Research progress of stem cell therapy for ischemic stroke
Abstract
Ischemic stroke is a serious cerebrovascular disease with high morbidity, disability and mortality. There is no doubt that the disease has a severe impact on the physical and mental health and quality of life of patients, as well as impose a heavy burden on families and societies. Unfortunately, there has been a lack of effective treatment. This overview reviews the pathophysiology of stem cell therapy in Ischemic stroke, and discuss its effects on neurogenesis, the latest clinical trials, and advances in tracking and monitoring of endogenous and exogenous stem cells.
Introduction
Cerebral stroke, one of the acute cerebrovascular diseases, in which brain. tissue is damaged by reason of the sudden rupture or occlusion of a blood vessel that prevents blood from flowing to the brain. Stroke includes Ischemic Stroke (IS) and hemorrhagic Stroke. Ischemic stroke accounts for about 80% of all Stroke cases (Writing GM, et al., 2016; Wenzhi W, et al., 2017). Previous studies on the risk factors of Ischemic stroke have shown that stroke often occurs acutely in a quiet state with complex etiology (Hui L, 2020). The common risk factors for stroke are extensive, including hypertension, dyslipidemia, abdominal obesity, alcoholism, heart disease, diabetes and smoking (Cipolla MJ, et al., 2018; Naohide K, et al., 2020; Guzik A, et al., 2017). In addition, atrial fibrillation, TIA, and microglia-related inflammatory responses are well known risk factors for stroke (Wan Z, et al., 2021; Guruswamy R, et al., 2017). The clinical manifestations of patients with this disease are mostly neurological impairment, such as memory decline, cognitive decline, and severe complications such as hemiplegia, depression, etc. Critical threat to the patient's health and quality of life (XiaoYan H, et al., 2020; Johnson CO, et al., 2019).
At present, the main objectives of the therapy of Ischemic stroke are to improve the blood flow supply to the brain and restore neurological function in the ischemic site (HongBin W, et al., 2015). During the acute phase of Ischemic stroke, mechanical thrombolysis (MT) and tissue plasminogen activator (tPA) can re-canalize occlusionary lesions. In the sub-acute to chronic stage, depending on the patient's condition or the intervention patient's stroke risk factors, therapeutic schedule may shift to long-term use of antiplatelet or anticoagulant drugs that promote neuroremodeling (M BM, et al., 2020; ZiHan Z, et al., 2021). Currently, however, the U.S. Food and Drug Administration (FDA) only approves intravenous thrombolysis and endovascular thrombectomy for Ischemic stroke. In both cases, the therapeutic window of Ischemic stroke is narrow, resulting in a large number of stroke patients not receiving correct treatment (Li Y, et al., 2019; Song T, et al., 2020; Wu S, et al., 2019; Li Z, et al., 2019). Although rehabilitation therapy has a certain effect, its function reminds limited (S KE, et al., 2020). Therefore, it is extremely necessary to seek new treatment methods to improve the neurological function and reduce the mortality of Ischemic stroke patients.
In recent years, stem cell therapy has attracted more and more attention on account of its satisfactory results in some preclinical and clinical studies (Connor S, et al., 2017; Ling W, et al., 2017). The ability of cells in the central nervous system to differentiate and regenerate was affected by Ischemic stroke, and injured cells cannot be completely repaired and replaced by endogenous cells. Stem cells have the characteristics of self-renewal and multidirectional differentiation (Yinong H, et al., 2018; Alexander B, et al., 2017). Neural function can be restored through mechanisms such as cell differentiation and replacement, immune regulation, neural circuit reconstruction and release of neurotrophic factors (Detante O, et al., 2018; E MH, et al., 2018; Miroslaw J, et al., 2015).
There is evidence that stem cell therapy plays an important role in prolonging the time of drug intervention, improving neurological function, reducing the volume of cerebral infarction, promoting the regeneration of nerve cells, and improving the prognosis of patients with Ischemic stroke. It has shown bright prospects in clinical and preclinical studies.
However, the clinical transformation of stem cell therapy for ischemic stroke has not been fully realized. Mainly due to the type of stem cells, route of infusion, dosage, and the ability to trace stem cell migration and survival for a long time. This article looks back the pathophysiology of ischemic stroke, the application of different stem cell therapies in Ischemic stroke, the study of stem cell therapy and its effects on neurogenesis and mechanisms, as well as the development of endogenous and exogenous stem cell tracking and monitoring techniques.It is expected to provide help for early clinical application (Figure 1).
