Effects of transcranial direct current stimulation on pain perception and working memory
Funding sources:
This study was supported by a research grant of the Prof. Schmidtmann Foundation in Marburg.
Conflicts of interest:
None declared.
Abstract
Previous studies have shown that non-invasive stimulation of the dorsolateral prefrontal cortex (DLPFC) could modulate experimentally induced pain and working memory (WM) in healthy subjects. However, the two aspects have never been assessed concomitantly. The present study was set up to investigate the effects of transcranial direct current stimulation (tDCS) of the DLPFC on thermal pain and WM in the same population of healthy volunteers. In a randomized and balanced order of different sessions separated by 1 week, 20 min of 2 mA anodal, cathodal or sham tDCS were applied to the left or right DLPFC in two separate experiments. Twelve healthy volunteers were enrolled for each stimulated hemisphere. Warm and cold detection thresholds, heat and cold pain thresholds as well as heat pain tolerance thresholds were measured before, during and following tDCS. WM was assessed by a 2-back task applied once during cortical stimulation. Anodal tDCS of the right DLPFC led to an increase of tolerance to heat pain. The 2-back task revealed fewer outliers during cathodal tDCS of the left DLPFC. The present data show an involvement of the DLPFC in the processing of pain and WM. There was no correlation between these findings, suggesting that the analgesic effects of cortical stimulation are not associated with cognitive processing. However, this conclusion is difficult to affirm because of some limitations of the study regarding the parameters of stimulation or a ceiling effect of the 2-back task for instance.
1. Introduction
The cognitive component of pain represents the integration of different components within the psychosocial context, including anticipation, attention, long-term memory and working memory (WM). Related neural processes involve a cortical network through the frontal and parietal cortices (Apkarian et al., 2005; Tracey and Mantyh, 2007). For instance, the parietal primary sensory cortex (S1) contributes to short-term memory of sensory information (Harris et al., 2002). Regarding pain-related WM, the dorsolateral prefrontal cortex (DLPFC) plays a major role (Albanese et al., 2007). A positron emission tomography (PET) study led to the hypothesis that the perceived pain is modulated via descending inhibitory systems originating in the DLPFC (Lorenz et al., 2003). This study showed that DLFPC activation correlates negatively with perceived pain, in association with midbrain activation for the left DLPFC and anterior insular cortex activation for the right DLPFC (Lorenz et al., 2003).
Cortical function can be transiently influenced by non-invasive cortical stimulation. Cathodal transcranial direct current stimulation (tDCS) and low-frequency repetitive transcranial magnetic stimulation (rTMS) decrease cortical excitability, whereas anodal tDCS and high-frequency rTMS increase cortical excitability, at least when applied to the primary motor cortex (M1) in healthy subjects (Chen et al., 1997; Maeda et al., 2000; Nitsche and Paulus, 2000). In addition, both stimulation techniques were found to modify regional cortical blood flow of distant cortical areas connected to the stimulation site, depending on the location of the cortical target and on the parameters of stimulation (Lang et al., 2005; Knoch et al., 2006).
Previous studies performed in healthy volunteers showed that DLPFC could be a valuable target for non-invasive cortical stimulation to modulate either WM or experimentally induced pain. First, anodal tDCS over the left DLPFC was found to ameliorate WM function (Fregni et al., 2005; Ohn et al., 2008) or to reduce electrically induced pain (Boggio et al., 2008). Second, low- or high-frequency rTMS delivered to the right or left DLPFC was found to reduce cold- or heat-induced pain (Graff-Guerrero et al., 2005; Borckardt et al., 2007).
Under the assumption that cognitive function influences pain perception as shown in dementia as well as in experimental studies (Willer et al., 1979; Terkelsen et al., 2004; Kunz et al., 2009), we aimed to test whether tDCS of the DLPFC could influence the perception of experimentally induced thermal pain and verbal WM. In a parallel group design, cold and warm/heat detection, pain and tolerance thresholds were assessed before, during and after a 20-min session of 2 mA anodal, cathodal or sham tDCS delivered to the contralateral right or left DLPFC. The effects on verbal WM were assessed by using a number-based 2-back task applied during cortical stimulation.
