MRI tracking/detection of bone marrow mesenchymal stromal cells transplantation for treatment of ischemic cerebral infarction
Abstract
Background
Cerebral stroke is the second leading cause of death with high mortality and morbidity worldwide, currently it lacks effective therapies to improve the prognosis. This study was aimed to explore the role of bone marrow mesenchymal stem cells (BMSCs) transplantation in the recovery of brain structure and function after ischemic cerebral infarction by magnetic resonance imaging (MRI).
Methods
By applying internal carotid artery embolization, the ischemic cerebral infarction model in rats was established. MRI was performed to detect the imaging changes in the brain tissue after modeling, and the successful modeling was evidenced by the presence of obvious high-signal infarct areas in the brain. BMSCs were then injected into the lateral ventricles of rats, and the recovery of brain tissue and function were quantitatively evaluated by T2-weighted image (T2WI) and voxel-based morphology (VBM) after 28 days.
Results
The results showed that BMSCs were cell subsets with multiple differentiation potentials. Deficits caused by Ischemic cerebral infarction were relieved by BMSCs transplantation, including increase in damaged cerebral tissue and recovery of cerebral function. In addition, the combined imaging technology of VBM and T2WI quantitatively revealed the effectiveness of BMSCs in repairing damaged brain tissue structure and function.
Conclusion
Taken together, the results revealed that the transplantation of BMSCs into the lateral ventricle was beneficial to repair the structure and function of the damaged brain tissue after ischemic cerebral infarction. Moreover, the combination of VBM and T2WI technology can detect the level of brain injury in ischemic cerebral infarction dynamically and noninvasively, and evaluate the recovery of structure and function of damaged brain tissue.
Introduction
Cerebral infarction (CI), also known as cerebral ischemic stroke (CIS), refers to the localized ischemic necrosis or softening of brain tissue caused by brain blood supply disorders, ischemia, and hypoxia. Cerebral stroke is the second leading cause of death in the world according to the latest data published by the World Health Organization. Among them, ischemic cerebral infarction accounts for more than 70% of all cerebrovascular diseases (Gorelick PB, et al., 2020; Kuriakose D, et al., 2020). Mounting studies have shown that interruption of blood supply to the brain for more than 30 minutes would develop irreversible damage to the nerve cells of brain tissue. The longer ischemia time, the more serious reperfusion injury of brain tissue after reperfusion (Sims NR, et al., 2010). The consequences of cerebral ischemia and hypoxia are far-reaching and lasting, eventually leading to short-term or long-term neurological dysfunction and disability as well as high mortality, which brings heavy burdens on the family and society (Rapsomaniki E, et al., 2014; Naess H, et al., 2010). As a result, how to promote the survival and regeneration of neurons in the injured area, recovery the damaged brain tissue, enhance the neurological function, and improve the prognosis are the major research hot topics in the treatment of ischemic brain infarction (Abeysinghe HCS, et al., 2016; Becerra-Calixto A, et al., 2017; Cui C, et al., 2016). At present, the clinical therapeutic strategies for CI are mainly symptomatic supportive treatments; however, these treatments cannot improve long-term neurological dysfunction. Thus, it is of great significance to explore new detection technologies and treatment methods based on main pathophysiology for CI.
Bone marrow mesenchymal stem cells (BMSCs) are a subset of cells with multipotent differentiation potential (Friedenstein AJ, et al., 1976). Woodbury D et al. proved that BMSCs not only have the ability to differentiate multi-directionally, but also to break the traditional restriction of germ layer differentiation (Woodbury D, et al., 2002). Recently, increasing evidences showed that BMSCs transplanted into the brain infarction model can migrate to the injured site and differentiate into neurons, which promoted angiogenesis, anti-glial cell proliferation and anti-apoptosis at the injured site (Jiang W, et al., 2014; Nakajima M, et al., 2017). Many reports demonstrated that BMSCs play an important role in the development of the nervous system and even the survival or repair of neuronal cells (Bonsack B, et al. 2020; Li C, et al. 2019; Shen H, et al., 2020). In addition, the animal experiments have shown that BMSCs transplantation can notably improve the neurological function and prognosis in rats with acute ischemic brain injury (Xie P, et al., 2019; Sammali E, et al., 2017).