Flowchart of the review
Pathophysiology of ischemic stroke
Acute ischemic stroke can disrupt the BBB and cause neuroinflammation, which can lead to dysfunction. Immediate treatment goals in most cases of ischemic stroke focus on patient stabilization and rapid restoration of cerebral blood flow. This requires rapid assessment prior to treatment and early intervention to restore maximum brain tissue reperfusion (Sloane K, et al., 2019). So, the pathophysiological changes after stroke have been extensively studied in the past few decades.
The blood-brain barrier (BBB) destroyed
The BBB is the barrier between plasma and brain cells formed by brain capillary walls and glial cells, and between plasma and cerebrospinal fluid formed by choroid plexus. These barriers can prevent some substances (mostly harmful) from entering the brain tissue by blood, thus maintaining the basic stability of the environment in the brain tissue, which has important biological significance to maintain the normal physiological state of the central nervous system (Daneman R, et al., 2015).
After Ischemic stroke, it leads to nerve cell death and the release of factors such as damage-related molecular patterns (DAMP), which results in local inflammation in the damaged brain area. Secondary brain injury is aggravated by increased BBB permeability, microvascular failure, brain edema, oxidative stress and directly induced nerve cell death (Shi K, et al., 2019). Recent studies have shown that neutrophils accumulate in the periinfarct cortex after ischemic stroke, and that neutrophilic external traps (NETs) are generated intravascular and parenchymal at 3 to 5 days. Peptide-arginine deiminase 4 (PAD4) is an essential enzyme for NET formation. Overexpression of this enzyme will give rise to increased NET formation, decreased neovascularization, and incremental destroy to the BBB. Therefore, NET reduction can lessen the damage of the BBB
Glial activation
Neuroinflammation after stroke is a sterile form of inflammation that increases chemokine production of glial cells, thereby activating glial cells. Glial cells, other than neurons, are widely distributed in the central and peripheral nervous systems. There are mainly astrocytes, oligodendrocytes (together with the former called macroglia) and microglia. Glial cells has complicated structure and expression of the secretion of rich product, it contains most of the neurotransmitter, neuropeptides and hormones and neurotrophic factor receptors, ion channels, the neural activity of amino acid affinity carrier recognition molecules, cells, and can secrete a variety of neural active substances (growth factors and neurotrophic factors and cytokines, etc.). Glial cells connect and support various nerve components, distribute nutrients, and participate in repair and phagocytosis.
Astrocytes, mainly found in central nervous system astrocytes, are the most abundant population in the brain. It is the key cell after the disease of central nervous system (Verkhratsky A, et al., 2016). Importantly, astrocytes are the cellular component of nerves and the connections between nerves and blood vessels in the brain. In addition, at the ends of the smallest arteries and cerebral parenchymal arterioles, there may be enclosing astrocyte processes and endopodia.
Microglia play a key role in the homeostasis of the central nervous system (Zhang W, et al., 2021). The morphology of microglia is intensively plastic and closely related to its biological function.In normal brain tissue, microglia are highly branched, with third-order and fourth-order branches, and the intercellular branches rarely overlap. The branching microglia are often referred to as “resting microglia” (Wong W, et al., 2013). They can be activated and release inflammatory cytokines by recognizing various external stimuli such as glycoproteins, aggregators, cytokines, chemokines and inflammatory microenvironments (Fan Y, et al., 2018). These cytokines give rise to severe neurotoxicity, resulting in long-term and lasting damage through overactivation of microglia (Zhang S, et al., 2019). Microglia are involved in a series of neurodegenerative diseases. Microglial activation and neuroinflammation are the main features of neuropathology (Ma Y, et al., 2017; Pekny M, et al., 2019). It mediates endogenous immune responses to central nervous system injury and disease, thereby playing a neuroprotective or neurotoxic role. After ischemic stroke, microglia are activated and release inflammatory cytokines such as interleukin-1β, tumor necrosis factor -α, interleukin-6, etc (Jayaraj RL, et al., 2019).
In chronic inflammation, microglia are activated over a long period of time and then continuously release a series of inflammatory mediators, leading to oxidative stress response. Microglia-mediated chronic inflammatory response is generally considered to be harmful to the body, resulting in neurological tissue damage.Microglial activation showed functional abnormalities, including increased levels of pro-inflammatory cytokines and reactive oxygen species, and increased mitochondrial content (Koellhoffer EC, et al., 2017).
Stem cell therapy for ischemic stroke
Stem cells are self-regenerating cells with permanent life, capable of developing into any other cell, and thus facilitating the repair and regeneration of damaged tissue. According to the developmental stage of stem cells, they can be divided into embryonic stem cells and adult stem cells. According to the possibility of genesis, stem cells are divided into omnipotent, pluripotent and specialized stem cells. Stem cells are immature cells that are not fully differentiated and have the potential function of regenerating various tissues and the human body. They are called “universal cells” in medical circles.
