Research progress of IGF-1 and cerebral ischemia
Abstract
Cerebral ischemic disease is a group of diseases that cause insufficient blood supply to the cerebrum, cerebellum or brain stem for different reasons, resulting in corresponding nervous system symptoms. Cardiovascular disease is the leading cause of death in the world. Among them, the death caused by cerebral ischemia accounts for the vast majority, and it is one of the fatal diseases in the middle-aged and elderly at present. Epidemiologic studies have projected increasing mortality due to cardiovascular disease worldwide (about 23.3 million people by 2030) because of the aging population. However, related studies have shown that insulin-like growth factor I (IGF-1) is a multifunctional cell proliferation regulator. It plays an important role in cerebral ischemia. It is effective in promoting cell differentiation, proliferation and individual development. Studies have shown that IGF-1 signaling pathway is a key pathway controlling cell growth and survival. There may be five mechanisms in cerebral ischemia: prevention of intracellular calcium overload, inhibition of the upregulation of nNOS, IGF-1upregulations activating HIF-1α, regulation of Bcl-2 to resist apoptosis, and enhancement of vascular endothelial function. Three critical nodes in the IGF-1 signaling pathway have been described in cardiomyocytes: protein kinase Akt/mammalian target of rapamycin (mTOR), Ras/Raf/extracellular signal-regulated kinase (ERK), and phospholipase C (PLC)/inositol 1,4,5-triphosphate (InsP3)/Ca2+. IGF-1 plays an important role in cerebral ischemia and myocardial ischemia, mainly by activating downstream of IGF-1, controlling cell death and differentiation or transcription work, improving the function of heart muscle cells, reducing the myocardial cell apoptosis induced by myocardial infarction, regulating endogenous protection and restoration of cerebral ischemia injury, thus protecting cerebral and myocardial injury. Related studies have shown that bcl-2 exerts great influence on both cerebral ischemia and myocardial ischemia. Therefore, the relevant pathways and targets of cerebral ischemia and myocardial ischemia and the role of IGF-1 in protecting the heart are reviewed in this paper.
Introduction
Cerebral ischemic disease is a group of diseases that cause insufficient blood supply to the cerebrum or brain stem for a variety of reasons, resulting in corresponding nervous system symptoms. The most common cause is vascular blockage, which can be caused by two common causes: first, plaque shedding, and micro thromboembolism caused by arteriosclerosis; second, platelet aggregation caused by anemia, heart disease, myocarditis, thrombocytopenia and other reasons, resulting in high blood viscosity and thrombus formation; Cerebral ischemia may also be caused by some of its own diseases, such as hypertension, hyperlipidemia, diabetes and complications of diabetes, etc. Cardiovascular disease is the leading cause of death in the world. Among them, the death caused by cerebral ischemia accounts for the vast majority, and it is one of the fatal diseases in the middle-aged and elderly at present. Epidemiologic studies have projected increasing mortality due to cardiovascular disease worldwide (up to 23.3 million people by 2030) because of the aging population (Hausenloy DJ, et al., 2013). As we all know, the damage of neurons is irreversible, cerebral ischemia and hypoxia will lead to a large number of neuronal deaths in a short time, which will bring the threat of death. Insulin-like growth factor (IGF) system plays an important role in the development and growth of central nervous system (CNS) (Bake S, et al., 2016). IGF-1 can regulate cell growth and is a potential drug for the treatment of traumatic brain injury (Zhao B, et al., 2017). In hypoxic/ischemic neonatal mice, IGF-1 can promote interneuronal development, suggesting that it is a promising strategy for the treatment of premature encephalopathy (EoP) (Vaes JEG, et al., 2020). So more detailed and comprehensive evidence related to IGF-1 has been provided for the clinical treatment of cerebral ischemia or myocardial ischemia, and even innovative research ideas have been explored. This article reviews cerebral ischemia and its relationship with IGF-1, as well as the role of IGF-1 in cerebral ischemia.