In the evaluation of the mechanism and efficacy of BMSCs in the repair of cerebral ischemia injury, most of the literature in China and abroad explored the repair mechanism and efficacy through immunohistochemistry, immunofluorescence, Western blot and cell pathology. In recent years, with the rapid development of medical imaging, Magnetic resonance imaging (MRI) technology has been used to dynamically and noninvasively observe the changes of functional magnetic resonance imaging (fMRI) efficacy evaluation of cerebral ischemic injury area after stem cell intervention, which provided an imaging basis for the mechanism of stem cell treatment and clinical efficacy evaluation (Korbakis G, et al., 2016; Rimmele DL, et al., 2014; Karunamuni RA, et al. 2019; Visser MM, et al., 2019; Nael K, et al., 2015). Among them, Voxel-based morphological (VBM) analysis allows brain region segmentation and post-processing of MRI images, thus the individual data of each brain region can be analyzed with individual data in voxels. Based on the different intensities of voxels, the brain tissue is divided into grey matter, white matter and cerebrospinal fluid, then the density or volume of these parts is measured to quantitatively analyze the morphological changes in brain tissue (Gauthier LV, et al., 2008; Yin D, et al., 2013). So, with the popularity of high magnetic field intensity MRI and the development of brain imaging technology, this safe and non-invasive method can be used to conduct non-invasive and efficient brain structure and function research on living bodies, expecting that it would help early clinical stage with diagnosis, early intervention and early treatment by providing useful information and efficient support.
The present research explored the exact role of BMSCs in CI by establishing CI model in rats. The BMSCs were injected into rats at the lateral ventricle. Afterwards, combined with MRI technology to detect the structure and function recovery of the damaged brain tissue in rats. The present study was designed to validate the specific significance of BMSCs in CI-induced neurological deficits and to research the value of MRI in evaluating the damage and cell apoptosis of ischemic cerebral infarction in rats.
Materials and methods
Animals and grouping
The animal experimental design of this study has been approved by the Animal Care & Welfare Committee of Kunming Medical University. All experimental processes complied with the guidelines for the care and use of laboratory animals published by the National Institutes of Health. The Sprague-Dawley (SD) rats aged 8-week (300 ± 20 g, male) were provided by Animal Centre of Kunming Medical University, and housed with alternate 12/12 light/dark environment at humidity of 40-70% and temperature of 22-24°C. A total of 12 SD rats were randomly divided into Sham group (rats were only subjected to same anesthesia and arterial exposure), Control group (CI rats were injected with the same dose of normal saline at the same site) and BMSC group (CI rats were injected BMSCs, 2x105 cells, totaling 5 μl).
Establishment model of CI
SD rats were anesthetized with an intraperitoneal injection of 2% pentobarbital sodium (0.3 ml/100 g), and then fixed on the operating table in the supine position. After disinfected the skin, an incision was made in the middle of the neck, followed by the left common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). Silk sutures were tied around the CCA distal and proximal ends and ECA for use. ICA was clamped with an arteriole clip temporarily, and then CCA and ECA were ligated proximal to the heart. A beveled incision was cut from the CCA ligature and the knot, while a strand plug was inserted through the ICA, and tied the tethering wire with a thin wire around the distal end of the CCA gently. The thread was gently put with ophthalmic tweezers, and calculated distance from the arterial bifurcation. When the insertion depth reached 18 mm, the slimline at the distal end of CCA was tightly fastened. The aseptic environment was maintained strictly during surgical procedures.
BMSCs cell culture
One-month-old adult rats were used for primary BMSCs extraction. Briefly, adult rats were sacrificed by intraperitoneal anesthesia with 2% pentobarbital sodium (0.3 ml/100 g) and sterilized in 75% ethanol for 3 minutes (min). Then, the femur was taken out aseptically, the remaining muscle was removed and the end was cut off. Afterwards, the culture fluid was injected into the bone marrow cavity with a 1 ml needle and rinsed three times, the cell suspension was collected, filtered through a 200-mesh syringe and inoculated in a bottle. Then, the cells were incubated with SD rats BMSCs special medium under the condition of 5% carbon dioxide. On the 5th day, half of the fluid was changed, 5 passages of cells were harvested for subsequent experiments after observing the growth and morphology of the cells.
Transplantation of BMSCs into the lateral ventricle
BMSCs were injected into lateral ventricle 5 min after cerebral artery embolization, and the injection dose of BMSC group was 2x105 cells, totaling 5 μl. The control group was injected with the same dose of normal saline at the same site. The injection coordinates were 1.0 mm from the bregma, 1.5 mm on the left side, and 4 mm depth of needle insertion, the process was operated under warm and sterile conditions.
