Vascular endothelial (VE)-cadherin is an endothelial-specific cell adhesion molecule expressed at junctions between endothelial cells. It is important in regulating vascular permeability, cell proliferation, apoptosis, and vascular endothelial growth factor receptor function,59 making its expression a good indicator of endothelial integrity and functionality.60 Therefore, HUVECs were seeded onto surface-modified silk with or without rDV and cultured for 10 days in the presence of serum and growth factors. Cells were then fixed and stained for vinculin or VE-cadherin, F-actin, and nuclei. Representative confocal images of each stain and merged images are shown in Figure 5B,D, respectively.
By Day 10 post-seeding, all silk surfaces with or without rDV-biofunctionalization showed well-spread HUVECs as observed with the F-actin staining. However, there were clear differences in confluence and cell–cell marker expression. In the absence of rDV-biofunctionalization, silk did not support the formation of a confluent layer of HUVECs while both EDC/NHS-silk and PIII-silk supported the formation of a near-confluent cell layer (Figure 5B). Following rDV-biofunctionalization, all silk biomaterials supported the formation of a confluent cell layer on the biomaterial surface. Qualitative analysis of the confocal images of vinculin expression showed distinct differences in the amount and pattern of vinculin expression. For cells grown on untreated silk, a minimal amount of expression was observed, primarily confined around the nucleus of the cells. Following either EDC/NHS or PIII surface modification, vinculin expression increased in HUVECs, with positive staining found throughout the cytoplasm. Following rDV-biofunctionalization, there was an increase in vinculin expression for cells on all silk surfaces with expression dispersed throughout the cytoplasm, like in EDC/NHS-silk and PIII-silk. To study the effect of rDV and immobilization condition on the vinculin-actin cytoskeleton link, the co-localization of vinculin and actin was quantified in HUVECs (Figure 5C). Prior to rDV biofunctionalization silk and EDC/NHS-silk showed little colocalization, with only 19.8% ± 9.3% and 27.9% ± 6.6% of actin colocalizing with vinculin. However, HUVECs on PIII-silk showed a significantly higher percentage of colocalization compared with silk, with 38.8% ± 12.3% of actin colocalizing with vinculin (p < .05). Following rDV biofunctionalization, the colocalization of actin with vinculin increased slightly to 28.9% ± 9.0% for rDV-silk, 30.1% ± 5.5% for rDV-EDC/NHS-silk, and 51.1% ± 5.3% for rDV-PIII-silk. However, there was no significant increase for any silk surface compared to the unbiofunctionalized counterpart. However, it should be noted that the colocalization observed on rDV-PIII-silk was significantly higher than silk (p < .001), rDV-silk (p < .05), and rDV-EDC/NHS-silk (p < .05), suggesting that rDV-PIII-silk supported excellent vinculin-actin co-localization.
VE-cadherin expression on silk surfaces with or without rDV showed a similar trend to vinculin expression. In the absence of rDV, HUVECs adhered on silk were not confluent and had little VE-cadherin expression which was confined to near the nucleus of the cell, similar to vinculin (Figure 5D). HUVECs on EDC/NHS-silk and PIII-silk showed improved cell coverage and VE-cadherin expression, with diffuse expression observed throughout the cell cytoplasm and some at the intercellular junctions. Following rDV-biofunctionalization, all silk surfaces showed an increase in VE-cadherin expression and localization to cell–cell junctions rather than diffuse expression in the cytoplasm. Quantification of the VE-cadherin localization (Figure 5E) showed that there was a significant increase in VE-cadherin staining at intercellular junctions on rDV-biofunctionalized silk surfaces compared to their non-functionalized counterparts. Prior to rDV-biofunctionalization, silk, EDC/NHS-silk, and PIII-silk had a low percentage of VE-cadherin localization to intercellular junctions at 1.1% ± 1.2%, 7.5% ± 4.1%, and 10.3% ± 8.8%, respectively. However, after rDV-biofunctionalization, all silk surfaces showed a significant increase in the percentage of VE-cadherin localization to intercellular junctions, with rDV-silk, rDV-EDC/NHS-silk and rDV-PIII-silk showing a significantly higher 32.6% ± 18.6% (p < .01), 30.1% ± 16.0% (p < .05), and 49.0% ± 23.6% (p < .001) localization, respectively.