Currently, there have been many reports on stem cell therapy for Ischemic stroke, and the potential for treating Ischemic stroke has been evaluated (Tang YH et al., 2019). It mainly includes endogenous stem cells and exogenous stem cells (Zhang Z, et al., 2016). The former is mainly the endogenous neural stem cells, exists in humans and other mammals, the central nervous system are mainly distributed in the hippocampus, venation from subependymal region, and under the condition of ischemia hypoxia endogenous neural stem cells can proliferate and migrate, and the neurons and glial cells differentiation, and repair of the central nervous system (Zheng W, et al., 2013; Christian KM, et al., 2014); Exogenous stem cells include exogenous nerve stem, embryonic stem cell, mesenchymal stem cell and induced pluripotent stem cell. Both endogenous and exogenous neural stem cells can migrate to the injured area after ischemic brain injury and differentiate into new neurons and glial cells, so as to repair the tissue and restore the function after central nervous system diseases, especially after ischemic stroke.
Endogenous Neural Stem cells (En-NSCs)
The treatment of Ischemic stroke disease through endogenous stem cells, primarily the activation of En-NSCs. En-NSCs mainly exist in the nervous system of human or other mammals. They are normally located in the hippocampus, choroid plexus and subependymal region, it can provide new neurons for olfactory and hippocampal nerves. When Ischemic stroke disease occurs, NSCs can migrate to the site of injury and differentiate into new neurons, neuroglia cells and oligodendrocytes, so as to repair the damaged tissue and achieve functional recovery.
Hea reported that endogenous stem cell activation occurs in response to external factors, including neurotrophic factors, hematopoietic growth factors, magnetic stimulation, and enrichment environment. And neural function repair induced by endogenous stem cells may soon become a reality (Hea YJ, et al., 2016). It has also been found that M1/M2 phenotype microglias release pro-inflammatory and anti-inflammatory cytokines, thereby inducing the activation of endogenous NSCs. M2-type microglia derived TGF-α enhanced proliferation and neural differentiation of neural stem/progenitor cells (NSPCs) after Ischemic stroke disease (Yong CJ, et al., 2017).
Endogenous neural stem cell activation for therapy solves the problem of origin of NSCs and avoids the damage caused by cell transplantation and immune rejection after transplantation. However, the ischemic environment affects the survival of new nerve cells, so the number of proliferating nerve cells is small and their capacity is limited.
Exogenous stem cell
Exogenous Neural Stem cells (Ex-NSCs)
NSCs exist in the mammalian nervous system and can be differentiated into neurons, astrocytes, oligodendrocytes, etc(C BA, et al., 2018; Kirsten O, et al., 2019; Linda O, et al., 2020). Exogenous NSCs are transplanted into the brain of Ischemic stroke patients through in vitro culture and amplification, thus mediating brain injury repair.The MCAO rat model be used to determine whether NSCs migrated to the ischemic region after stroke, and injected NSCs into the hippocampus one day after stroke. The results showed that the cells migrated to the site of injury and the infarct volume decreased (Hong Z, et al., 2015).
It has been reported that NSCs may reduce the activation of microglia, and the expression of pro-inflammatory factors (tumor necrosis factor -α, interleukin (IL)-6, IL-1β, monocyte chemoattractor protein-1, macrophage inflammatory protein-1 α) and adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1). The expression levels of BDNF, Nestin, Sox10, Doublecortin, β-III tubulin, GFAP and IL-6 are increased to improve the mechanism of BBB injury (Salehi MS, et al., 2020; Lei Huang., 2014). CTX0E03 is a permanent human neural stem cell line from which a drug was developed for allogeneic therapy (CTX-DP). Dheeraj Conducted a phase 1 clinical trial of CTX-DP, and the observation results showed that there were no adverse events in 11 patients with chronic stroke treated with intracerebrain CTX-DP (Dheeraj Kalladka., 2016). This novel cell therapy for ischemic stroke is feasible and safe. The feasibility and potential therapeutic response of intracranial implantation of allogeneic human neural stem cell CTX0E03 in the subacute chronic recovery period of stroke were inspected. There were no cell-related adverse events at 12 months of follow-up.This indicates that the implantation of human neural stem cells into the brain is feasible in multi-center studies (Keith W Muir., 2019).
