New progress of isoflurane, sevoflurane and propofol in hypoxic-ischemic brain injury and related molecular mechanisms based on p75 neurotrophic factor receptor
Abstract
Hypoxic ischemic brain injury (HIBI) is one of the most common clinical disorders, especially in neonates. The complex pathophysiology of HIBI is an important cause of disability and even death of patients, however, being without effective clinical treatments. Common anesthetics (such as isoflurane, propofol and sevoflurane) have an adverse impact on neuronal cells for HIBI via the regulation of p75 neurotrophic factor receptor (P75NTR). In order to protect the injured brains and study the effect of underlying treatments, it is particularly significant to understand and master the developmental mechanism of anesthetics for the occurrence of HIBI related molecular mechanisms. Therefore, this paper will mainly review the corresponding pathogenic and protective mechanisms about HIBI binding to the research progress of the role of P75NTR. In conclusion, the effects of neuroprotection and injured nerves are involved in the expression and activation of P75NTR, mainly increased P75NTR mRNA, protein levels and calpain-dependent for propofol, and inducing neuronal apoptosis for isoflurane and sevoflurane, and we look forward to that connection with P75NTR, common anaesthetic and HIBI may be a new direction of research and gain perfect outcomes in the future.
Introduction
Hypoxic ischemic brain injury (HIBI) refers to the brain lesions caused by various reasons of ischemia and hypoxia, such as brain edema, cerebral hemorrhage and hydrocephalus putting pressure on brain tissue and indirectly blocking absorption of oxygen, which further leads to a series of brain diseases or systemic pathological changes (Martínez-Biarge M, et al., 2014). For newborns, HIBI mostly occurs during the perinatal period, the common causes of adult hypoxic ischemic (HI) are stroke, shock and heart attack, causing cerebral palsy, epilepsy, seizures and learning limitations, and chronic disability (Li C, et al., 2017; Huang L, et al., 2018, Kojima T, et al., 2013). Its main pathological mechanisms include that the balance disorders of the calcium ion and the damage to DNA, proteins and lipids from superfluous free radical and others mechanisms (Kristian, et al., 1998; Leist, et al., 1997).
In fact, the generation mechanism of HIBI is complex. The growth and development of human brain neurons are characterized by stages with different changes in different periods. When drugs are involved or exposed, the effects on neurons will become more unfathomable and unpredictable. Moreover, the time window between the end of ischemic injury and neuronal death is thought to be related to the activation of competitive programs for gene expression, some of which promote cell survival while others promote neuronal death (Papadopoulos MC, et al., 2000). All anesthetics influence neuronal homeostasis (Karmarkar SW, et al., 2010; Staib-Lasarzik I, et al., 2014) and anesthetic exposure to the immature brain may induce neuronal cell death which raise a general concern that sedation or anesthesia of children (Jevtovic-Todorovic V, et al., 2013). In contrast to the developing brain, healthy adult brain tissue is resistant to anesthesia-related toxicity (Zhu C, et al., 2010). The results of these studies all have pointed to a close connection with the changes in neurotrophin signaling and subsequent activation of p75 neurotrophic receptor (P75NTR) when anesthesia-induced changes in neurotrophin signaling and subsequent activation of P75NTR, leading to neuronal cell death by preferential signaling of the neurotrophin precursor brain-derived neurotrophic factors (proBDNF) via P75NTR activation (Head BP, et al., 2009; Popic J, et al., 2012; Dzhala V, et al., 2012). But that doesn't mean all general anesthesia have a negative effect on nerve cells. In recent years, some common anaesthetics (such as isoflurane, sevoflurane, and propofol) have also proved that they have different effects on on neuronal integrity, associating with proBDNF and proapoptotic P75NTR (Kehl F, et al., 2014; Sebastiani A, et al., 2016; Colucci-D'Amato L, et al., 2020; Schallner N, et al., 2014).