Morphology and pathology of cerebral ischemia
The occurrence of the disease is inseparable from the changes of histomorphology and pathology of the body. In clinical treatment of cerebral ischemia, we should first fully understand the morphological and pathological changes of the corresponding brain tissue after the occurrence of cerebral ischemia, and further understand the mechanism of its damage. in order to diagnose and treat the disease accordingly. The brain is the most important organ of the human body, the weight of the brain accounts for 2% of the body weight, but the blood flow it needs accounts for 15% of the cardiac output and 20% of the cardiac output, and its oxygen consumption accounts for 25% of the total oxygen consumption of the body. Some studies clearly confirmed that the infarction center of rats with permanent focal cerebral ischemia was sectioned by hematoxylin-eosin staining. Under the microscope, obvious ischemic changes were observed in 1.5 hours. There were red neurons and neutrophil infiltration in the parenchyma in 12 h. Necrosis and a large number of leukocyte infiltration were observed at 72 h (Tatlisumak E, et al., 2009). Acute ischemic neuronal injury is characterized by axonal preservation and selective neuronal injury caused by a large amount of glutamate release, but delayed neuronal death is related to the decreased expression of anti-apoptotic growth factor, microtubule-associated protein and tubulin. At the morphological level, ischemia can be detected by neuronal necrosis, microglial proliferation and astrocytes in fragile areas of the brain. In the case of permanent ischemia, so-called pale nerve cell injury can be observed, and in the case of partial perfusion, so-called dark nerve cell injury and apoptosis can be detected (Oechmichen M, et al., 2006). Some researchers conducted a comprehensive neuropathological examination on the autopsy data of 20 patients with traumatic asphyxia (TA) caused by different factors. The results showed that there was a tendency to increase brain weight in patients with TA, with severe cerebral vascular congestion, perivascular hemorrhage and occasional β-APP deposition, which was consistent with early axonal destruction (Al-Sarraj S, et al., 2017). Some studies have shown that if a patient has hypoxic-ischemic encephalopathy (HIE) before death, the disappearance of cerebral hemispheric sulcus and the loss of contrast at the basal ganglia level can be found by computer tomography (PMCT) after brain death (Shirota G, et al., 2016). From the above results, it can be found that different parts of the brain have different morphological and pathological changes after cerebral ischemia.
After mastering the morphological changes of cerebral ischemia, we will systematically introduce and describe IGF-1, as well as its related changes and functions after cerebral blood.
Changes of IGF-1 expression after cerebral ischemia
Insulin-like growth factor
IGF system consists of IGF-I, IGF-II, IGF-I receptor (IGF-IR), IGF-II receptor (IGF-IIR) and IGF binding protein (IGFBP) (Suleiman MS, et al., 2007). It participates in a variety of physiological functions and is an important cytokine in the process of tissue cell proliferation, differentiation and maturation. Among them, the role of IGF-1 is particularly important. IGF-1 is essentially a kind of basic peptides synthesized and secreted by the liver under the action of hypothalamic growth hormone (GH), which contains more than 70 amino acids. Other non-hepatocyte types also produce IGF-1, which acts locally as autocrine and paracrine hormones (Troncoso R, et al., 2014). It has 50% homology with insulin (De Meyts P, et al., 2002). It can mediate the growth-promoting effect of growth hormone on the body. IGF-1 exists in brain tissue and is a necessary growth regulator for postnatal brain development. The effect of IGF-I is mediated by binding to IGF-IR on the cell surface (Suleiman MS, et al., 2007) Insulin-like growth factor-1R has two extracellular α subunits with ligand binding sites. Each α-subunit is coupled to one of two membrane-scanned β-subunits, which contain an intracellular domain with inherent tyrosine kinase activity (Adams TE, et al., 2000). Binding to IGF-IR ligands results in conformational changes, which leads to the activation of tyrosine kinase domain and automatic phosphorylation of tyrosine and serine residues (LeRoith D, et al., 1995). Once stimulated, IGF-IR causes phosphorylation of proteins such as insulin receptor substrate (IRS)-1 and IRS-2 (Sun XJ, et al., 1995).
Distribution of IGF-1 in brain tissue
AmtulZ et al examined the histological presence of IGF-1 and insulin receptor substrate (IRS1) in anatomically different brain circuits of rats. The results showed that they were mainly distributed in the ipsilateral striatum and the distal areas associated with striatal injury. These areas include subcortical white matter, motor cortex, thalamus, dentate gyrus, septal hippocampal nucleus, periventricular area and Broca horizontal diagonal band of basal forebrain (Amtul Z, et al., 2018). In addition, in a randomized controlled trial, the level of IGF-1 and the expression of IGF-1 receptor (IGF-1R) activity in rat hippocampus were detected (Wadowska M, et al., 2015). Furthermore, some studies have confirmed that the gene expression of IGF-1 and IGF-1 receptors is expressed in all parts of the brain and is mainly concentrated in cerebral vessels. In addition, astrocytes are considered to be the main local source of IGF-1 in the developing brain (Janowska J, et al., 2020). During ischemia, IGF-1 can promote receptor-mediated endothelial cell anchoring to reduce harmful substances through the cell monolayer, so as to exert its vascular protective function(Bake S, et al., 2019). IGF-1 is involved in neurogenesis, angiogenesis, stimulating cell proliferation, and repairing the injury response of the central and peripheral nervous system, and has antioxidant, anti-inflammatory and protective effects on the central nervous system (Bake S, et al., 2016).
