Effects of Medical or Surgical Castration on Erectile Function in an Animal Model
Abstract
ABSTRACT: The goal of this study was to investigate the effects of medical castration (luteinizing hormone-receptor hormone [LH-RH] agonist treatment) or surgical castration on erectile function in an animal model. New Zealand White male rabbits were either kept intact (control); surgically orchiectomized; or treated for 2, 4, or 8 weeks with the LH-RH agonist leuprolide acetate (107 μg/kg/mo). At 2 weeks, plasma testosterone levels of orchiectomized and leuprolide acetate—treated animals were 12.8% and 57.4% of intact control animals, respectively. Erectile function was assessed by continuously recording systemic arterial pressure (SAP) and intracavernosal blood pressure (ICP) and determining the ICP:SAP ratios in response to electrical stimulation of the pelvic nerve at varying frequencies (2.5–32 Hz). Androgen deprivation by surgical (orchiectomy) or medical (leuprolide acetate) castration reduced ICP at all frequencies tested but did not alter SAP. Administration of the phosphodiesterase type 5 inhibitor vardenafil (10 μg/kg) did not enhance ICP in surgically orchiectomized or leuprolide acetate—treated animals. Nitric oxide synthase and arginase activities in the corpus cavernosum were not significantly altered by surgical or medical castration. Further, Masson trichrome staining of erectile tissue from androgen-ablated animals showed a reduction in smooth muscle content. These data demonstrate that androgen deprivation achieved by surgical or medical castration adversely affects penile hemodynamics and erectile function without producing significant changes in the activities of nitric oxide synthase or arginase. We conclude that androgen deprivation produces structural alterations in the corpus cavernosum leading to corporal veno-occlusive dysfunction.
Androgens play an important functional role in the development, growth, and maintenance of male secondary sexual characteristics (Dorfman and Shipley, 1956). However, the physiological role of androgens in male sexual function and the pathophysiological role of androgen deprivation in male sexual dysfunction remains controversial. Recent studies have proposed a direct relationship between free testosterone and corporal veno-occlusive hemodynamic integrity in men with erectile dysfunction (Aversa et al, 2000; Becker et al, 2000).
Several clinical studies have demonstrated that androgen deprivation via administration of a luteinizing hormone-releasing hormone (LH-RH) agonist is strongly associated with erectile dysfunction (Peters and Walsh, 1987; Rousseau et al, 1988; Eri et al, 1994; Marumo et al, 1999). However, there is a lack of understanding of the mechanisms of androgen modulation of male erectile function. Thus, the goal of this study was to investigate the effects of androgen deprivation via medical (LH-RH agonist administration) or surgical castration on erectile function in a rabbit model. We assessed biochemical and histological changes in penile erectile tissue, as well as in vivo hemodynamic parameters.
Materials and Methods
Surgical and Medical Castration of Animals
The Institutional Animal Care and Use Committee of the Boston University School of Medicine approved this study. New Zealand White male rabbits (4.5–5.0 kg) were divided into 5 groups. One group was kept intact (control; n = 4). Another group of animals (n = 4) was anesthetized with an i.m. injection of ketamine (35 mg/kg) and xylazine (5 mg/kg) and underwent bilateral castration through 3-cm scrotal incisions. Erectile function was assessed 2 weeks after surgery in orchiectomized animals. The 3 other groups of animals were administered the LH-RH agonist leuprolide acetate (107 μg/kg/mo) for 2 weeks (n = 4), 4 weeks (n = 5), or 8 weeks (n = 5) before assessment of erectile function. The dose of leuprolide acetate was determined from the conventional dosing regimen for patients with prostate cancer and normalized to mean body weight.
Measurement of Plasma Testosterone
Blood samples were drawn before orchiectomy or leuprolide acetate treatment and then again on the day of erectile function assessment. Plasma was processed from each sample, extracted with ether, and used in a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Assay Designs, Ann Arbor, Mich).