BDNF and corresponding receptors have been concerned and experimented by lots of researchers because of their profound roles in brain plasticity, enhancing neuronal survival, migration and differentiation, supporting neurogenesis and improving outcomes in adult ischemic and neonatal HI models (Marini et al., 2007; Yasuhara et al., 2010; Han et al., 2012; Rosenkranz et al., 2012). However, BDNF interacts with two entirely distinguishing types of receptors, Trks and P75NTR. The former was discovered in the member of the tumor necrosis factor receptor superfamily, the former was regarded as a low-affinity receptor for nerve growth factor (NGF) (Radeke MJ, et al., 1987). It binds to neurotrophic proteins, including NGF, neurotrophic protein 3, BDNF, and neurotrophic protein 4 (He XE, et al., 2004). Besides, in Chen's study, he and his colleagues found that P75 NTR could promote the occurrence of nerve injury by promoting neuroinflammatory response, nerve apoptosis and synaptic plasticity and other mechanisms, and thus destroy the integrity of neural network and structure under the condition of chronic ischemia and hypoxia (Chen, et al., 2020). Therefore, the gene expression, activation P75NTR plays an irreplaceable role for the pathways involving that promoting neuronal survival and death. Currently, there is no effective clinical treatments to mitigate HI-induced brain injury, due to the lack of understanding of the neural networks associated with HI- induced neurodegeneration and its mechanisms (Liu S, et al., 2015). There are few studies related to P75NTR and HIBI, as well as little literature content about this area. However, the P75NTR expression level and activation are two of the key correlation between anesthesia and the injured brain or immature neurons (Sebastiani A, et al., 2016). In the treatment of HIBI, the application of anesthesia has become a major topic to be urgently solved in perioperative medicine. Therefore, the purpose of this review is to conclude the recent research status of HIBI and the research progress and molecular mechanism of isoflurane, propofol and sevoflurane related to HIBI will be reviewed in the following section, which potentially become one of the research directions in the future.
HIBI
Hypoxic-ischemia (HI) injury remains one of the most common causes of brain damage in newborns. In the United States, neonatal HI brain injury occurs from 1 to 4 births per 1,000 living borns, accounting for approximately one quarter of neonatal deaths worldwide (Kurinczuk, et al., 2010). During an HI attack, the supply of oxygen and glucose to the neonatal brain is briefly depleted, inducing to a state of energy depletion or inefficiency, which thought to be the main cause of the damage. This attack triggers a series of harmful cellular events, including dysfunction of the transcellular ion pump and the accumulation of excitatory glutamate and oxygen free radicals (Perlman, et al., 2006; Yildiz, et al., 2017). After brief resuscitation, there may be secondary damage, including inflammation, mitochondrial dysfunction, and cell death (Perlman, et al., 2006; Johnston, et al., 2001; Vannucci, et al., 2000). Hypoxic-ischemia-induced neuronal death has been found to have two stages (Vannucci, et al., 2004). Severe HI damage leads to primary energy depletion, leading to primary neuronal death, which is associated with a significant decrease in energy (adenosine triphosphate (ATP)) and an increase in intracellular lactic acid production. Low ATP levels lead to the failure of energy-dependent cell membrane ion channels that maintain cell integrity, leading to acute intracellular influx of calcium and sodium and cell membrane depolarization, as well as extracellular accumulation of glutamate. In addition, the accumulated lactic acid directly increases the level of reactive oxygen species (ROS). These changes eventually lead to rapid cell swelling and necrosis. Delayed neuronal death accounts for most of the final brain cell loss after HI injury ( ).
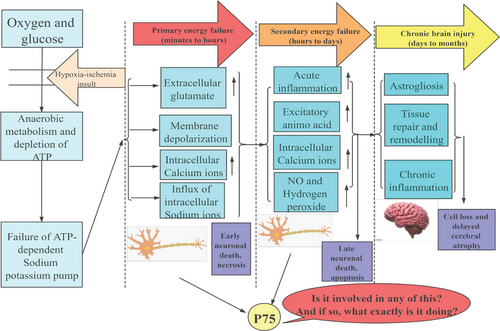
The pathogenesis of hypoxic-ischemic encephalopathy. Neonatal hypoxic ischemia causes brain tissue to be deprived of energy substrates, oxygen and glucose, and causes cells to convert to anaerobic metabolism. Reductions in ATP lead to the failure of energy-dependent cell membrane ion channels, resulting in acute intracellular influx of calcium and sodium and depolarization of the cell membrane and accumulation of extracellular glutamate. Thus, this cascade leads to cell swelling and death of necrotic cells. If the initial insult is prolonged or severe, it can lead to secondary delayed energy depletion within hours, and most cell death is due to apoptosis. At this stage, excitotoxicity, inflammation and continued intracellular calcium uptake and release of reactive oxygen species are observed. After the second stage of injury, another stage of chronic injury can occur within days and last for months or even years. This stage includes astrocytosis, chronic inflammation, and tissue repair and remodeling, which further leads to brain cell loss and brain atrophy.