At present, there are many studies on exogenous NSCs, and animal models are relatively mature. However, exogenous NSCs cannot be transplanted in the same body on account of the limited source, and there are problems such as immune rejection. Further research is needed for clinical application.
Embryonic Stem cells (ESCs)
ESCs are isolated from early embryos or primitive gonads. It has the advantages of infinite reproduction, self-renewal and multidirectional differentiation. ESCs can be induced to differentiate into almost all cells in vitro and (Z RM, et al., 2019). So, this is a hope for basic scientific for a variety of difficult diseases.
One million neural stem cells were isolated from mouse embryos and transplanted into the corpus callosum and striatum of the contralateral ventricle 24 hours after stroke induction for detection. At 2 and 4 weeks after transplantation, transplanted cells from all three regions were attracted to the ischemic core and the largest number of attracted cells were observed after transplantation to the striatum. The proliferation of transplanted cells increased with the decrease of Caspase-3 activity (Kosi N, et al., 2018). Chen successfully differentiated hESC into human oligodendrocyte progenitor cells (hOPC) in vitro. In addition, local injection near the injured area in the middle cerebral artery occlusion (MCAO) model showed significant repair of the damaged area and improvement of neurological function in rats’ model (Li C, et al., 2020). But the study of ESCs has been a controversial issue. The first is ethical issues, because ESCs research requires the destruction of embryos,which is unethical. Secondly,problems such as tumorigenicity, immune rejection, induction of epigenetic changes and genetic changes have also attracted attention (Z RM, et al., 2019).
Mesenchymal stem cells (MSCs)
MSCs are pluripotent stem cells that can differentiate into different cell types and can be obtained from almost any tissue type. It can be classified into bone marrow MSCs (BM-MSCs), umbilical cord, and adipose tissue-derived mesenchymal stem cells (AD-MSCs) according to their different sources (Zhang L, et al., 2017). Among them, BM-MSCs are one of the best candidates for ischemic stroke stem cell therapy due to their advantages of easy acquisition, low immunogenicity and less ethical considerations (Young BO, et al., 2016; Sarmah D, et al., 2018;Steinberg GK, et al., 2016). Some evidence suggests that MSCs can affect the pathological process of ischemic stroke by inhibiting apoptosis, secreting neurotrophic factors, inducing angiogenesis, regulating the immune system, orienting differentiation into nerve cells and astrocytes, and supplementing and replacing cells damaged by ischemia (Li G, et al., 2016; Sohni A, et al., 2013; Wang F, et al., 2018).
One study focusing on a Meta-analysis of preclinical studies of MSCs for Ischemic stroke disease showed that MSCs significantly improved all functional outcomes (Satani N, et al., 2019). In the study of animal models, Mu evaluated the behavioral recovery of rats through a series of behavioral tests, and confirmed the role of the early administration of human-derived AD-MSCs in the behavioral recovery of Ischemic stroke (Jingwei M, et al., 2019). Duan used spion loaded cationic polymer to label Green fluorescent protein (GFP)-expressed MSCs to determine whether MRI can accurately reflect the long-term survival fate and underlying mechanism of MSCs in ischemic stroke treatment (Xiaohui D, et al., 2017). Results showed that MSCs can improve functional prognosis of stroke and reduce infarct volumeMSCs may play a therapeutic role by secreting paracrine factors rather than by differentiating into neurons and/or glial phenotypes to guide cell replacement. Yuan claimed that hMSCs transplantation could improve the function of MCAO rat model by using MRI tracing technology (Yuan X, et al., 2019). It has been demonstrated that stroke induces a recovery process by activating MSCs. It was also found that using the serum obtained from the acute phase of stroke to cultivate MSCs can form a new MSCs activation method, which is feasible and valid for neurological recovery of stroke (Moon GJ, et al., 2018).
MSCs can induce neurovascular and synaptic genesis by generating cells with a neural lineage, and activate endogenous neural stem cells to restore function by producing cytokines and nutritional factors. In addition, MSCs treated stroke by regulating cerebral blood flow (CBF), BBB, and other neuroprotective mechanisms, such as decreased apoptotic inflammation and increased astrocyte survival. However, factors such as low survival rate of cultured and amplified MSCs and poor survival retention and transplantation of transplanted stem cells are major challenges that limit the efficacy of MSC in stroke treatment.