Measurements of Systemic Arterial and Intracavernosal Blood Pressure
Animals were anesthetized with an i.m. injection of ketamine (35 mg/kg) and xylazine (5 mg/kg). Anesthesia was maintained with 0.2-mL i.v. bolus injection of pentobarbital (25 mg/mL) as needed. A 20-gauge angiocatheter was placed into the carotid artery for measurement of systemic arterial blood pressure (SAP). A 21-gauge minicatheter was placed near the base of the penis for measurement of intracavernosal pressure (ICP). A midline abdominal incision was made to expose the perivesical space. The internal pudendal artery was identified and the distal branch to the prostate, bladder neck, and cavernosal bodies was localized. The cavernosal nerve bears relation to the cavernosal artery on the postero-lateral surface of the prostate. Using platinum wire electrodes, we electrically stimulated the cavernosal nerve at varying frequencies (2.5–32 Hz) with a train of square waves at 10 V and a pulse width of 0.8 milliseconds for a total duration of 30 seconds. Animals subjected to orchiectomy or 8 weeks of leuprolide acetate treatment were administered the selective phosphodiesterase type 5 (PDE 5) inhibitor vardenafil (10 μg/kg i.v.; Choi et al, 2002) and pelvic nerve stimulation was repeated after 20 minutes. At the end of the protocol, animals were killed by i.v. administration of sodium pentobarbital (50 mg/kg), the penis was removed, and the cavernosal bodies were dissected out. A portion of the cavernosal tissue was fixed in 10% formalin buffered with 75 mM phosphate for histology, and the rest was frozen in liquid nitrogen for biochemical assay.
Preparation of Tissue Extracts
Penile cavernosal tissues from different animals within each group were pooled and pulverized. The resulting tissue powder was combined 1:4 (wt:vol) with ice-cold 20 mM HEPES buffer pH 7.4 containing 0.25 M sucrose and protease inhibitors (Sigma Chemical Company, St Louis, Mo). The mixture was homogenized on ice with a Brinkmann PT3000 polytron and the homogenate was centrifuged at 800 × g for 20 min at 4°C. The supernatant was used for enzyme assays, as described below. Soluble protein concentration was determined by the Lowry assay.
Determination of Nitric Oxide Synthase Activity
Nitric oxide synthase (NOS) activity in the total tissue extract was determined by conversion of l-[14C(U)]arginine (313 mCi/mmol; NEN Life Science Products, Boston, Mass) to [14C]citrulline and nitric oxide (NO), as previously described (Kim et al, 1993; Traish et al, 1999). Briefly, aliquots of the tissue extract were incubated with tetrahydrobiopterin (3 μM), calmodulin (30 units/mL), l-arginine (50 μM), l-[14C]arginine (2 μCi/mL), reduced nicotinamide adenine dinucleotide phosphate (NADPH; 20 mM) and calcium chloride (1 mM) at 37°C for 45 minutes. Parallel samples were incubated at 2°C. Citrulline was separated from arginine by ion exchange columns (1 mL) of AG50W-X8 resin (Bio-Rad Laboratories, Hercules, Calif) and quantified by scintillation counting of radioactivity. Enzymatic activity was normalized to total soluble protein in the tissue extract. Unless specified, all reagents were purchased from Sigma.
Determination of Arginase Activity
Crude tissue extracts were prepared from homogenates of rabbit corpus cavernosum tissue as described above. Arginase enzyme activity in cytosolic extracts was assessed by the Rüegg and Russell method as previously described by Kim et al (2001). Briefly, 10 μL of tissue extract (triplicate aliquots) were incubated in buffer (75 mM glycine pH 9.0, 0.25 mM MnCl2) containing 300 000 disintegrations per minute of [14C-guanidino]-l-arginine (51.5 mCi/mmol; NEN Life Science), 4 mM unlabelled l-arginine in a final volume of 100 μL. Samples were incubated for 60 minutes at 37°C and reactions were terminated by the addition of 400 μL of 0.25 M acetic acid pH 4.5, 7 M urea, and 10 mM l-arginine. After the addition of 500 μL of water, samples were passed through a 0.5-mL column of Dowex 50W-X8 resin (Bio-Rad, Hercules, Calif). Tubes were rinsed twice with 500 μL of water and both rinses were poured onto the columns. Columns were washed with an additional 1 mL of water and all effluent was collected in 20-mL vials. After the addition of 16 mL of Liquiscint (National Diagnostics, Atlanta, Ga), radioactivity was quantified by liquid scintillation spectroscopy. Urea production (pmol/min) was normalized to total soluble protein in the tissue.