To explore the pathophysiology of neonatal HI brain injury, several animal models have been developed over the past decade (Yager, et al., 2009). The Rice-Vannucci model (Levine, et al., 1960) was the most widely accepted, including unilateral common carotid artery ligation followed by hypoxic (8-10% O2) therapy. Utilizing these models, the study identified some unique features of HI brain damage in neonates, which may be related to immature nervous system development. The immature brain has limited activity of antioxidant enzymes (e.g., glutathione peroxidase, copper-zinc superoxide dismutase) before and after birth (Sheldon, et al., 2004). Therefore, the newborn brain is more susceptible to oxidative damage caused by HI damage (Sheldon, et al., 2004). In addition, there is a “continuous” phenotype of cell death in the injured neonates, characterized by a mixture of apoptosis and necrosis (Northington, et al., 2007). Stroke is still the leading cause of chronic disability in adults and is also a cause of hypoxic ischemic brain injury in adults. Meanwhile, stroke treatment is not sufficient to be seen. Tissue plasminogen activator (tPA) remains the only FDA-approved drug for the treatment of acute ischemic stroke. In addition to tPA, emerging evidence suggests that endovascular therapy is beneficial and promising (Cougo-Pinto, et al., 2015). However, due to the influence of various reasons, its use is not very extensive. At the same time, even after prolonged rehabilitation, most survivors are unable to achieve full neurological and functional recovery and have to face several obstacles to a normal life (Qureshi, et al., 2013). Similarly in HI injury of the newborns, inadequate blood supply can trigger a series of pathophysiological events which leads to nerve cell death after ischemic stroke. The mechanisms, including excitotoxicity, mitochondrial dysfunction, protein misfolding, oxidative stress, and inflammatory responses. Although these pathways were initially considered to be delegitive in the development of nerve injury, some pathways have also been displayed to be beneficial for brain recovery such as neurogenesis . Astrocytosis with HIBI can have a significant impact on functional recovery. In Mingming Chen's study, we were able to find significant upregulation of astrocyte activation and nerve growth factor receptor (P75NTR) in the vicinity of the lesion. Not only that, they also found a profound change in the cell cycle of the astrocytes near the lesion, switching from the mitotic quiescent (G0) phase to the G 2 / M and S phases. After P75NTR knockdown in mouse astrocytes, the cell cycle in response to cortical puncture progressed to the G 2/M and S phases and CDK 2 protein levels were inhibited. Contrastly, overexpression of P75NTR in mouse astrocytes was sufficient to promote cell cycle progression. Eventually, their results show that upregulation of P75NTR in astrocytes after brain injury induces cell cycle entry by promoting CDK2 expression and promoting astrocyte proliferation.
In addition, P75NTR expression has been shown to increase in the brains of patients with AD. P75NTR is A receptor for Aβ, which can arise neuronal death and degeneration of nerve endings in the brain by binding to P75NTR. P75NTR is cleaved by enzyme during metabolism, releasing its extracellular segment (P75 NTR-ECD). Experimental results show that P75NTR-ECD, as the Aβ binding site of P75NTR, can inhibit Aβ aggregation into oligomer and fiber, promote Aβ fiber deaggregation, clear Aβ in the brain, anti-Aβ toxicity (Hao L, et al., 2014). Its pharmacokinetic properties make it a promising clinical development tool for the treatment of Alzheimer's disease. Due to the advantages of currently commonly used clinical anesthetics such as sevoflurane, isoflurane and propofol, such as fewer side effects and convenient control of the depth of anesthesia, we will focus on how these anesthetics can reduce the damage caused by HIBI in the future.