MRI scan of rats' brain
All RI scans were performed by a 7.0 T magnetic resonance scanner (Bruker Biospec 70/30, Ettlingen, Germany) to collect rat brain MRI images. The general procedure is as follows: The rats were anesthetized with 2% pentobarbital sodium (0.3 ml/100 g) using a 16 cm horizontal scanning frame and a volume or surface coil. The rats were placed in an organic glass scanning bed to fix the brain and the body temperature, heart rate and respiration were continuously monitored. Moreover, the heating blanket was used to maintain the body temperature of rats at about 37°C. MRI scanning parameters of rat brain structure were: T2 weighted image, coronal T2WI scanning, parameter setting, Rapid Imaging with Refocused Echoes (RIRE) was 4; Time Repetition (TR) was3000 ms; Echo Time (ET) was 12 ms, Field of View (FOV) was 3.2 cm*3.2 cm; layer thickness was 0.5 mm; layer spacing was 0.75 mm and 25 layers; matrix size was 384*384, spatial resolution was 0.083*0.083*0.75, and the scanning time was 12 min.
VBM image analysis technique
VBM analysis is based on Matlab R2020a software platform. All structural image data are first expanded 10 times by DPABI software, which is convenient for SPM12 toolbox (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) for subsequent processing (Yan CG, et al., 2016). SPM12 toolbox is used to preprocess the collected extended structural images, including: 1. Manual correction: manual correction of all 3D images according to the pre-and post-joint; 2. Tissue segmentation and standardization: SPM12 was used to convert all individual T2WI to standard three-dimensional space according to the rat brain structure template (Valdés-Hernández PA, et al., 2011), and then divided into grey matter, white matter and cerebrospinal fluid. After the optimal template is generated by DARTEL registration method, the segmented image is averaged to obtain the grey and white template of the sample. The segmented image is standardized to the template again, and then segmented and extracted again. The grey and white images of all individuals after segmentation are standardized to 1.5 mm*1.5 mm*1.5 mm. Grey matter volume (GMV), white matter volume (WMV), cerebrospinal fluid volume (CSFV) and whole brain volume (WBV) were calculated; 3. Image modulation: The volume change caused by non-linear transformation in the standardization step is adjusted to obtain the relative volume of each voxel after correction; 4. Tissue smoothing: Gaussian kernel (full width at half-maximun (FMWH) = 6 mm) with full width at half-maximun (FMWH) = 6 mm is used for spatial smoothing of the obtained images, so as to reduce the anatomical differences of brain structures of different individuals and improve the signal-to-noise ratio, as well as ensure that the data conform to the normal distribution. The masks and final results in all the analysis processes were presented based on the rat anatomical map and rat standard template made by Valdés-Hernández et al., and the coordinate localization is referred to the rat brain stereotaxic map made by Paxinos and Watson (Paxinos G, et al., 1986).
Statistical analysis
The comparison of GMV between the two groups was using by statistical analysis module of DPABI software for statistical analysis. The comparison between the two groups was using two sample t test, while each voxel test level was set to p < 0.001. By using single tail t test, block test level was set to p < 0.05, cluster > 100 voxels of the brain area, the difference was statistically significant. The final result was rendered by DPABI Viewer. Brainnet Viewer software was used to make brain graphics (Mingrui X, et al., 2013).
Results
MRI manifestations of rats’ brain
Bruker Biospec 7T MRI was used to examine rats in the Sham group, Control group and BMSC group on the 28th day after arterial embolization. The results showed that compared with the Sham group, the Control group rat’s coronal T2WI showed significantly high signal infarct area in the ischemic side of the rat, and the signal intensity of the infarct location increased; compared with the Control group, the T2WI of the coronal surface of the rat brain in the BMSC group showed that the signal intensity of the ischemic side brain tissue increased (Figure 1).