2. Materials and methods
2.1 Subjects
Twenty-six healthy volunteers were recruited. Among them, two participants were withdrawn from the study after the first stimulation due to transient mild headache. One subject presented holocephal transient headache following cathodal tDCS of the right DLPFC, while the other subject developed headache following sham tDCS of the left DLPFC. Thus, 24 subjects (12 for each side of stimulation; left DLPFC stimulation: female: 6, male: 6; right DLPFC stimulation: female: 10, male: 2) between the age of 20 and 25 years [mean (years) ± SE; left DLPFC stimulation: 25.1 ± 3.4; right DLPFC stimulation: 23.5 ± 3.7] completed the protocol. The sample size was chosen according to previous investigations showing significant effects of tDCS/rTMS on either experimental pain or WM with similar group sizes (Fregni et al., 2005; Graff-Guerrero et al., 2005). All participants were right-handed according to the Edinburgh handedness inventory (Oldfield, 1971). None of the subjects had taken regular medication, especially no analgesic medication for at least 24 h prior to the sessions. Furthermore, patients suffering from chronic pain, neurological or psychiatric disorders, as well as subjects with cranial metal implantations or former brain surgery, were excluded from participation. All subjects gave written informed consent. The study protocol was approved by the local institutional review board.
2.2 Threshold determination
Thermal thresholds were measured using a Peltier-based contact stimulation device (Medoc TSA-2001, Ramat Yishai, Israel) with a 30 × 46 mm2 contact thermode applied to the forearm contralateral to the stimulated hemisphere. Warm and cold sensation thresholds (WSTs and CSTs) were measured from a baseline neutral temperature of 32 °C, whereas heat pain, cold pain and heat pain tolerance thresholds (HPTs, CPTs and HPTTs) were measured from a baseline temperature of 35 °C according to a previous study (Lautenbacher et al., 2005). Thresholds were determined from gradual temperature change at a linear rate of 0.7 °C/s, by using the method of limits (Djaldetti et al., 2004) described as follows. The participants were instructed to indicate the level at which the sensation of warmth and cold starts by tapping a mouse key. The same procedure was then performed for the level at which warmth or cold turned into pain, and for the level at which the tolerance to heat pain was reached. Three trials were performed at the beginning of the session to familiarize the subjects with the procedure and then five trials were performed for each threshold determination (means were calculated for analyses) before, during and following cortical stimulation (HPTT only included three trials). Upper and lower limits for CPT and HPT/HPTT were fixed at 0 °C and 54 °C, respectively. The participants were allowed to discontinue the measurements at each time of the experiment as stated in the information letter.
2.3 Assessment of working memory
A 2-back single-number-based task was employed for the assessment of WM in an ‘online’ paradigm. Subjects were presented with a pseudo-random set of 10 numbers (0–9). The stimuli were generated using the TAP software version 2.0 (Zimmermann and Fimm, 1995). Each number was displayed on a computer monitor for 30 ms. A different number was displayed every 3 s. White numbers were presented on a black background. Subjects were required to respond (press key) if the presented number was the same as the number presented two stimuli previously. A total of 15 correct responses within a reaction time of 2 s were possible (total numbers: 100). If the reaction occurred after this time window, the response was counted as omission. In addition, outliers were defined according to the mean and standard deviation of the reaction time of each trial (>2.35 × standard deviation above the mean reaction time). A trial session was performed with a total of three correct responses (total numbers: 20) before the beginning of the investigation. Reaction time, errors, outliers and omissions were measured and transferred to the statistical analysis software for further calculations.
2.4 tDCS
Direct current was delivered by using a saline-soaked pair of surface sponge electrodes with a surface of 35 cm2, and applied using a battery-driven, constant current stimulator (Schneider Electronic, Gleichen, Germany) with a maximum output of 10 mA. The DLPFC was stimulated with the anode (cathode) placed over F3 or F4 according to the International 10–20 EEG system for electrode placement and the cathode (anode) over the contralateral supra-orbital area as previously performed (Fregni et al., 2005). One neuroimaging study has demonstrated that F3 and F4 were correctly localized over the DLPFC (Herwig et al., 2003). Stimulation intensity was set at 2 mA for 20 min according to a previous study on chronic pain, while another study showed that an intensity of 2 mA was more effective than 1 mA to modulate WM (Boggio et al., 2006; Fregni et al., 2006b). The sham procedure was performed as usual, by turning off the stimulator after the subjects felt the initial tingling sensation for 5 s (Nitsche et al., 2003).