Propofol and HIBI
As mentioned above, the pathological mechanism of HIBI is complex, in which excessive extracellular glutamate is released when the patient suffers from cerebral ischemia, which in turn leads to excitatory toxicity and cell death (Choi, et al., 1990). Glutamate transporters (Gegelashvili, et al., 2001), such as glial glutamate transporter-1 (GLT-1), can rescue the excitatory toxicity caused by glutamate mediation. GLT-1 is a transporter that, among other things, regulates glutamate homeostasis in extracellular fluid, but lacks an enzyme that breaks down glutamate (Danbolt, et al., 2001). Not only that, GLT-1 also removes excess glutamate and reduces neuroexcitatory toxicity (Ouyang, et al., 2014). Studies have shown that ischemia reperfusion results in a significant decrease of GLT-1 in hippocampal CA1 region (Bruhn, et al., 2000). In order to avoid more severe conditions in patients, the scientists found that ischemic preconditioning and transient subfatal cerebral ischemia can reverse the excitatory toxicity caused by reduced GLT-1 levels, thereby protecting neurons from ischemic damage and causing additional pain to patients (Gong, et al., 2014). As a common anesthetic, propofol is widely applied in clinical practice and has become the preferred drug in clinical anesthesia. Propofol has previously been shown to have neuroprotective effects in numerous studies (Harman, et al., 2012; Zhou, et al., 2013), its mechanism of action is varied in Table 1. (G S Hollrigel, et al., 1996; HY Wang. et al., 2009; Yoshinori Kotani. et al., 2007) Hypoxia-induced hippocampal neuron damage can also be attenuated by propofol (Zhou, et al., 2013). In addition, some experiments have shown that propofol can up-regulate GLT-1 expression, resulting in a decrease in glutamate concentration in the hippocampus of depressed rats (Zhu, et al., 2015). Otherwise, propofol reduced NMDA-induced vasodilation and superoxide production. Therefore, propofol also reduces the excitatory toxicity caused by NMDA (N-methyl-D-aspartic acid). In the study of Hong-Yan Gong, postprocessing of propofol in rats with transient middle cerebral artery occlusion can inhibit neuronal apoptosis during ischemia/reperfusion. Astrocyte apoptosis induced by peroxynitrite was also attenuated by propofol treatment. The hypoxia-induced reduction in GLT-1 expression was attenuated in vitro by propofol treatment. Similarly, propofol has been shown to alleviate decreased GLT-1 expression in depressed rats (Zhu, et al., 2015). Propofol therapy significantly reversed the decrease of cell viability and the increase of cell apoptosis induced by GLT-1 down-regulation. However, when ischemia is extended to 20 min, GLT-1 causes glutamate release and triggers neuronal death. In addition, the brain protective target of propofol may become a new clinical target for the treatment of ischemic brain injury (Rui, et al., 2020). More experiments are sought to explore the mechanism of disparate pathophysiological states of propofol on neurons and its correlation with HIBI.