Masson Trichrome Staining of Tissue Sections
Fixed tissues were embedded in paraffin, sectioned (6 μm), and placed on Colorfrost Plus glass slides (Fisher Scientific, Pittsburgh, Pa). Tissue sections were deparaffined with CitriSolv (Fisher Scientific) and rehydrated in graded ethanol solutions (100%–70%). Sections were then placed in Bouin fixative for 1 hour at room temperature, transferred to 4% ferric ammonium sulfate for 5 minutes at 50°C, and rapidly rinsed with distilled water at 50°C. Sections were stained with 1% hematoxylin at 50°C for 30–60 seconds and destained in 2% ferric ammonium sulfate at room temperature until only nuclei retained stain. After washing in running water for 10 minutes, slides were immersed in 0.1% acid fuchsin for 1 minute and gently rinsed by repeatedly immersing in water 5 times. The slides were then placed in 1% phosphomolybdic acid for 10 minutes and then stained for 90 seconds in 0.25% aniline blue/0.5% phosphomolybdic acid. The slides were washed in water until the rinses became clear and then dehydrated in graded ethanol, cleared with CitriSolv, and coverslipped using Permount (Fisher Scientific). Images of tissue sections at 100× were captured with a digital camera.
Data analysis
Plasma testosterone values did not change between 2 and 8 weeks after leuprolide acetate treatment. Thus, plasma testosterone data from leuprolide acetate—treated animals were combined. Testosterone data for both surgically and medically castrated animals were analyzed by paired t-test. All other data were analyzed by analysis of variance (ANOVA). If the ANOVA P value was <.05, multiple paired comparisons were made using the Tukey-Kramer test. Paired comparisons were considered to be significantly different if P ≤ .05.
Results
Effect of Orchiectomy or Leuprolide Acetate Treatment on Plasma Testosterone Levels
At 2 weeks, plasma testosterone in orchiectomized animals decreased 87%, relative to the testosterone concentration measured before orchiectomy (Fig. 1A). Leuprolide acetate treatment also significantly decreased testosterone levels 2 weeks after administration (Fig. 1B). This decrease was maintained through 8 weeks. However, unlike orchiectomy, leuprolide acetate reduced plasma testosterone by approximately 43%.
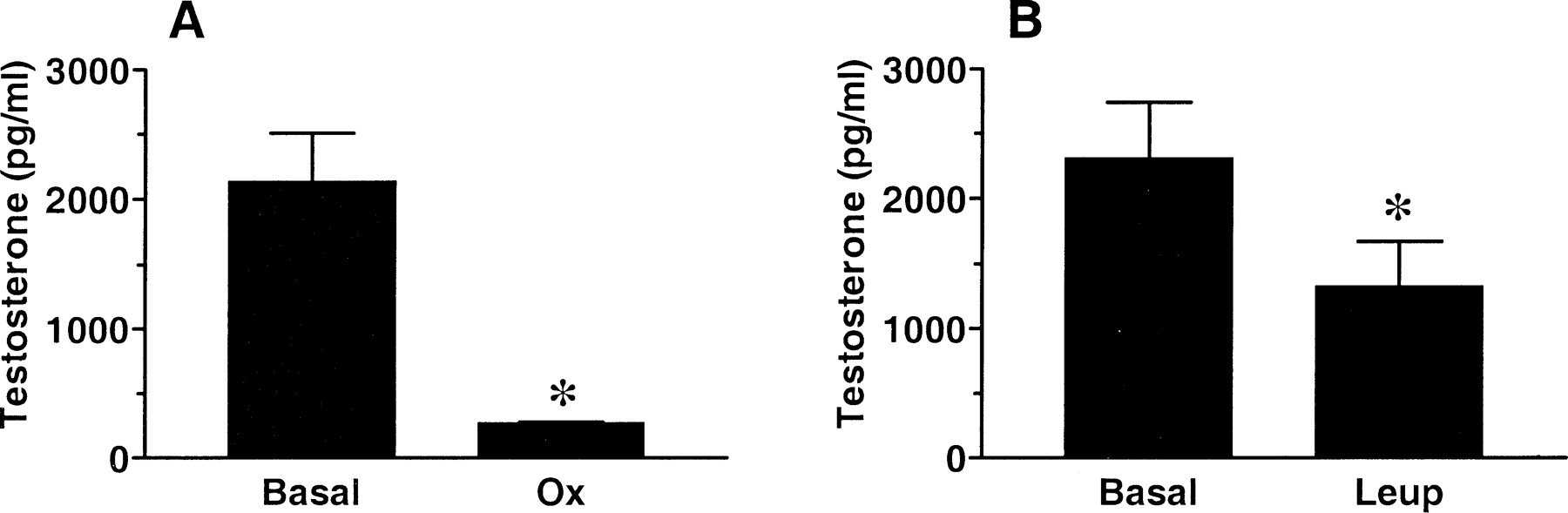
. Effects of orchiectomy and LH-RH agonist treatment on plasma testosterone. Blood samples were drawn from rabbits before surgical or medical androgen ablation (basal) and then again at 2, 4, or 8 weeks after orchiectomy (Ox) or initial leuprolide acetate administration (Leup). Testosterone concentration in plasma was determined by a commercially available ELISA kit. Because testosterone values remained stable between 2 and 8 weeks after leuprolide acetate administration, all data from the leuprolide acetate groups were combined. Data are means ± SEM and were analyzed by paired t-test (*P < .05).