2.5 Procedure (Fig. 1)
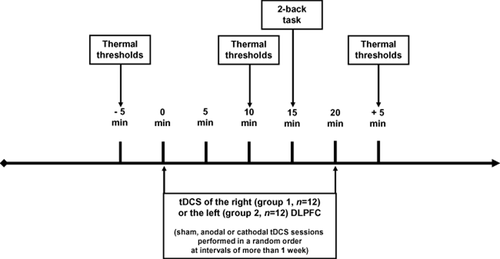
Experimental design: assessment of thermal thresholds and working memory (2-back task) before, during and after transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC).
Experiments were performed in a quiet and normally illuminated room. The volunteers were seated in a comfortable armchair. Thermal thresholds were first measured 5 min before, and then 10 min after tDCS onset. The 2-back task was performed 15 min after tDCS onset for up to 5 min. Finally, thermal thresholds were measured again for 5 min after the period of 20-min tDCS. Two groups of 12 subjects were assessed in parallel, the first group being stimulated over the right DLPFC and the second one over the left DLPFC. In both groups, three different sessions of cortical stimulation (sham, anodal and cathodal tDCS) were performed in a balanced and randomized order separated by an interval of 1 week. In all cases, thermal thresholds were measured at the forearm contralateral to the stimulated hemisphere.
2.6 Statistical analysis
The Statistic Package for Social Science version 15 (IBM SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Since the Kolmogorov–Smirnov test revealed that some parameters had not a normal distribution, we performed non-parametric tests. The Friedman test for repeated measures comparisons was used in each group (right or left DLPFC stimulation) to study the influence of stimulation condition (sham, anodal and cathodal tDCS) on thermal thresholds at each time point (before, during and after tDCS). Significant results were further analysed using the Wilcoxon signed-rank test. This test was also used to compare the 2-back task results obtained during sham, anodal and cathodal tDCS. Statistics were expressed as mean ± standard deviation and statistical significance was Bonferroni adjusted.
3. Results
Descriptive statistics regarding thermal thresholds at the different points in time to tDCS for the various conditions of cortical stimulation (sham, anodal and cathodal tDCS) are presented in Table 1a,b for the right and the left DLPFC stimulation, respectively. The 2-back task results are presented in Table 2.
| Threshold | Time point | Sham tDCS | Anodal tDCS | Cathodal tDCS | p |
|---|---|---|---|---|---|
| (a) TDCS of the right DLPFC | |||||
| CST (°C) | Before | 29.91 ± 1.73 | 30.15 ± 1.53 | 30.71 ± 0.77 | 0.262 |
| During | 29.85 ± 1.96 | 29.89 ± 1.49 | 30.31 ± 0.67 | 0.447 | |
| After | 30.00 ± 1.12 | 29.85 ± 1.51 | 29.84 ± 1.23 | 0.138 | |
| WST (°C) | Before | 34.26 ± 1.05 | 33.75 ± 0.48 | 34.13 ± 0.61 | 0.315 |
| During | 37.68 ± 2.40 | 37.80 ± 2.21 | 36.86 ± 1.76 | 0.401 | |
| After | 37.67 ± 2.34 | 37.48 ± 3.77 | 37.09 ± 1.78 | 0.297 | |
| CPT (°C) | Before | 22.15 ± 5.81 | 21.08 ± 7.24 | 20.38 ± 6.97 | 0.717 |