Changes of IGF-1 after cerebral ischemia
For newborns, hypoxic-ischemic (HI) brings great injury to them including hypoxic-ischemic encephalopathy. It can lead to neurodegeneration of the brain, leading to mental retardation, hyperactivity, cerebral palsy, epilepsy and neuroendocrine disorders. In neonatal cerebral ischemia, the level of igf-1 is decreased. Through clinical controlled experiments, some researchers have proved that the levels of IGF-1, GH and NGB in serum are changed in neonatal hypoxic-ischemic encephalopathy. The levels of IGF-1 and NGB are related to the prognosis of hypoxic-ischemic encephalopathy (HIE) (Liu G, et al., 2018). Some researchers found that compared with hypoxia group, IGF-1 in IGF-1 hypoxia group could increase cell survival rate and reduce cell apoptosis (Zhao B, et al., 2017). In addition, IGF-1 could improve the downregulation of phosphorylated AKT, MAPK and ERK induced by hypoxia to prevent anti-apoptosis and increases the survival rate of hypoxia-induced neural stem cells (NSCs) (Chiu HY, et al., 2017). A prospective clinical study of newborns found that compared with the control group, the level of IGF-1 was measured within 6 hours after birth, or the level of IGF-1 was measured again on the 3rd day in the hypoxic-ischemic encephalopathy (HIE) group and the control group. The results showed that the level of serum IGF-1 in the hypoxic ischemic encephalopathy group was significantly lower than that in the control group (Umran RM, et al., 2016). These are the changes of IGF-1 after neonatal cerebral ischemia and the decrease of IGF-1 after adult cerebral ischemia. It has a lot to do with the prognosis. Some studies have investigated the expression of IGF-1 in middle cerebral artery occlusion / reperfusion (MCAO/R) in rats. The results showed that the level of IGF-1 decreased after injury and reached the peak at 24 hours after reperfusion (Hu M, et al., 2009). Another study on hypoxic-ischemic (HI) rats showed that the serum levels of growth hormone (GH), insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) were significantly decreased in the subacute phase of HI (Kartal Ö, et al., 2016). Moreover, some studies have verified whether the levels of plasma IGF-1 and IGFBP-3 can predict the functional prognosis of 3 months in 404 patients with acute stroke. Finally, it was confirmed that low insulin-like growth factor-1 levels were independently associated with a reduced risk of adverse outcomes. Low level of insulin-like growth factor binding protein-3 was independently associated with poor prognosis. Low level of IGFBP-3 and high level of IGF-1 in subacute stage predicted poor prognosis 3 months after stroke (Armbrust M, et al., 2017). These studies suggest that IGF-1 levels decrease after cerebral ischemia in both newborns and adults.
When cerebral ischemia is caused by myocardial ischemia, the level of IGF-1 decreases. This change is the result of the joint effect of myocardial ischemia and cerebral blood ischemia, so we have made the following summary and thinking.