Induced pluripotent stem cells (iPSCs)
iPSCs are derived from somatic cells, which are reprogrammed into pluripotent stem cells by introducing specific transcription factors into terminally differentiated somatic cells. Under certain conditions, the cells are reversed to a totipotent state, or to form embryonic stem cell lines. The concept of iPSCs was first proposed in 2006 by Japanese scientist Shinya Yamanaka in Cell (Takahashi K, et al., 2006). iPSCs have similar advantages to ESCs, but iPS technology does not use embryonic cells, so there is no ethical problem. In addition, iPS technology can be used to produce proprietary stem cells from the patient's own somatic cells, so there is no problem of immune rejection (Ojeh N, et al., 2015; Aline V, et al., 2018).
Emily found that in a porcine Ischemic stroke injury model, iPSCs-derived NSCs transplantation improved tissue recovery through neuroprotective regeneration and cell replacement mechanisms. Longitudinal multi-parameter magnetic resonance imaging (MRI) after treatment showed reduced changes in white matter integrity cerebral perfusion and brain metabolism in infarcted tissue. Histological evaluation showed that iNSC treatment also produced neuroprotection, reduced microglial activation, and stimulated endogenous neurogenesis (Baker EW, et al., 2017). The therapeutic effects of iPSCs and NSCs on ischemic stroke were evaluated by 18F-FDG PET imaging combined with neurological examination and immunofluorescence stainingMoreover, in the subacute and chronic stages of ischemic stroke, extensive changes in protein expression after iPSCs transplantation were mainly related to oxidative stress of neuronal survival axon remodeling and mitochondrial function (Yao C, et al., 2020). Immunohistochemical analysis by Wang showed that the transplanted stem cells survived and migrated to the area of ischemia, and most of the stem cells expressed protein markers of the cells of interest. The results showed that the metabolism of MCAO rats recovered after iPSC and ESC transplantation (Jiachuan W., 2015).
Although iPSCs can be differentiated into neurons, endothelial cells or astrocytes to improve the therapeutic effect, iPSCs have the risk of tumorigenesis (Satoshi S, et al., 2020). The therapeutic effect of stem cell therapy on Ischemic stroke disease has been generally recognized, and this treatment has entered the clinical trial stage. But the cell types, infusion methods and cell doses need further exploration (Hess DC, et al., 2017). Treatment is also different at various times of stroke (Qian O, et al., 2019; Zhang G-L, et al., 2019; B BG, et al., 2020). When injected intra-arterial, stem cells can be found in the brain, but there are safety questions. Intravenous infusion is minimally invasive, easy to operate and has few side effects, so it will be used more in clinical studies (Bárbara A, et al., 2017; Cui, L-L, et al., 2015). Studies have shown that trans-arterial stem cell infusion can be performed safely during the sub-acute phase of Ischemic stroke disease. However, studies with larger cohorts are needed to validate the results (Bhatia V, et al., 2018). In principle,the cell dose should be minimal to achieve the highest efficacy (Shichinohe H, et al., 2017).
Today, efforts are being made to improve the effectiveness of stem cell therapy for Ischemic stroke disease and reduce the rate of adverse reactions. Such as the use of growth factors, drugs, ischemia, hypoxia and electrical stimulation, as well as measures to improve the efficiency of stem cell expansion in vitro (Hilal R, et al., 2018; Moon GJ, et al., 2018). In recent years, extra-cellular vesicles have been suggested to have similar effects to stem cells, with a lower risk (Li W-Y, et al., 2021; Hongqi X, et al., 2017). Extra-cellular vesicles derived from stem cells have emerged as a new therapeutic approach (Tian T, et al., 2021; Young BO, et al., 2019). In this study, we compared the infusion dose and infusion mode of stem cells for ischemic stroke (Table 1). The advantages and disadvantages of commonly used stem cell therapy for Ischemic stroke are shown in Table 2.