| Source | Mechanism of action | Impact Factor | Function type | Animal type/cell type | Medicine concentration |
|---|---|---|---|---|---|
| G. S. Hollrigel. et.al.1996 | Activation of GABA A receptors, including the specific binding subunits of propofol, plays A role in inhibiting neuronal death caused by brain chemistry and acute mechanical injury. | 2.265 | Propofol and GABA | Adult rats | 5 microM Propofol |
| N Stratford. et.al.1998 | Propofol can improve the antioxidant capacity of human plasma, and various experimental models have shown that propofol can directly eliminate ROS, inhibit the generation of free radicals and lipid peroxidation, so as to protect brain cells from the effects of oxidative stress. | 3.375 | Propofol and Antioxidant Property | Patients | 1% Propofol |
| HY Wang. et.al.2009 | Propofol at anesthetic dose can protect pyramidal neurons in the CA1 subfield of hippocampus and prevent delayed neuronal death caused by global cerebral ischemia. In addition, 30 min infusion of propofol at the beginning of reperfusion significantly reduced neuronal apoptosis in middle cerebral artery occlusion (MCAO) rats during reperfusion. |
2.733 | Propofol and Anti-Apoptosis | Rats | 1%; Propofol 10, 20, 35 mg x kg(-1) |
| Yoshinori Kotani. et.al.2007 | The propofol formulation contains EDTA, which exacts neuroprotective effects in permanent MCAO and OGD-induced cell ischemia models by chelating residual brain zinc. | 5.681 | Propofol and EDTA | mice | Propofol EDTA (0.005% (w/v) EDTA) |
P75NTR levels become extremely re-expressed likely by trauma-induced activation of developmental-like programs for survival, regeneration, and reestablishment of lost network connections in the central nervous system (CNS) when suffered from trauma-induced brain injury (TBI-induced) ( Shulga A, et al., 2012; Shulga A, et al., 2013). To be declared, HIBI is not an exception. And there is animal experiments proving propofol increased P75NTR mRNA and protein levels supporting the hypothesis of a TBI-induced expression posttraumatic propofol neurotoxicity, and was mediated via P75NTR pathway, which result in a higher sensitivity for anesthesia-related toxicity. In addition, due to propofol exposure, the relative levels of P75NTR ligand proBDNF went up. However, it is worthwhile heeding that early application of propofol (within 6 h of injury) and almost constant expression of P75NTR did not affect brain injury, while delayed (24 h after injury) significantly increased high expression. The results suggested that the expression pattern of P75NTR may be related to the observed neurotoxic effects, suggesting a time-dependent sensitivity to anaesthetic-related toxicity (Sebastiani A, et al., 2016). Moreover, in cell culture studies and animal investigations, cell death-related enzymes could be induced with the help of anesthetics increasing cytosolic calcium levels (Yang H, et al., 2008; Wang H, et al., 2011) such as calcium-regulated cysteine proteases (calpains). Ischemia and other various pathologic conditions, caused the production of cal pains, while propofol application at 24 hours after injury strongly enhanced calpain-dependent proteolysis and activation of P75NTR mediated increased Ca2+ uptake (Dechant G, et al., 2002). Thus, elevated calpain-dependent is also thought to be a potential mechanism of propofol-induced neurotoxicity in the developing brain and is related to structural changes, changes in synaptic plasticity, and activation of other cell death processes (Milanovic D, et al., 2010)
Isoflurane and HIBI
HIBI can conduce to a variety of pathological conditions and may appear during neurological, cardiac surgery and anesthesia. The biggest challenge is how to protect the brain from hypoxic injury and to treat hypoxic ischemic brain injury in clinical practice. Numerous studies have clarified that hypothermia and preischemic therapy have shown protective effects on the brain, but both are complicated and difficult to be implemented in clinical practice, while drug therapy is more common and practical in clinical practice (Feiner, et al., 2005). Recently, the neuroprotective effects of anesthetics have attracted a great attention from clinicians. Isoflurane is a halogenated volatile ether used in inhalation anesthesia and is extensively applied in clinical practice. Neonatal hypoxic-ischemic injury occurs in the immature brain which leads to delayed cell death through excitatory toxicity and oxidative stress. Despite the trend in the use of isoflurane in human medicine has begun to decline, even many hospitals have replaced it with sevoflurane, desflurane and the intravenous anesthetic propofol, isoflurane is still commonly used in veterinary anesthesia. Isoflurane plays a neuroprotective role in the study of hypoxic-ischemic brain injury in neonates (Burchell, et al., 2013). Isoflurane has been studied in animal models of a variety of diseases, such as lipopolysaccharide-induced (LPS) acute lung inflammation (Chung, et al., 2013), acute lung injury (Harr, et al., 2012), glucose oxidative stress (Kinoshita, et al., 2012), renal ischemia/reperfusion injury (Kim, et al., 2007), and cardiac injury (Lang, et al., 2013). In these models, isoflurane has been shown to have neuroprotective effects and has the ability to ameliorate various adverse symptoms (Table 2).