The role of IGF-1 in cerebral ischemia
Overexpression of IGF-1
IGF-1 is a well-known growth factor, which has a definite neuroprotective effect on cerebral ischemia. Some researchers have demonstrated that the sustained high expression of IGF-1 and IGF-1R in the hippocampal CA1 region of young gerbils after transient cerebral ischemia (TCI) may be related to the decrease of neuronal death after TCI (Yan BC, et al., 2018). With the characteristics of neuronutrition and angiogenesis, IGF-1 can be used to promote nerve regeneration after cerebral infarction, and it has become a potential method for the treatment of stroke. In the experiment, they achieved long-term brain IGF-1 overexpression by stereotactic injection of adeno-associated virus vector (AAV). Adeno-associated virus vector green fluorescent protein (GFP) or saline was injected as control. As shown by immunohistochemical staining compared with AAV-GFP or saline treatment post-ischemic gene transfer of IGF-1 significantly increased blood vessel density in peri-infarction and injection area 8 weeks after stroke. Compared with AAV-GFP, AAV-IGF-1 treatment effectively increased neurogenesis. These results confirmed that the ischemic postconditioning of IGF-1 effectively promoted the neurogenesis and vascularization in the chronic phase of cerebral infarction in mice with cerebral ischemia (Zhu W, et al., 2009). IGF-1 is a multiple growth factor, which has protective effect on acute ischemic brain injury. In addition, the methods of adeno-associated virus (AAV)-mediated IGF-1 overexpression and control experiment were also used. Sensorimotor test showed that the motor ability of rats in the IGF-1 gene transfection group was significantly better than that in the control group. Functional recovery is accompanied by a reduction in the volume of cerebral infarction. Immunohistochemical analysis of endothelial cell marker CD31 showed that IGF-1 gene transduction could effectively increase the formation of neovascularization around infarction and injection needle area compared with AAV-green fluorescent protein transduction. In addition, compared with AAV-green fluorescent protein therapy, AAV-IGF-1 treatment promoted neurogenesis in the subventricular zone. These results suggest that the overexpression of IGF-1 promotes the long-term functional recovery after cerebral infarction. The improvement of functional performance is parallel to the enhancement of neovascularization and nerve regeneration (Zhu W, et al., 2008). The application of IGF-1 after ischemic stroke can reduce the infarct size and increase the sensorimotor function (Serhan A, et al., 2020).
Lack of IGF-1
In a clinical control study of 32 patients with stroke, it was found that the serum levels of vascular endothelial growth factor (VEGF), IGF-I and hepatocyte growth factor (HGF) were measured on admission. Compared with age-matched healthy subjects (n = 15), the serum levels of VEGF and HGF in patients with cerebral infarction were higher than those in the control group, while the level of IGF-I was lower than that in the control group (Okazaki H, et al., 2014).
Through the experiment of Galectin-3 gene knockout mice (Galectin-3KO), it has been proved that the defect of activation / proliferation of microglia is related to the increase of the range of ischemic injury, and the two-fold increase in the number of apoptotic neurons is related to the decrease of IGF1 level (Lalancette-Hébert M, et al., 2012). The decrease of IGF-1 and the up-regulation of matrix metalloproteinase-9 may be involved in the damage of blood-brain barrier and white matter (WM)/axon after cerebral ischemia caused by ATP-binding cassette transporter A Mel-1 deficiency (Cui X, et al., 2015). Hypoxic-ischemic (HI) is a widely used animal model to simulate preterm or perinatal subfatal hypoxia, including hypoxic-ischemic encephalopathy. It can lead to neurodegeneration of the brain, causing mental retardation, hyperactivity, cerebral palsy, epilepsy and neuroendocrine disorders. Some studies have found that in the acute phase of hypoxia, the brain volume increases significantly, which is related to the decrease of growth hormone (GH) and IGF-1 secretion. During subacute hypoxia, neuronal survival rate and brain volume decreased significantly, accompanied by increased apoptosis in hippocampus and cortex. During this period, the levels of serum growth hormone (GH), insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) decreased significantly. And during subacute hypoxia, the development of brain and body is obviously retarded (Kartal Ö, et al., 2016). Aging is the main risk factor for cerebrovascular disease. Growth hormone (GH) and its synthetic metabolic medium insulin-like growth factor (IGF)-1 decrease with age, which has been proved to cause vascular dysfunction. In addition, lower GH/IGF-1 levels are associated with higher stroke mortality in humans. This suggests that the decrease of GH/IGF-1 level is an important factor in the increased risk of cerebrovascular disease (Yan H, et al., 2014) . Therefore, the lack of IGF-1 may play an adverse role in brain diseases and may be related to the increased risk of cardiovascular and cerebrovascular diseases.
The signaling pathway of IGF-1 after cerebral ischemia
Prevents intracellular calcium overload
Energy metabolism disorder leads to ATP deficiency and can induce calcium overload in nerve cells during cerebral ischemia. While IGF-1 can observably prevent the decrease of -28kd positive neurons of calcium binding protein, thus stabilizing the concentration of intracellular calcium ions and preventing intracellular calcium overload (Guan J, et al., 2000). In addition, Chik et al (Chik CL, et al., 1997) found in the study of mouse pineal cells that IGF-1 can prevent the opening of L-type calcium channels, thus reducing the influx of calcium ions. Thus, IGF-1 inhibits calcium overload by regulating intracellular calcium-binding protein levels.IGF-1 also prevents glutamate decarboxylase inactivation and protects neuronal cells from glutamate-induced neurotoxicity and secondary intracellular calcium overload. Research has also shown that IGF-IR activation is associated with increased cardiomyocyte size, inhibition of apoptosis, regulation of glucose metabolism and intracellular Ca2+ concentration..