| First author/Year of publication | Type of stem cell | Dose of cells | The way of injection | Animals |
|---|---|---|---|---|
| Bhasin A [2017] | MSCs | 3.0-9.0 × 104 | IV injection | Human with IS |
| Bárbara Argibay [2017] | MSCs | 1.0 × 106 | IA/IV injection | Male Wistar rats |
| Jiachuan Wang [2015] | iPSC or ESC | 1.0 × 106 | Stereotaxic injection | Male SD rats |
| Shiwei Du [2014] | BMSCs | 3.0×106 | IA/IV injection | Male SD rats |
| Vivian W. Lau [2018] | iNPC | 5.0 × 106 | Stereotaxic injection | Male Pigs |
| Dheeraj Kalladka [2016] | hNSCs | 2.0×106, 5.0×106, 10×106, 20×106 | Stereotaxic injection | Human with IS |
| Lei Huang [2014] | hNSCs | 1.0 × 105 | Stereotaxic injection | Male C57BL/6J mice |
| Mohammad Saied Salehi [2020] | BM-MSCs or EPI-NCSCs | 2.0 × 106 | IA/IV injection | Male SD rats |
| Jingwei Mu [2019] | AD-MSCs | 2.0 × 106 | IV injection | Male SD rats |
| Yao Chen [2021] | iPSCs or NSCs | 2.0 × 106 | Stereotaxic injection | Male SD rats |
| Hong Zhang [2016] | iPSCs;NSC | 1.0 × 106 | Stereotaxic injection | Male SD rats |
| Keith W Muir [2020] | hNSCs | 20 × 106 | Stereotaxic injection | Human with IS |
| Nina Kosi [2017] | ESCs | 1.0 × 106 | Stereotaxic injection | Mouse |
| Emily W [2017] | iPSCs | Not motioned | Stereotaxic injection | Male Pigs |
| Li Chen [2020] | hESCs | Not motioned | Stereotaxic injection | Male SD rats |
| Xuegang Yuan [2019] | hMSCs | 1.0 × 106 | IA injection | Male SD rats |
| Moon GJ [2018] | hMSCs | 2.0 × 106 | IV injection | Male SD rats |
| Classification | Mechanism | Advantages | Disadvantages | |
|---|---|---|---|---|
| Exogenous stem cell | ESCs | Immunological regulation and alleviation of post-stroke inflammation; | A large number of amplifications; Multiple potential; Multidirectional differentiation; Multiple potential; | Ethical issues; Storage issues; Security issues; |
| EX-NSCs | Directional differentiation into nerve cells and astrocytes, complement and replace cells damaged by ischemia; | Multidirectional differentiation; Migrate to the damaged area; Strong repair ability; High survival rate; Broad resource; | Limited source; Immune rejection; Purity problem; | |
| MSCs | Secretion of neurotrophic factors and cytokines activates | Easy to access and save; No immune rejection; Differentiation into neurons; | Too few study samples; | |
| iPSCs | endogenous neural stem cells | Multidirectional differentiation; No ethical issues; | Oncogenesis; | |
| Endogenous stem cell | EN-NSCs | The activation and proliferation of endogenous nerve stem cells mediate the repair of brain injury | Migrate to the damaged area; Promotes blood vessel and nerve regeneration; | Functional recovery potential and mechanism are unclear; Low efficiency; Content is less; |
Application value of molecular imaging technology in ischemic stroke
Stem cell therapy for ischemic stroke is regarded as a promising clinical treatment strategy. Stem cells can promote the functional recovery of damaged tissue after ischemic stroke by various mechanisms, such as secretion of neurotrophic factors, immunomodulatory function, neuroprotective ability, stimulation of endogenous neurogenesis and new nerves and blood vessels. However, clinical transformation of stem cell therapy has not been fully achieved, in part due to insufficient ability to follow up stem cell migration and survival in the body over time. With the rapid development of molecular imaging technology, the current molecular imaging technology can provide a non-invasive, repeatable and quantitative tracking method for stem cell therapy. The implantation or transplantation of stem cells in the same living recipient can be monitored, providing important information on their biological distribution, migration dynamics, differentiation process and regeneration potential. Currently, imaging methods are combined with other methods to facilitate the follow-up of stem cell transplantation in stroke patients, including MRI and nuclear medicine imaging.
Magnetic resonance imaging (MRI)
Cellular MRI is considered an attractive and clinically translatable tool for longitudinal tracing of stem cells on account of its non-invasive, deep tissue penetration, lack of irradiation, superior spatial resolution, as well as the advantages of lofty sensitivity, low toxicity and biocompatibility (Xiaohui D, et al., 2017). Multiparameter MRI can observe the therapeutic effect of transplanted cells by showing infarct tissue size, white matter integrity, cerebral blood perfusion, and changes in brain metabolism (Baker EW, et al., 2017).