| General models | ||||||
|---|---|---|---|---|---|---|
| Source | Isoflurane Protection | Impact Factor | Animal Models | Animal type/cell type | Medicine concentration | The associa-tion with p75 |
| Lang, X. et.al. 2013 | The release of LDH and CK-MB was decreased by PKCε/ ALDH2 pathway, and the infarct size was decreased | 4.065 | Cardiac Injury | Male Sprague-Dawley (SD) rats | isoflurane of 1.0 (2.1%) | × |
| Chung, I.S. et.al. 2013 | Inhibitory ROS burst, NF-κB activation and pro-inflammatory cytokines | 4.049 | LPS-Induced Actute Lung Inflammation (Rat) | Male Sprague-Dawley rats | 1.0 minimum alveolar concentration of isoflurane | × |
| Kim, M. et.al.2007; Lee, H.T. et.al. 2004 | Reduces renal insufficiency and tubular necrosis by the SK/S1P approach | 2.925;2.871 | Renal ischemia/reperfusion injury | Rats | 1.2% isoflurane | × |
- Note: √ means that there are articles related to P75, and × means that there are no articles related to P75
Isoflurane has shown neuroprotective effects in a variety of stroke models, including subarachnoid hemorrhage (SAH), cerebral artery occlusion (MCAO), intracerebral hemorrhage, craniocerebral injury, and neonatal hypoxic-ischemic brain injury (Table 3). Results of one experiment showed that exposure to isoflurane for 2 h did not induce neuronal apoptosis in adult human brains, indicating that delayed astrocyte processes were reduced after isoflurane exposure. This conclusion may explain why some volatile anesthetics confer neuroprotective effects after experimental strokes (Dallasen, et al., 2011). Additionally, isoflurane has certain toxicological effects on the human body. It has certain influence on the cognitive function of elderly patients after operation. Chen Yao-Xiong's experiment has showed that isoflurane anesthesia may cause transient postoperative cognitive dysfunction in elderly patients after laparoscopic cholecystectomy, and the mechanism of action may be related to the up-regulation of peripheral blood 6 expression (Yao-xiong, et al., 2016). In addition, the side effects of isoflurane include suppression of the respiratory center, lowering blood pressure and causing arrhythmia. As a matter of fact, there is a convincing number of evidence to strongly support the neuroprotective effects of isoflurane in different models) Although the clinical benefit of isoflurane inhalation is promising, its influence on long-term outcomes of patients is still uncertain in the developing brain and adult cerebral ischemic injury under certain circumstances (time, duration for the treatment and concentration of isoflurane, as well as depending on the severity of the insult). Mechanisms of neuroprotection, induced by isoflurane are complex and complicated. Inhibiting apoptosis is one of the more important mechanisms. The first reported that reducing to neuronal apoptosis in rats after isoflurane preconditioning, other studies determined that isoflurane reduced cell apoptosis primarily via inhibition of apoptotic molecules such as caspase-3 and caspase-8 (Li et al., 2008b, 2013b), related to the P75NTR-mediated apoptosis isoflurane increased P75NTR signaling. P75NTR modulates synaptic transmission, axonal elongation, and growth cone collapse, and it initiates neuronal apoptosis. Dendritic complexity in P75NTR mice, which is increased substantially, can be reduced by P75NTR overexpression (Zagrebelsky M, et al., 2005), indicating a negative modulatory effect of P75NTR on dendritic spine development. In addition, to enhance proBDNF signaling via the P75NTR. ProBDNF is also crucial in the process (Head BP, et al., 2009). Thus, these data support that isoflurane can enhanced proBDNF/P75NTR-mediated apoptosis.
| Source | Isoflurane Protection | Impact Factor | Animal Models | Animal type/cell type | Medicine concentration | The association with p75 |
|---|---|---|---|---|---|---|
| Altay, O. et.al. 2012 | The S1P pathway was used to prevent neuronal apoptosis and BBB destruction after SAH in the ipsilateral hemisphere | 7.078 | Subarachnoid hemorrhage | CD-1 mice | SAH+1% isoflurane ;SAH+2% isoflurane |
× |
| Li, H. et.al. 2013 | NF-κB activation was attenuated to improve neurological function and reduce the production of inflammatory cytokines | 5.815 | Occlusion of the middle cerebral artery | male Sprague–Dawley rats | 2.0% isoflurane | × |
| Khatibi, N. H. et.al. 2011 | Reduces brain edema, apoptotic cell death and neurological deficits | 4.145 | Cerebral hemorrhage | CD1 mice | 1.5% isoflurane | × |
| Statler, K.D. et.al. 2006 | Increased CA3 neuronal survival and improved histopathology | 2.666 | Traumatic brain injury | Adult, male rats | 1% isoflurane | × |
| Zhou, Y. et.al. 2010 | Reduce infarct size, improve neurobehavioral prognosis and increase PAKT | 6.633 | Neonatal hypoxic-ischemic brain injury | Sprague-Dawley rats | 2% isoflurane | × |
- Note: √ means that there are articles related to P75, and × means that there are no articles related to P75.