Regulation of nitric oxide synthase (NOS) activity
Nitric oxide (NO) can aggravate brain injury, and IGF-1 can significantly inhibit the upregulation of nNOS and alleviate neuronal injury (Sharma HS, et al., 1998). Tagami et al (Tagami M, et al., 1997) found that IGF-1 can prevent NO-mediated apoptosis of cortical neurons after ischemia-reperfusion in spontaneously hypertensive stroke-prone rats. Therefore, IGF-1 can achieve its neuroprotective effect by inhibiting the activity of nNOS, thus playing a protective role in the brain.
The role of HIF-1α
Hypoxia-inducible factor (HIF) is an important transcription factor that senses oxygen concentration in cells and modulates adaptive responses to hypoxia (Semenza GL, et al., 1992). HIF-1α is an oxygen-regulating subunit of HIF. When cells are hypoxic, HIF-1α is transferred from cytoplasm to nucleus and forms dimer HIF-1 with HIF-1β. HIF-1 binds to hypoxia-induced gene hypoxia response element (HRE). Promote hypoxia-induced gene transcription and induce a series of cell responses to hypoxia. In addition, recent studies have shown that CA repeating microsatellites and HIF1α influence each other's transcriptional activity during the regulation of IGF1 expression, suggesting an interaction between them (Geng H, et al., 2020). Chavez et al (Chavez JC, et al., 2002) suggested that IGF-1 is upregulated after ischemic injury, initiating and maintaining the continuation of HIF-1α accumulation. Therefore, HIF-1 α activation may be part of the mechanism by which IGF-1 promotes cell survival after cerebral ischemia.
Anti-apoptosis by regulating Bcl-2
Anti-apoptosis was induced by increasing the expression of anti-apoptotic protein Bcl-2 and decreasing the expression of pre-apoptotic protein Bax (Qin SQ, et al., 2020). In addition, IGF-1 can also block apoptosis signal transduction by blocking the activation of Caspsase-9 (Poulaki V, et al., 2002) and Caspase-8. In the apoptosis induced model established in vitro, IGF-1 can effectively inhibit the apoptosis of fibroblasts induced by the overexpression of c-MYC (Harrington EA, et al., 1994). When hearts are suffering from ischemia-reperfusion injury, the IGF-1 downstream signaling like Akt will be phosphorylated to prevent apoptosis in rats' hearts (Yamashita K, et al., 2001). Akt activation in cardio myocytes inhibits AMP-activated protein kinase (AMPK) phosphorylation and function. This action requires both phosphatidylinositol-3-OH-kinase (PI3K) and MAPK kinase (MEK1)-dependent pathways to lead to the activation of the transcription factor cAMP response element-binding protein (Mehrhof FB, et al., 2001). In the experimental myocardial infarction, IGF-1 bound to peptide nanofibers activated Akt, decreased activation of caspase-3, and increased expression of cardiac troponin I in cardio myocytes (Davis ME, et al., 2006). In a model of hypoxia-induced apoptosis of cultured neonatal cardiomyocytes, IGF-I-stimulation was followed by a PI3K-dependent phosphorylation of AKT and BAD and an MEK1-dependent phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 (Mehrhof FB et al., 2001). These demonstrate that both PI3K- and MEK1- dependent pathways activation induces the expression of the antiapoptotic factor bcl-2 in the cardiac ischemia.