Wen synthesized a novel class of nanoscale polymer vesicles loaded with superparamagnetic iron oxide nanoparticles (SPIONs) with adjustable positive charges on the membranes for standard MSCs. Using these polymers, efficient markers of stem cells were achieved in a simple and controlled manner, with no direct adverse effects on cell viability, apoptosis and multiline differentiation. The transplanted cells retained the marker and lived for up to six weeks. On this basis, the biological distribution and migration of transplanted stem cells can be monitored by MRI and optical imaging under the background of cerebral ischemia injury. Intracerebral injection of anti-CD15-SPIONs molecular probe can be used to achieve targeted MR imaging of intracerebral NSCs in adult mice.After intraventricular administration, endogenous NSCs rapidly detected low signal within 1 day after injection.And persist longitudinally for at least 1 week in the same living animal. Histologically, these nanoparticles targeted the cell surface and extracellular environment of CD15-positive NSCs. This targeted imaging strategy has the advantage of rapidly monitoring endogenous NSCs subsets in the adult brain (Zhong, et al., 2015). Duan found that stem cells labeled with SPION-loaded polymersomes could be used to verify the biological distribution and migration of transplanted cells with MRI . The dynamic changes of low signal volume in MRI can reflect the trend of cell survival and apoptosis, but the long-term survival of cells may be overestimated due to the presence of iron-bearing macrophages around the cell graft (Xiaohui D, et al., 2017). Yuan used T2-weighted sequences to display MPIO labeled hMSCs, and magnetic resonance imaging showed that the initial biological distribution of hMSCs injected via artery was directed to the stroke hemisphere.
The 21.1-T ultra-high field magnet was used to improve the sensitivity and spatial resolution, and 1H and 23Na sequence imaging was used to monitor the size of stroke lesions in the same animals, providing direct evidence for the effects of hMSCs injection after MCAO on injury and functional recovery (Xuegang BS, et al., 2019). Avigdor has demonstrated that separate sodium and stem cell tracers are enhanced in ultra-high fields in a rat model of stroke and has developed robust single-scan diffuse-weighted imaging using ADC spatio-temporal coding (SPEN) to cope with these challenging field strengths (Avigdor L, et al., 2020). Klara demonstrated that pll-coated, γ-Fe2O3 labeled cells are suitable for MR detection, do not affect the differentiation potential of human induced pluripotent stem cell-derived neural precursors (iPSC-NPs), and are suitable for cell therapy of experimental models of central nervous system diseases (Jiráková K, et al., 2016).
Positron emission computed tomography (PET)
Pathological changes of ischemic stroke mainly involve changes in local cerebral blood flow, cerebral glucose metabolism, local oxygen metabolism and cerebral blood volume, etc. Which can be analyzed by imaging with 15O, 18F and other labeled tracers of PET in small animals, so as to evaluate the course of cerebrovascular disease. PET can measure the changes of cerebral blood flow early and judge whether the tissue of cerebral ischemia area is alive or not, which is of great value to the course of disease and the evaluation of therapeutic effect (Yingying L, et al., 2017; Meina H, et al., 2019; Bixiao C, et al., 2019; Hyafil F, et al., 2016). In recent years, Micro PET has also been used in cerebral ischemia (Xiaohui D, et al., 2017). In addition, PET has its unique advantages in tracing stem cell transplantation. Through injecting NSCs or iPSCs into MCAO rat models and performing a series of weekly small animal PET imaging of 18F-FDG to find dynamic metabolism and functional recovery (Figure 2) (Hong Z, et al., 2015).
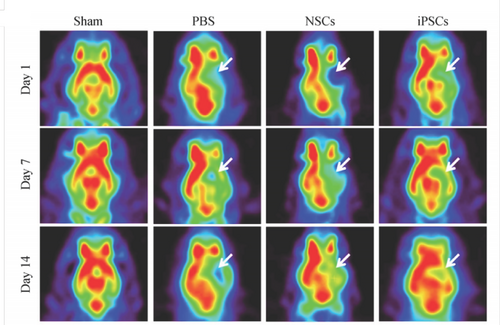
Representative 18F-FDG PET images after a series of stem cell transplants. (Modified from the reference of Hong Z, et al., 2015). Note: The white arrow indicates the stroke area.
Because ischemic stroke is a neuroinflammatory disease characterized by activation of microglia, which can be demonstrated by position emission tomography (PET) (Varley J, et al., 2015). the 18 kDa (TSPO) protein is the preferred target for imaging progression of microglia inflammation. TSPO is expressed in the mitochondrial membrane and is present at extremely low concentrations in healthy human brains, but significantly upregulation of TSPO in brain injury and inflammatory response has become the gold standard (Alam MM, et al., 2017). TSPO has become the best PET imaging target for neuroinflammatory events in neurodegenerative diseases. Backhaus demonstrated non-invasive imaging of activated microglia/macrophages using PET-MRI with [18F] DPA-714 targeting TSPO to potentially aid in differential diagnosis treatment monitoring and biopsy programs (Backhaus P, et al., 2020). Maria Ardaya also demonstrated the use of cell proliferation marker 30-Deoxy-30 -[18F] Fluorothymidine ([18F] FLT) PET imaging can evaluate gliogenesis after transient middle cerebral artery occlusion (MCAO) in rats (Ardaya M, et al., 2020).