Sevoflurane and HIBI
Previous studies have shown that sevoflurane post-treatment provides neuroprotection after hypoxic-ischemic injury. In Qiu-Shi Gao's experimental study, they induced hypoxic-ischemic injury using the classical Rhys-Vanozzi model. First, they treated neonatal rats (7 days after birth) with 2.4 % sevoflurane for 30 minutes after hypoxic-ischemic injury. The final results showed that sevoflurane post-treatment did significantly improve learning and memory function, reducing astrocytosis and glial scarring, increased the number of dendritic spines, and protected the histological morphology of the hippocampus. Based on this study, it is obviously to obseerve that sevoflurane can effectively reduce the proliferation of astrocytes in the hippocampus and reduce the learning and memory impairment caused by glial scarring formation after hypoxic-ischemic injury. Among them, it is speculated that the potential mechanism may be related to the up-regulation of DJ-1 expression, or the decrease of hypoxia-inducible factor 1α ubiquitination and the stability of hypoxia-inducible factor 1α expression (Jia, et al., 2020). In addition, in Wang's study, they used the Morris Water Maze to test spatial learning and memory after using sevoflurane. Tunel analyzed neuroprotective effects and p-Akt levels in sevoflurane and control groups. The results showed that postprocessing not only improved spatial learning and memory ability, but also reduced apoptotic cells. Penicillin wortmannii counteracts the neuroprotective effect and prevents the increase of p-Akt. Finally, their study further suggests that this neuroprotective effect may be partially attributable to activation of the PI3K/Akt pathway and inhibition of neuronal apoptosis. Therefore, sevoflurane treatment can prevent focal cerebral ischemia-reperfusion injury through the PI3K/Akt pathway. Generally, the neuroprotective mechanisms of sevoflurane and isoflurane are similar as inhibiting apoptosis. Apoptosis is a major process of delayed neuronal death in the ischemic brain. P75NTR-mediated apoptosis has been mentioned in this article. Otherwise, in models of brain ischemia, sevoflurane accommodated the balance between apoptotic proteins and anti-apoptotic proteins by increasing expression of anti-apoptotic proteins (Bcl-2 and c-Fos) and reducing expression and activation of apoptotic protein and activation of apoptotic proteins, that is likely to be potentially related to P75NTR binding to neurotrophic proteins.
Summary and outlook
HIBI is still a considerable cause of disability and death for newborns and adults, experiments are continuing to be conducted to reduce this incidence, but being without effective clinical treatments to mitigate it, the narcotic drugs mentioned above are currently commonly used in clinical practice. The effects of neuroprotection and injured nerves seem to be more or less involved in the expression and activation of P75NTR, mainly increased P75NTR mRNA, protein levels and calpain-dependent for propofol, and inducing neuronal apoptosis for isoflurane and sevoflurane. Therefore, we draw special attention of how to effectively use to reduce the incidence of HIBI a certain extent. More importantly, it would be inspiring if medical practitioners could further investigate these preexisting connections and explore valid experimental results, and we look forward to that connection may be a new direction of research and gain perfect outcomes in the future.
Ethical statement
Not applicable.
Acknowledgments
Not applicable.
Conflict of interest
There is no conflict of interest in this study.
Funding
None.
Transparency statement
The authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Yi Zhu contributed to the conception of the study; Hong-Su Zhou, Dong-Qin Chen contributed significantly to analysis and manuscript preparation, Di Zhou, Nan Zhao, Liu-Lin Xiong revised manuscript; Issac Deng, Xin-Fu Zhou, Zhao-Qiong Zhu approved and finalized this work.