IGF-1 is a potent vascular growth factor
IGF-1 is a potent vascular growth factor (Lopez-Lopez C, et al., 2004). IGF-1 enhances vascular endothelial function through anti-inflammatory and anti-apoptotic properties (Conti E, et al., 2004). Zhu W et al (Zhu W et al., 2008) showed that IGF-1 promoted blood vessel and nerve regeneration and prevented brain atrophy after brain injury in ischemic reperfusion brain tissue (Figure 1) (Table 1).
| Function | Signal pathway | Result | Reference |
|---|---|---|---|
| Prevents intracellular calcium overload | IGF-1 can prevent the decrease of -28kd positive neurons of calcium binding protein, prevent the opening of L-type calcium channels | Reducing the influx of calcium ions., stabilizing the concentration of intracellular calcium ions and preventing intracellular calcium overload | (Guan J, et al., 2000) (Chik CL, et al., 1997) |
| IGF-1 can prevents glutamate decarboxylase inactivation | Protects neuronal cells from glutamate-induced neurotoxicity and secondary intracellular calcium overload | (Chik CL, et al., 1997) | |
| Regulation of nitric oxide synthase (NOS) activity | NO can aggravate brain injury and IGF-1 can inhibit the upregulation of nNOS | Alleviate neuronal injury | (Sharma HS, et al., 1998) |
| The role of HIF-1α | When cells are hypoxic, forms dimer HIF-1 with HIF-1β. HIF-1 binds to hypoxia-induced gene hypoxia response element (HRE) | Promote hypoxia-induced gene transcription and induce a series of cell responses to hypoxia | (Semenza GL, et al., 1992) |
| Anti-apoptosis by regulating Bcl-2 | Anti-apoptosis was induced by increasing the expression of anti-apoptotic protein Bcl-2 and decreasing the expression of pre-apoptotic protein Bax. | Anti-apoptosis | (Qin SQ, et al., 2020) |
| Block apoptosis signal transduction by blocking the activation of Caspsase-9 | Anti-apoptosis | (Poulaki V, et al., 2002) | |
| The IGF-1 downstream signaling Akt occurs phosphorylation | Prevent apoptosis in rats’ hearts | (Yamashita K, et al., 2001) | |
| IGF-1 is a potent vascular growth factor | Through anti-inflammatory and anti-apoptotic | It can enhance vascular endothelial function | (Conti E, et al., 2004) |
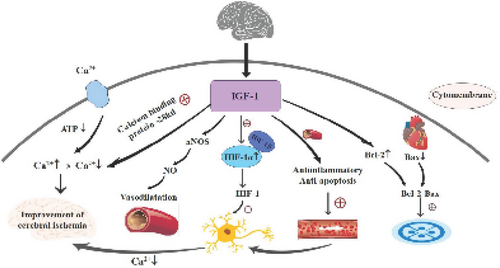
The signaling pathway of IGF-1 after myocardial ischemia. These are schematic diagrams of three cerebral myoischemic pathways. IGF-1 (insulin-like growth factor I), NOS (nitric oxide synthase), NO (nitric oxide), HIF (Hypoxia-inducible factor), HIF-1α (Hypoxia-inducible factor-1α), HIF-1β (Hypoxia-inducible factor-1β), Bcl-2 (anti-apoptotic protein Bcl-2), Bax (pre-apoptotic protein Bax).
The signaling pathway of IGF-1 after myocardial ischemia
Cardiac IGF-1 signaling
Three critical nodes in the IGF-1 signaling pathway have been described in cardiomyocytes: protein kinase Akt/mammalian target of rapamycin (mTOR), Ras/Raf/extracellular signal-regulated kinase (ERK), and phospholipase C (PLC)/inositol 1,4,5-triphosphate (InsP3)/Ca2+ (Troncoso R, et al., 2013).
PI 3-kinase/Akt signaling
IGF-1 activates PI 3-kinase and Akt in cardiomyocytes. Activated PI 3-kinase and Akt are sufficient to protect hypoxic cardiomyocytes against apoptosis in vitro (Matsui T, et al., 1999). Cardiomyocytes derived from human embryonic stem cells (ESCs) proliferate extensively in vitro, and their proliferation appears to be mediated primarily via the PI 3-kinase/Akt signaling pathway, using the IGF-1 receptor as one upstream activator (McDevitt TC, et al., 2005). Then, overexpression of Akt produced cardiac hypertrophy at the molecular and histological levels, and increased cardiomyocyte cell size and concentric LV hypertrophy significantly (Condorelli G, et al., 2002). IGF-I/PI3-kinase accelerates the accumulation of autophagic vacuoles and subsequent autophagic cell death during glucose deprivation, revealing the opposing role of IGF-I/PI 3-kinase in two distinct types of programmed cell death (apoptotic and autophagic cell death) (Aki T, et al., 2003). Thus, the PI 3-kinase/Akt signaling pathway governs survival in the settings of cardiac stress and hypertrophic growth.