Chemokine C-X-C motif receptor 4 (CXCR4) is a seven-transmembrane helical G-protein coupled receptor. CXCR4 has been shown to play a role in stroke, autoimmune disease, and myocardial infarction, and CXCL12 is upregulated after infarction (Hummel S, et al., 2014; Ruscher K, et al., 2013). Burke developed a highly stable 64 Cu-labeled small molecule PET reagent for imaging CXCR4 chemokine receptors in humans and mice. The degree of infarction after stroke was assessed (Burke B, et al., 2019). Braun monitored proliferation in the niches of neural stem cells by PET using the radioactive tracer 3′-deoxy-3′-[18F] fluoro-L-thymidine ([18F] FLT) (Braun R, et al., 2016). Wang used [18F]-FDG Micro PET to trace the effect of stem cell transplantation on glucose metabolism in cerebral ischemia rats.It was found that stem cell transplantation could significantly promote the neurological recovery after cerebral ischemia in rats (Wang J, et al., 2013). Du compared the therapeutic effect of bone marrow stem cells injected through artery and vein in MCAO rats, and monitored the intracarterial injection process and changes of brain metabolism by using SPECT and PET respectively, and found no decrease in cerebral blood flow.It is suggested that intra-arterial infusion is a safe and efficient method for bone marrow stem cell transplantation (Du S, et al., 2014). At present, the combination of PET and CT or MRI in small animals can obtain the energy metabolism and anatomical structure information of the examined tissues at the same time, which has great effect on the study of animal models. However, due to the high requirements of PET detection on environment and technicians, and the expensive cost of equipment, there are generally few basic studies on the application of Micro PET in cerebral ischemia models. Due to the supernal cost of research, sufficient sample size cannot be provided and the study is not carried out in depth. And the direct labeling of MRI can make the imaging signal weaken or become undetectable as the cells divide. The reason for this confusion between labeling and imaging is that the dead cells still produce signals until the macrophages clear the cell debris.
Current problems
First of all, most experiments are still limited to animal experiments, and the performance of stem cell inhibition therapy in animal models cannot fully represent its changes in human body. In addition, the animal model of ischemic stroke relatively simple, and ischemic stroke usually prone to the elderly and accompanied by a variety of complications. Although some clinical trials have been carried out, satisfactory results are difficult to obtain due to the short follow-up time and small sample size (Bhatia V, et al., 2018; Jie F, et al., 2018; Fang W, et al., 2018; Hess DC, et al., 2017; Bhasin A, et al., 2017). Secondly, the selection, purification, dosage and infusion route of stem cells, as well as how to prevent tumor formation and rejection after transplantation are all problems that need to be solved.
Thirdly, the mechanisms of stem cell transplantation in the treatment of ischemic stroke remain unclear. In addition to the common mechanisms of cell replacement, neuroprotection and nutrition, inflammation and immune regulation, whether the mechanisms of different types of stem cell transplantation in the treatment of ischemic stroke are different remains to be further explored. Fourthly, the use of molecular imaging techniques to evaluate the efficacy of ischemic stroke and follow up the monitoring of stem cells should be paid attention to. Finally, the safety of stem cell transplantation in the treatment of ischemic stroke is also a concern before it is widely used in clinic.
Summary and outlook
Ischemic stroke disease can contribute to irreversible nerve damage, and the time window for surgical intervention and medication were limited. So, there's a lot of hope for stem cell therapy. In this paper, we summarized and compared the advantages and disadvantages of several stem cells, but different infusion methods and cell dose need to be further explored. So, a lot of research needs to be done to find the best stem cells and treatment methods.
Moreover, most of the studies on Ischemic stroke are still in the animal test stage, and the selection of good animal models of Ischemic stroke disease also is an issue that we need to pay attention to. In this paper, MRI cell imaging and Micro PET were used to trace stem cells in animals and whether stem cells survived were discussed. Therefore, in order to enable stem cell therapy to be applied to clinical treatment of ischemic stroke as early as possible, we need to select an animal model closest to the patient's condition and select a stem cell with strong repair ability and good prognosis, and then evaluate it with appropriate tracer techniques. So, essentially, more research of ischemic stroke disease was needed before stem cell therapy can be used clinically in Ischemic stroke patients.
Ethical statement
Not applicable.
Acknowledgements
Not applicable.
Conflict of Interest
The authors have no competing financial interests in relation to the work presented.
Funding
Not applicable.
Transparency statement
The writing process is open and transparent.
Authors' contribution
Ting Li drafted the manuscript; Gao-Hong Zhu provide the framework and modified it. All authors read and approved the final manuscript.