Effect of Androgen Deprivation on Pelvic Nerve-Stimulated Penile Erection
A frequency response increase in ICP:SAP ratio was observed in intact animals with ICP:SAP values increasing from 0.48 at 2.5 Hz to 0.8 at 32 Hz. A marked decrease in ICP:SAP was observed in surgically orchiectomized animals (Fig. 2). Similarly, LH-RH agonist treatment for 2, 4, or 8 weeks markedly reduced the ICP:SAP ratio (Fig. 2). The decrease in ICP:SAP ratio was significantly pronounced at the lower frequencies. After 8 weeks of LH-RH agonist treatment, the ICP:SAP ratio was similar to that obtained with surgical castration. Androgen deprivation via surgical or medical ablation did not result in significant changes in systemic systolic and diastolic blood pressure in all animal treatment groups.

. Effects of androgen deprivation on nerve-stimulated penile erection. SAP and ICP were continuously recorded in anesthetized male rabbits that were intact (controls, n = 4), orchiectomized (Ox, n = 4), or treated with leuprolide acetate for 2 weeks (L2, n = 4), 4 weeks (L4, n = 5), or 8 weeks (L8, n = 5). The maximum rise in ICP was determined at the indicated frequencies of pelvic nerve stimulation and normalized to SAP. Data are means ± SEM (*P < .05 compared with controls).
Effect of PDE Type 5 Inhibitor in Androgen-Deprived Animals
As shown in Figure 3, i.v. administration of the selective PDE type 5 inhibitor vardenafil (10 μg/kg) to surgically castrated or LH-RH agonist—treated animals did not increase ICP:SAP values to those observed in control animals. On average, the SAP decreased 6.9 ± 0.9 mm Hg after vardenafil administration.

. Effects of PDE 5 inhibitor on nerve-stimulated penile erection in androgen-deprived animals. SAP and ICP were continuously recorded in anesthetized male rabbits that were intact (controls, n = 4), orchiectomized (Ox, n = 4), or treated with leuprolide acetate for 8 weeks (L8, n = 5). The maximum rise in ICP was determined at the indicated frequencies of pelvic nerve stimulation and normalized to SAP. Nerve stimulation was repeated 20 minutes after i.v. administration of the PDE 5 inhibitor vardenafil (Var, 10 μg/kg). Data are means ± SEM.
Effects of Androgen Deprivation on NOS and Arginase Activity in Rabbit Corpus Cavernosum
Measurement of total NOS activity in corpus cavernosum cytosol from castrated animals showed no significant change in NOS activity compared with that from intact or LH-RH agonist-treated animals (Fig. 4A). Total arginase activity in corpus cavernosum extract from castrated animals showed no significant change compared to cytosol from intact or LH-RH agonist-treated animals (Fig. 4B).