Ras/Raf/ERK pathway
IGF-1 induces cell growth and proliferation through the Ras/Raf/ERK pathway in concert with the Akt/mTOR pathway which mediates protein synthesis (Fuentes EN, et al., 2011). This signaling along with Akt regulates the survival in cardiac stress.
(PLC)/ (InsP3)/Ca2+
We have observed that IGF-1 induces a rapid increase in nuclear Ca2+ that is independent of cytosolic Ca2+ and regulates gene transcription by promoting migration of a plasma membrane receptor in close to the nucleus (Ibarra C, et al., 2013). IGF-IR activation is associated with increased cardiomyocyte size, inhibition of apoptosis, regulation of glucose metabolism and intracellular Ca2+ concentration (Latronico MV, et al., 2004). This pathway mainly regulates metabolic adaptability and gene transcription (Figure 2).
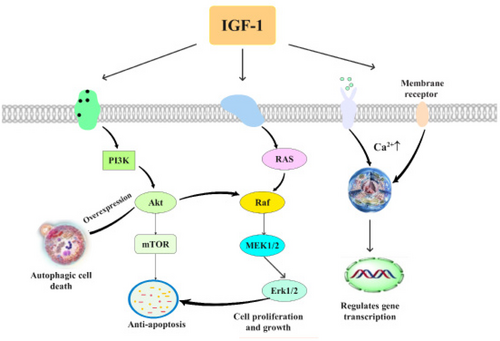
The signaling pathway of IGF-1 after myocardial ischemia. This is a schematic of the three pathways of myocardial ischemia, respectively: PI 3-kinase/Akt signaling, Ras/Raf/ERK pathway, (PLC)/ (InsP3)/Ca2+ passageway.
IGF-1 is the target of miRNAs associated with cerebral ischemia
Many experimental studies have confirmed that different kinds of miRNAs play a role in cerebral ischemia by acting on IGF-1. In 2018, researchers found that miR-320 could promote apoptosis and increase cerebral infarction volume and edema volume by inhibiting the expression of IGF-1mRNA and protein in cerebral ischemia-reperfusion injury model in mice with left middle cerebral artery occlusion (MCAO). This suggests that miR-320 may be involved in the regulation of cerebral ischemia-reperfusion injury by inhibiting IGF-1 pathway (Liang L, et al., 2018).
In addition, in an experiment on hypoxia-glucose deprivation-reperfusion (OGD/R), the researchers found that miR-186-5p could reduce the expression of IGF-1. At the same time, miR-186-5p inhibitor could promote cell viability and IGF-1 expression. Luciferase activity assay confirmed that IGF-1 is a direct target gene of miR-186-5p and an insulin-like growth factor necessary for the development of the nervous system. These results suggest that miR-186-5p may be an unfavorable factor in inducing neuronal apoptosis and inhibiting IGF-1 in ischemic stroke model and predict that miR-186-5p may be a diagnostic marker and potential therapeutic target in patients with ischemic stroke (Wang R, et al., 2018). In addition, miR-15a is closely related to acute cerebral ischemia of IL-6 and IGF-1. It can be used as a potential biomarker and therapeutic target for stroke (Lu WJ, et al., 2018). The level of miR-151b in blood was negatively correlated with IGF-1, and the level of miR-27b-3p was negatively correlated with IGF-1 and insulin-like growth factor binding protein-3. It is suggested that miR-151b and miR-27b-3p can be used as blood biomarkers in the diagnosis of ischemic stroke (Cheng X, et al., 2018) (Table 2).