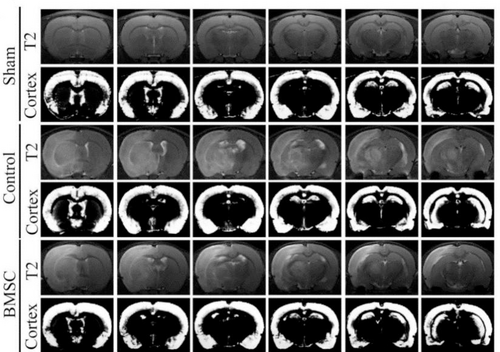
CI model was successfully produced. T2WI of the coronal surface of the CI model rat’s brain. MRI observes the area of CI. From top to bottom, they are divided into Sham group, Control group and BMSC group. From top to bottom, each group is T2WI and cortical segmentation.
3D reconstruction of rats’ brain MRI
According to the MRI results, the rats brain stereogram reconstruction was performed. The results showed that compared with the Sham group, the CI volume of the Control group increased significantly (Figure 2, p < 0.001); Compared with the Control group, 3D reconstruction showed that the CI volume of rats in the BMSC group did not change significantly (Figure 2).
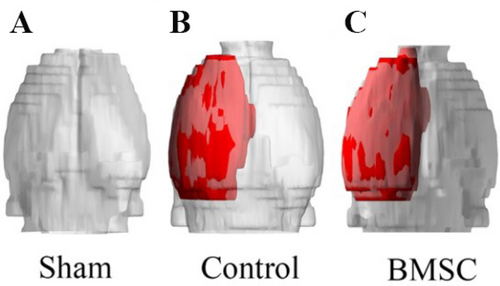
CI model rats brain 3D reconstruction image to detect rats’ brain tissue ischemic volume. (A) 3D reconstruction image of rat brain in sham operation group. (B) 3D reconstruction of rat brain in the Control group. (C) 3D reconstruction image of rat brain in BMSC group.
Effect of BMSCs injection on repairing brain infarction in rats
In order to verify the effects of BMSCs treatment on brain structure and function of rats after CI, VBM examination was performed on 28 d after CI. The results showed that:
(1) Compared with the Sham group, the total brain volume (Figure 3), grey matter volume (Figure 3) and cerebrospinal fluid volume (Figure 3) of rats in the Control group did not change significantly; the brain infarction volume significantly increased (Figure 3, p < 0.001); the white matter volume significantly reduced (Figure 3, p < 0.05).
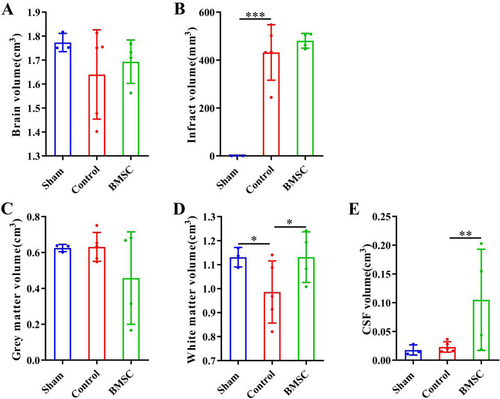
The DPABI software was used to process and analyze the VBM scan results, and conduct qualitative and quantitative analysis of rats’ brain tissue. (A) Mapping of total rat brain volume. (B) Mapping of CI volume in rats. (C) Plotting the volume of rat brain grey matter. (D) Mapping of rat brain white matter volume. (E) Mapping the volume of rat cerebrospinal fluid. * represents p < 0.05, ** represents p < 0.01, *** represents p < 0.001.
(2) Compared with the Control group, the total brain volume (Figure 3), brain infarction volume (Figure 3), and grey matter volume (Figure 3) of rats in the BMSC group did not change significantly; the white matter volume increased significantly (Figure 3, p < 0.05); the volume of cerebrospinal fluid increased significantly (Figure 3, p < 0.01).
Brain activation regions based on VBM analysis in BMSC group
Brain activation region based on VBM in two groups of rats: Compared with the Control group, the grey matter volume of BMSC group was significantly increased in the right inferior frontal gyrus, occipital fusiform gyrus, left anterior cingulate, Sub-Gyral, lingual gyrus, and Sub-lobar regions. Compared with the Control group, the grey matter volume of BMSC group significantly decreased in the right postcentral gyrus, the precentral gyrus region and the left precentral gyrus (Figure 4, Table 1, p = 0.001, α < 0.05).