Overall, silk did not promote a confluent layer of HUVECs, showing almost no actin-vinculin colocalization or cell-junction formation, as reflected in the low vinculin and VE-cadherin expression which was primarily localized around nuclei. Following surface functionalization, both EDC/NHS-silk and PIII-silk showed a higher vinculin and VE-cadherin expression compared to silk. From the previous cell adhesion and spreading results (Figure 2A), it can be reasonably expected that PIII-silk, unlike silk, would support the formation of a confluent cell layer due to the significantly higher level of adhesion and spreading, presumable due to changes in the silk surface chemistry following PIII treatment.29 However, it is interesting that EDC/NHS-silk also supported the formation of a near-confluent cell layer, likely due to immobilization of adhesion molecules from fetal bovine serum in these studies, relative to the adhesion and spreading studies. It is important to note that despite the near-confluent cell layer on both EDC/NHS-silk and PIII-silk, there was only a small fraction of cells that possessed defined intercellular junctions. Following rDV-biofunctionalization, silk surfaces supported similar percentages of vinculin colocalization with actin and supported strong VE-cadherin expression at the periphery, forming at cell junctions. This suggests that while EDC/NHS-silk and PIII-silk were able to support a confluent layer, the presence of rDV also resulted in the formation of a functional endothelium on silk biomaterials, most evident with silk before and after rDV-biofunctionalization. However, it should be noted that while there were intercellular junctions on cells grown on rDV-EDC/NHS-silk, they appeared relatively diffuse and did not form clear borders between neighboring cells compared to both rDV-silk and rDV-PIII-silk. Differences between cell–cell junction formations on rDV-immobilized silk surfaces are likely due to differences in rDV orientation and presentation, as previously observed in Figure 1F where silk and PIII-silk favor C-terminal presentation whereas EDC/NHS-silk favors N-terminal exposure. The C-terminal region of rDV houses the α2β1 integrin binding site that has previously been shown to mediate endothelial cell binding to perlecan and to rDV.8 GAG attachment site is also located in the C-terminal region of rDV and GAG-mediated growth factor signaling has previously been shown to support endothelial cell sprouting and angiogenesis in in vitro and in vivo assays.17 Therefore, it is possible that the improved availability of this region resulted in improved cell–cell junction formation in the current studies. While it may be tempting to speculate about PIII in the absence of rDV supporting functional endothelium with higher cell seeding densities or longer incubation times, the ultimate goal of this work is not to develop pre-endothelialized grafts, but rather acelullar grafts that can be used as an off-the-shelf product. In this case, a combination of rDV and PIII is the optimal condition, as PIII-silk in the absence of rDV is pro-thrombogenic,30 would likely support negative smooth muscle cell interactions, and does not support functional endothelium formation to the same extent as rDV-PIII-silk as shown here.
Taken together, these results show that rDV retained bioactivity when immobilized on all silk surfaces. Although rDV-biofunctionalization generally did not result in significant changes in cell adhesion and proliferation compared to their bare counterparts, all rDV-biofunctionalized silk surfaces supported the formation of functional endothelium in contrast to their unfunctionalized counterparts. Importantly, the results here show that even though PIII-silk enabled faster cell attachment and growth, only the presence of rDV (rDV-PIII-silk) resulted in higher levels of intercellular junction expression, suggesting that rDV-biofunctionalization promoted rapid and functional endothelialization.
4 CONCLUSION
Together the results show that PIII-treatment of silk enabled covalent immobilization and a higher density of rDV on silk surfaces compared to both untreated silk and EDC/NHS-silk. rDV orientation on PIII-silk was similar to that on silk and favored C-terminal epitope exposure at physiological pH where both the GAG chain and α2β1 integrin site are located; and unlike that on EDC/NHS-silk which showed preference for the N-terminal epitope exposure. The orientation of rDV on silk and PIII-silk could also be manipulated by altering the immobilization pH. More importantly, this study established that rDV remained bioactive when covalently attached to PIII-silk with rDV-PIII-silk samples showing an increase in short-term endothelial cell attachment and spreading compared to rDV-silk and rDV-EDC/NHS-silk. While the increase in cellular response was similar between rDV-PIII-silk and PIII-silk, the presence of rDV was important in the formation of functional endothelium where an increase in vinculin expression and intercellular junction formation, markers for the formation of a functional endothelium, were observed compared to the unfunctionalized counterpart. This complements previous work that showed that PIII-silk alone was pro-thrombogenic but maintained blood fluidity when biofunctionalized with rDV, establishing rDV-PIII-silk as a promising vascular material. Together, these results demonstrate the importance of the appropriate immobilization chemistry in biofunctionalization of silk biomaterials and provide evidence for the potential of rDV-PIII-silk as an acellular, off-the-shelf biomimetic vascular graft biomaterial. Further investigation of endothelialization in functional vascular interposition models in vivo is warranted.
ACKNOWLEDGMENTS
The authors would like to acknowledge funding support from the Australian Research Council (LP180100540, FT2101000668; Jelena Rnjak-Kovacina and LP190101003; Megan S. Lord). Kieran Lau was supported by the Australian Government Research Training Program Scholarship. The authors thank Ms. Maxine Halbheer for technical assistance with VE-cadherin data image analysis. The authors thank Dr. Yun Ye for technical assistance and use of the Anton Paar SURPASS 3 electrokinetic analyzer at the UNESCO Center of Membrane Science & Technology at UNSW Sydney. Confocal microscopy was partially performed using instruments situated in, and maintained by, the Katharina Gaus Light Microscopy Facility. Katharina Gaus Light Microscopy Facility is part of the Mark Wainwright Analytical Center at UNSW Sydney which is in part-funded by the Research Infrastructure program at UNSW. Open access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reporting in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