| MiRNAs | Function | Meaning | Reference |
|---|---|---|---|
| miR-320 | It can inhibit the expression of 1IGF-1 mRNA and reduce the content of IGF-1. | It can cause apoptosis in the area around cerebral infarction in left middle cerebral artery occlusion (MCAO) mice and increase the volume of cerebral infarction and edema. | (Liang L, et al., 2018) |
| miR-186-5p | It can reduce the expression of IGF-1. | It may be a diagnostic marker and potential therapeutic target for patients with ischemic stroke. | (Wang R, et al., 2018) |
| miR-151b and miR-27b-3p | Its blood level was negatively correlated with IGF-1. | It can be used as a blood biomarker for the diagnosis of ischemic stroke. | (Cheng X, et al., 2018) |
| miR-15a | It is closely related to IGF-1 and IL-6 in acute cerebral ischemia. | It can be used as a potential biomarker and therapeutic target for stroke. | (Lu WJ, et al., 2018) |
Bcl-2, cerebral ischemia and myocardial ischemia
Neonatal hypoxic-ischemic encephalopathy (HIE) is a clinically defined neurological condition with deficiency of oxygen, while the HIE often associated with cardiac dysfunction in term infants (Liu X, et al., 2011). And The degree of cardiac response in neonates with HIE is associated with the severity of hypoxia (Alp H, et al., 2011). Correlational research reported for the first time that acute cerebral ischemia significantly increased left ventricular end diastolic pressure (LVEDP), but decreased left ventricular systolic pressure (LVSP), which indicate that acute cerebral ischemia may specifically disturb cardiac function and calcium homeostasis, subsequently leading to Ca2+ overload and myocardial dysfunction (Sun L, et al., 2010). Similarly left focal cerebral ischemia can produce cardiac dysfunction, which is associated with the extent of left insular cortex damage (Min J, et al., 2009). We can conclude that there is a certain connection between the cerebral ischemia and myocardial damage, and brain ischemia produce cardiac damage. IGF-1, as a multifunctional cell proliferation regulation factor, is effective in promoting cell differentiation, proliferation and individual development. It is expressed in small amounts in normal brain tissue and promotes normal growth and brain development. And relevant data suggests that IGF-1 exerts a strong neuroprotective effect in acute ischemic injury, and endogenous levels of IGF-1 are upregulated after cerebral ischemia reperfusion, suggesting that IGF-1 may modulate endogenous protective and repair mechanisms in ischemic injury (Yan YP, et al., 2006). Studies shown IGF-1 plays an important role in cerebral ischemia through five signaling pathways, while the most important one may be to increase the expression of anti-apoptotic protein Bcl-2 (Long X, et al., 2019) and decrease the expression of apoptotic protein Bax, thus playing a protective role in cerebral ischemia. The most important pathway in cardiac ischemia may be pi3-kinase/Akt signaling pathway, which also induces the expression of anti-apoptotic factor Bcl-2 in cardiomyocytes to activate IGF-1 downstream and control cell death, differentiation or transcription. In conclusion, IGF-1 is associated with cerebral ischemia and myocardial injury, and its loss can lead to apoptosis of myocardial cells, impair the contractility of myocardial cells, and aggravate the degree of cerebral ischemia.
Summary and prospect
There is a great deal of evidence to support the role of IGF-1 in myocardial and cerebral ischemia (Baltazar-Lara R, et al., 2020). We mainly summarized the role of IGF-1 in cerebral ischemia. IGF-1 plays a role through the following pathways to prevent intracellular calcium overload, inhibit the upregulation of nitric oxide synthase, up-regulate the activation of hypoxia-inducible factor-1 α by insulin-like growth factor-1, regulate apoptosis-related gene bcl-2, and enhance vascular endothelial function. In the previous discussion, it has been shown that the PI3- kinase /Akt signal pathway may be the most important in myocardial ischemia. Here we believe that the main mechanism of IGF-1 in cerebral ischemia is to prevent intracellular calcium overload and regulate apoptosis-related gene bcl-2, and bcl-2 also plays a corresponding role in cardiomyocytes, so we think bcl-2 is the link that IGF-2 plays a role in myocardial ischemia and cerebral ischemia. The next step of research can start to develop corresponding drugs through the target of IGF-1to improve the damage caused by myocardial ischemia and cerebral ischemia (Figure 3).
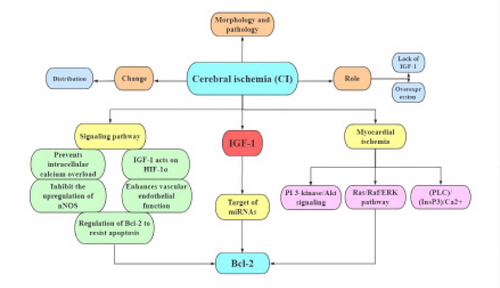
Summary for research progress of IGF-1 and cerebral ischemic. This figure is a summary of the full-text content.
Ethical statement
Not applicable.
Acknowledgements
Not applicable.
Conflict of interest
There is no conflict of interest in this study.
Funding
Not applicable.
Transparency statement
All the authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Shun-Lian Li and Jing Li contributed most analysis of the data, and wrote the initial draft of the paper. Hong-Su Zhou contributed to refining the ideas, carrying out additional analysis and finalizing this paper. Liu-Lin Xiong came up with the central idea, reviewed and edited this paper.