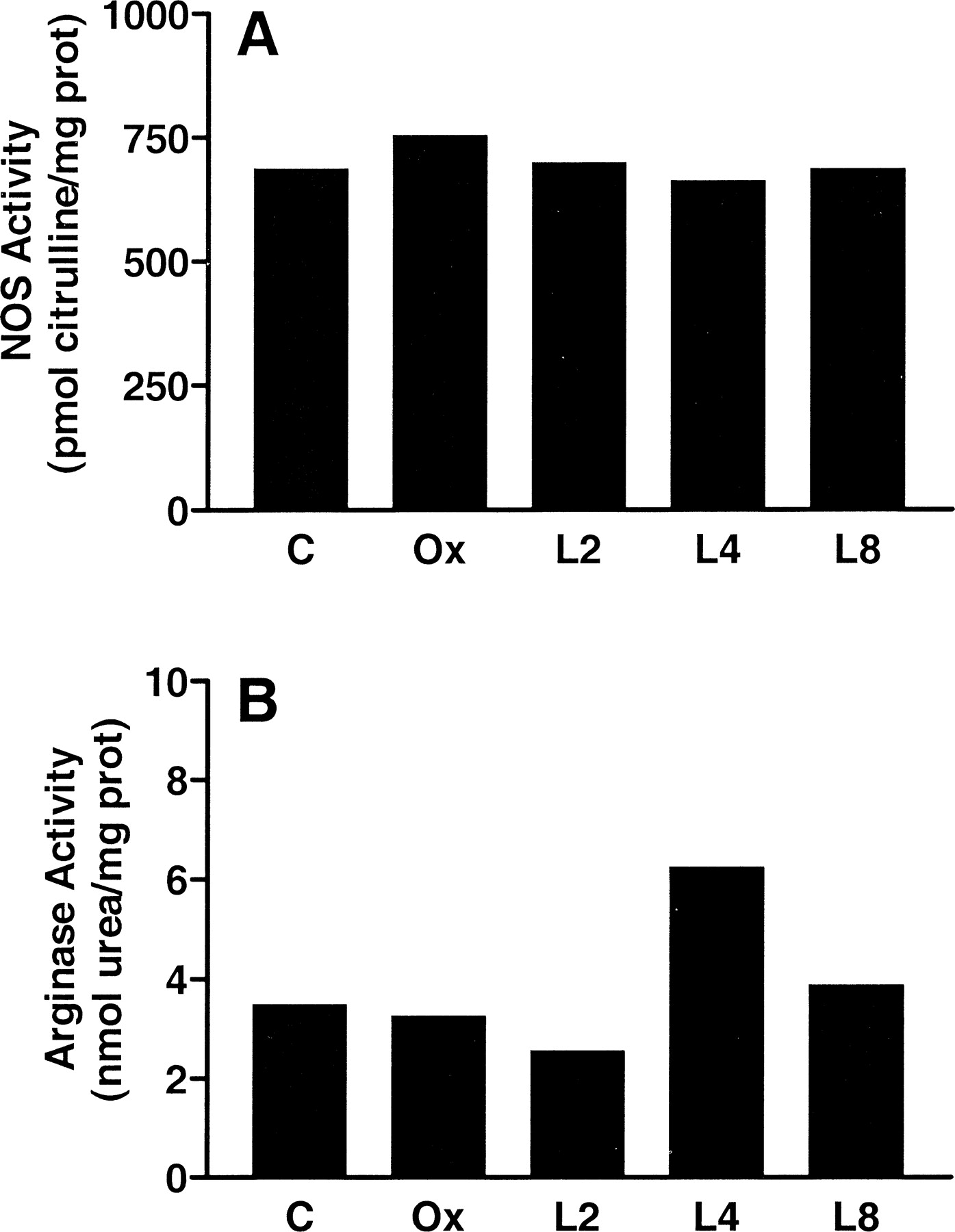
. Effects of androgen deprivation on total NOS and arginase activity in the corpus cavernosum. Total NOS activity was determined by formation of L-citrulline from L-arginine as described in Materials and Methods. Penile cavernosal tissue derived from each treatment group (C indicates intact control animals; Ox, orchiectomized; L2, 2 weeks of leuprolide acetate; L4, 4 weeks of leuprolide acetate; L8, 8 weeks of leuprolide acetate) was pooled and homogenized. The resulting tissue extracts were assayed for NOS or arginase activity. All data were normalized to total soluble protein and are the mean of 2 independent experiments.