| Structure | Brain | Peak MNI Coordinate | Number of Voxels | Peak Intensity | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Inferior Frontal Gyrus | R(right) | 17.5 | 17.5 | -18.75 | 583 | 10.7201 |
| Occipital Fusiform Gyrus | R | 23.75 | -50 | -11.25 | 372 | 7.3901 |
| Anterior Cingulate | L(left) | -5 | 43.75 | 5 | 442 | 10.8276 |
| Sub-Gyral | L | -31.25 | -66.25 | -3.75 | 229 | 6.5639 |
| Lingual Gyrus | L | -16.25 | -58.75 | -2.5 | 546 | 12.1244 |
| Sub-lobar | L | -31.25 | -41.25 | 1.25 | 255 | 8.8189 |
| Postcentral Gyrus | L | 55 | -17.5 | 20 | 188 | -6.7186 |
| Precentral Gyrus | L | -50 | 15 | 55 | 114 | -7.8315 |
| Precentral Gyrus | R | 48 | 10 | 56 | 100 | -7.8315 |
- MNI is Montreal coordinates.
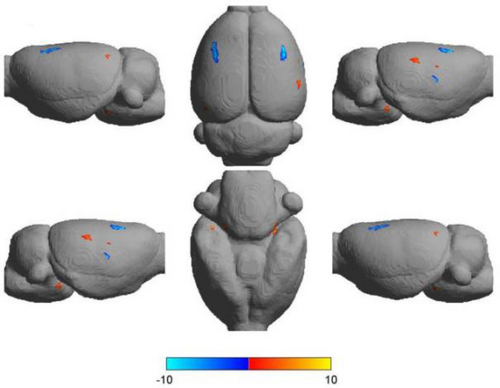
VBM analysis results of rats’ brain. The brain activation region (3D rendering map) of VBM analysis in BMSC group (p = 0.001, α < 0.05) was ranked from left to right as follows: left side, back side and right side; The lower row from left to right is: right side, ventral side, left side), blue represents atrophy, red represents increased.
Discussion
In this study, we demonstrated that the transplantation of BMSCs through the lateral ventricle was conducive to the survival and regeneration of neurons in the injured area of CI rats, and could boost the repair of damaged brain tissue and the improvement of neurological function. In addition, we also found that VBM imaging examination can sensitively observe the changes of brain tissue structure and functional recovery after ischemic injury in various regions of the brain of CI rats, indicating that VBM is an effective method for in vivo monitoring and evaluation of ischemic brain injury in vitro, and has certain value for the study of neurological diseases in clinical work.
In the present study, we established CI model by using SD rats according to previous studies (Longa EZ, et al., 1989), and used MRI to detect ischemic cerebral infarction caused by CI. As we all know, if the middle cerebral artery occlusion is not reversed in time, it will lead to local brain tissue including nerve cells, glial cells and associated fibers degeneration, necrosis or transient loss of function due to blood supply disorders (Sekerdag E, et al., 2018). Once the organization is damaged, it cannot be saved, and the individual will eventually be affected by long-term disability (Karen J, et al., 2014). Therefore, more forms of treatment are needed to improve the results (Vu Q, et al., 2014). At present, in addition to tissue-type plasminogen activator (t-PA) which can effectively treat very few patients with ischemic stroke in especial short time window, there are no other better treatment drugs for ischemic stroke. Although a lot of drugs have shown good therapeutic effects in animal experiments of ischemic brain injury, the clinical effects are not ideal.
To explore the role of BMSCs in CI, we transplanted BMSCs in the lateral ventricle of CI rats. The experimental results showed that the injection of BMSCs into the lateral ventricle of rats after CI modeling was beneficial to the repair of damaged brain tissue after ischemic cerebral infarction. Transplanted BMSCs mentioned in previous studies have the ability to differentiate into astrocytes and neurons (Yang H, et al., 2020; Xiao QX, et al., 2020). The white matter volume and cerebrospinal fluid volume of CI rats after BMSCs transplantation increased significantly. In recent years, many studies have reported that intracerebroventricular injection of BMSCs is a direct and efficient method for stroke patients, which can avoid a long circulatory system and directly affect the lesion site without crossing the blood-brain barrier, reducing the loss of stem cells and thus playing a better therapeutic role (Cui J, et al., 2017; Shichinohe H, et al., 2017). Evidence suggested that BMSCs can migrate and secrete various cytokines and growth factors (Lotfy A, et al., 2014; Kallmeyer K, et al. 2020; Zakaria DM, et al., 2021). In addition, these characteristics have immune regulation, angiogenesis, anti-inflammatory and anti-apoptotic effects, also help to regulate various acute and chronic pathological states. These mechanisms make BMSCs therapy play an important role in the repair of blood-brain barrier after ischemic stroke (Li Z, et al., 2019; Cunningham CJ, et al. 2018; Zhao XM, et al. 2019; Zachar L, et al., 2016). Thus, combined with our research results, all these mentioned studies suggested that BMSCs can promote the repair of brain tissue structure and function after CI in rats.
In this study, we also confirmed that BMSCs could promote the recovery of injured brain tissue structure and function after CI by using MRI combined with VBM imaging technology. Our results showed that the volume of brain grey matter, white matter and cerebrospinal fluid in CI rats after BMSCs transplantation all significantly increased, and the recovery of sensory and motor function of injured brain tissue was significantly better than that of the control group, indicating that MRI and VBM can be used to detect and quantify the recovery of brain tissue and function of CI rats after BMSCs transplantation. Previous studies have shown that CI not only causes changes in local grey matter structure, but also usually leads to the damage of white matter fiber bundles related to it (Ingo C, et al., 2020; Zuo LJ, et al., 2018). Currently, the mechanism and efficacy evaluation of BMSCs in repairing cerebral ischemic injury are mostly based on in vitro animal experiments or clinical observation, which cannot dynamically monitor the condition or treatment of patients, so its clinical application is limited. In addition, with the development of neuroimaging technology, the use of MRI dynamic, non-invasive observation of cerebral ischemic injury area changes in brain function and efficacy evaluation of the study opened up a new way to study the evolution of CI (Wang D, et al. 2020; Nakajo Y, et al. 2019). VBM, fMRI, DTI and other new technologies have been rapidly developed and popularized in recent years, which provide a powerful means to explore the changes of brain structure and function caused by CI (Wesley UV, et al., 2019; Hao XZ, et al., 2017; Mastropietro A, et al. 2019). VBM is an overall analysis of high-resolution anatomical images of the whole brain at voxel level (Wang X, et al. 2021; Chen G, et al. 2018). Taking the brain as a whole, the differences of brain anatomical structure among different subjects were analyzed (Mu SH, et al., 2017; Chen ZY, et al. 2018). By analyzing the changes of brain parenchymal density, the changes of local grey matter volume, white matter volume and brain tissue density were quantitatively calculated. In addition, since the region of interest does not need to be manually set and has high objectivity and accuracy, it is often used to study brain structural changes caused by central nervous system diseases (Fujimoto H, et al., 2017; Steinke J, et al. 2017). In our study, such new technologies were used to confirm the role of BMSCs in CI rats.
However, the present study has limitations. The main limitation of the study is that only MRI results are used to determine whether the CI model is successful, but no further behavioral score and histopathological examination of the animal model are confirmed. This study is a horizontal study without observing the dynamic evolution of brain structure and function in CI rats. Therefore, it is necessary to conduct longitudinal studies in future studies to increase the understanding of CI injury and recovery mechanism. The concentration of transplanted BMSCs was not tracked. Whether the concentration of BMSCs in different brain injury areas occurred variedly still needs further study.
In conclusion, our study shows that the transplantation of BMSCs in lateral ventricle is beneficial to the repair of damaged brain tissue structure and function after ischemic cerebral infarction. Moreover, VBM combined with MRI technology can realize detection of brain injury level of ischemic CI and evaluation of structural and functional recovery of injured brain tissue. This examination method is expected to be applied to the evaluation of curative effect of brain structural and functional recovery of ischemic injury area after stroke.
Ethical statement
The animal study protocol was legally approved by the Animal Care & Welfare Committee of Kunming Medical University (Approval No: kmmu2021534). All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Acknowledgements
We would like to thank Professor Ting-Hua Wang of Institute of Neurobiological Disease, West China Hospital, Sichuan University for the technical support.
Conflict of interest
There is no conflict of interest in this study.
Funding
This study was supported by grant from the National Natural Science Foundation of China (Grant Nos. 82001604, 82060243 and 81960214) and Joint Fund of Zunyi Science and Technology Bureau-Affiliated Hospital of Zunyi Medical University (No. HZ2020250).
Transparency statement
All the authors affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Nan Zhao and contributed the central idea; Nan Zhao, Yu-Hang Zhu and Rui-Ze Niu and designed the experiments; Chang-Yin Yu contributed to refining the ideas, carrying out additional analyses and finalizing this paper.