Effects of Androgen Deprivation on Structure of Rabbit Corpus Cavernosum
Tissue sections from control animals typically exhibited abundant areas of dense trabecular smooth muscle. Androgen ablation (medical or surgical) resulted in reduced trabecular smooth muscle content and increased connective tissue, as determined by Masson trichrome staining (Fig. 5). Trabecular smooth muscle bundles appeared thinner and less organized.
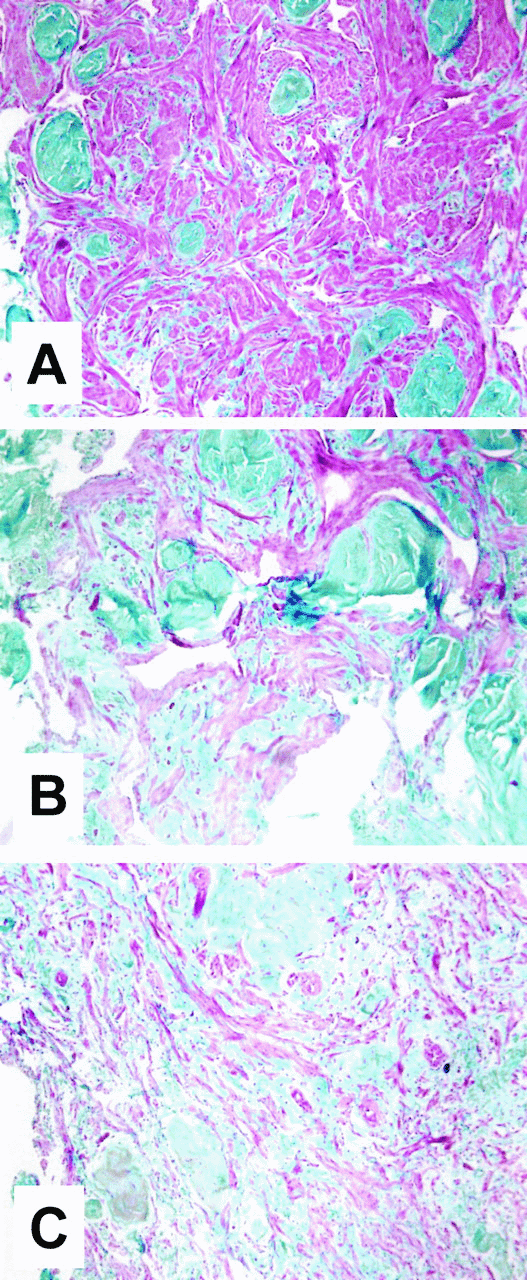
. Effects of androgen deprivation on trabecular smooth muscle and connective tissue content in the corpus cavernosum. Corpus cavernosum tissue from the various treatment groups was fixed, paraffin-embedded, sectioned (6 μm), and subjected to Masson trichrome staining procedure. Representative sections from the following groups of animals are shown: intact control (A), orchiectomy at 2 weeks (B), and leuprolide acetate at 8 weeks (C). All fields are at 100× magnification.
Discussion
Several clinical studies have indicated that 80%–100% of patients undergoing androgen deprivation via LH-RH agonist administration suffer complete or partial loss of erectile function (Peters and Walsh, 1987; Rousseau et al, 1988; Eri et al, 1994; Marumo et al, 1999). However, other studies indicated that LH-RH agonist treatment resulted in a minimal loss of erectile function (Rhoden et al, 2002a,b). Aversa et al (2000) recently reported that men with vasculogenic erectile dysfunction have lower levels of free testosterone than men with psychogenic erectile dysfunction. Further, free testosterone levels were correlated with erectile function independent of age. To date, no studies in an animal model have investigated the effects of medical castration on the physiological, biochemical, and hemodynamic mechanisms that regulate erectile function, including corpus cavernosum tissue structure and function.
Our findings suggest that androgen deprivation, irrespective of the method of androgen ablation, reduced erectile function. Although plasma testosterone concentration in medically castrated rabbits at 8 weeks was five-fold greater than in surgically castrated animals at 2 weeks, the attenuation of erectile function was similar in magnitude in both groups. The inability of leuprolide acetate treatment to reduce plasma testosterone to castrate levels may be due to differences in efficacy of the drug in rabbits versus humans. It is also likely that the effects of this LH-RH agonist cannot be linearly extrapolated based solely on body weight. Nevertheless, it is interesting to note that absolute or severe reduction in plasma testosterone need not occur for diminishment of erectile function. Since medically castrated animals did not exhibit a statistically significant reduction in erectile function until 8 weeks after LH-RH agonist treatment, the detrimental effects of androgen deprivation on erectile function appear to be cumulative over time.
The ICP reduction in androgen-deprived animals may be the result of either alterations in the synthesis and release of neurotransmitters, smooth muscle responsiveness to neurotransmitters, or the fibroelastic properties of the corpus cavernosum. Several studies using a rat model have reported that androgen deprivation results in reduction of NOS expression and activity (Chamness et al, 1995; Garban et al, 1995; Lugg et al, 1995; Zvara et al, 1995; Penson et al, 1996; Lugg et al, 1996; Schirar et al, 1997). However, these observations were not confirmed in rabbit corpus cavernosum (Holmquist et al, 1994), suggesting species differences in NOS regulation by steroid hormones. Our data also indicate no significant changes in NOS activity in cavernosal tissue from androgen-deprived animals with either medical or surgical castration. Furthermore, we did not observe any restoration of erectile function after administration of a potent PDE 5 inhibitor in surgically or medically castrated animals, suggesting that the NO pathway may not be significantly altered in androgen-deprived animals. Because we used only a single dose of vardenafil in this study, we cannot rule out the possibility that higher concentrations of PDE 5 inhibitor may have enhanced erectile function in androgen-deprived animals. However, as previously reported, the 10 μg/kg dose of vardenafil significantly enhanced erectile function in normal rabbits (Choi et al, 2002).
We further investigated whether androgens may limit the availability of the NOS substrate l-arginine by measuring the activity of arginase (Cox et al, 1999; Kim et al, 2001). Because we did not observe any significant change in arginase activity, it is unlikely that androgens modulate the substrate availability of l-arginine. Based on these data, as well as previous findings (Traish et al, 1999), we suggest that the compromised erectile function in medically and surgically castrated rabbits may be attributed to either reduced smooth muscle cell responsiveness or altered tissue composition. The veno-occlusive mechanism is critical for attaining and maintaining penile rigidity and is dependent on the integrity of neural, vascular, and endocrine components, as well as the fibroelastic properties of the cavernosal tissue (Nehra et al, 1996, 1998).
Mills et al (1998) suggested that androgen deprivation alters penile blood outflow in rats, resulting in reduced erectile function (reduced veno-occlusion). Clinical and animal studies have suggested that veno-occlusion is modulated by the balance between the smooth muscle and connective tissue content of the corpus cavernosum (Nehra et al, 1996, 1998; Moreland, 1998). It has been hypothesized that androgen deprivation may produce tissue atrophy and trabecular smooth muscle death, causing an imbalance in the ratio between smooth muscle and extracellular matrix, leading to veno-occlusive dysfunction. Previously, we have shown that androgen deprivation by surgical castration resulted in a significant decrease in trabecular smooth muscle content (Traish et al, 1999). Both smooth muscle content and erectile function were restored by testosterone treatment. The current study confirms that androgen ablation by medical or surgical castration results in changes in smooth muscle content and tissue atrophy.
Although a number of reports have clearly suggested that LH-RH treatment of patients with prostate cancer results in erectile dysfunction in the majority of patients, no studies have documented changes in physiological and biochemical parameters by LH-RH agonists in erectile tissue. Our study provides data indicating that LH-RH agonist treatment results in compromised erectile function due to altered cavernosal tissue structure. The results of the present study support the clinical observation that erectile dysfunction occurs secondary to LH-RH agonist treatment in patients with prostate cancer. Furthermore, because we did not observe any changes in NOS activity or improvement of erectile function with PDE 5 inhibitor administration, it is unlikely that PDE 5 inhibitors will be useful in patients with androgen insufficiency.
The effect of androgens on erectile function is complex. Androgens influence both the central and peripheral nervous system and penile erectile tissue structure and function. We suggest that in corpus cavernosum, androgens affect smooth muscle cell growth, connective tissue metabolism, and smooth muscle reactivity.