| During | 23.98 ± 5.32 | 21.09 ± 7.55 | 20.02 ± 8.93 | 0.174 | |
| After | 23.13 ± 6.09 | 21.48 ± 7.37 | 19.85 ± 8.83 | 0.338 | |
| HPT (°C) | Before | 42.10 ± 3.94 | 41.91 ± 4.05 | 42.95 ± 3.50 | 0.297 |
| During | 45.21 ± 2.33 | 44.42 ± 3.31 | 44.39 ± 2.55 | 0.076 | |
| After | 44.09 ± 3.26 | 44.69 ± 3.73 | 44.75 ± 2.75 | 0.558 | |
| HPTT (°C) | Before | 48.46 ± 1.87 | 48.15 ± 2.08 | 47.93 ± 2.49 | 0.401 |
| During | 48.95 ± 1.75 | 49.01 ± 1.82 | 48.89 ± 2.06 | 0.127 | |
| After | 48.84 ± 1.85 | 49.27 ± 1.75 | 48.78 ± 2.13 | 0.008 | |
| (b) TDCS of the left DLPFC | |||||
| CST (°C) | Before | 29.28 ± 1.74 | 29.44 ± 1.73 | 27.68 ± 3.94 | 0.105 |
| During | 29.12 ± 1.70 | 29.03 ± 1.66 | 27.22 ± 4.51 | 0.556 | |
| After | 28.52 ± 2.89 | 28.63 ± 2.24 | 27.26 ± 3.68 | 0.401 | |
| WST (°C) | Before | 34.80 ± 1.22 | 35.19 ± 2.00 | 35.78 ± 1.92 | 0.116 |
| During | 38.39 ± 2.93 | 38.75 ± 2.17 | 39.95 ± 3.34 | 0.401 | |
| After | 38.78 ± 2.45 | 39.84 ± 2.99 | 40.55 ± 3.25 | 0.667 | |
| CPT (°C) | Before | 22.86 ± 4.54 | 23.73 ± 5.41 | 18.91 ± 8.12 | 0.101 |
| During | 22.81 ± 5.94 | 23.42 ± 6.51 | 19.81 ± 9.11 | 0.826 | |
| After | 22.38 ± 6.49 | 22.70 ± 5.69 | 21.04 ± 7.90 | 0.097 | |
| HPT (°C) | Before | 42.14 ± 3.64 | 41.95 ± 4.60 | 43.86 ± 3.18 | 0.558 |
| During | 45.26 ± 2.84 | 44.93 ± 3.27 | 46.23 ± 2.50 | 0.455 | |
| After | 46.01 ± 1.87 | 45.85 ± 2.92 | 46.55 ± 2.77 | 0.472 | |
| HPTT (°C) | Before | 48.70 ± 2.38 | 49.21 ± 1.80 | 49.25 ± 1.89 | 0.640 |
| During | 49.46 ± 2.05 | 49.52 ± 2.05 | 49.86 ± 1.37 | 0.587 | |
| After | 49.89 ± 1.38 | 50.02 ± 1.66 | 50.20 ± 1.38 | 0.915 | |
- Significant results are marked as bold.
| 2-back task parameter | Sham tDCS | Anodal tDCS | P (Sham vs. Anod. tDCS) | Cathodal tDCS | P (Sham vs. Cath. tDCS) | |
|---|---|---|---|---|---|---|
| tDCS of the right DLPFC | Errors | 0.58 ± 0.90 | 0.92 ± 1.34 | 0.340 | 0.67 ± 1.23 | 1.000 |
| Omissions | 0.75 ± 1.48 | 0.83 ± 1.75 | 0.705 | 0.67 ± 1.23 | 0.705 | |
| Outliers | 0.50 ± 0.52 | 0.33 ± 0.49 | 0.414 | 1.00 ± 1.86 | 0.257 | |
| Reaction time (ms) | 568 ± 131 | 536 ± 109 | 0.666 | 561 ± 146 | 0.534 | |
| tDCS of the left DLPFC | Errors | 1.00 ± 1.04 | 0.50 ± 0.80 | 0.202 | 0.67 ± 0.65 | 0.234 |
| Omissions | 1.12 ± 1.27 | 1.08 ± 1.98 | 0.833 | 0.58 ± 0.90 | 0.107 | |
| Outliers | 0.67 ± 0.49 | 0.42 ± 0.51 | 0.257 | 0.25 ± 0.45 | 0.025 | |
| Reaction time (ms) | 551 ± 114 | 538 ± 99 | 0.695 | 593 ± 199 | 0.638 |
- Significant results are marked as bold.
3.1 Influence of tDCS of the right or left DLPFC on thermal thresholds
There were no significant differences in thermal thresholds at any time point (before, during and after tDCS) according to the stimulation condition (sham, anodal and cathodal tDCS of the right or left DLPFC), except for HPTTs tDCS of the right DLPFC (χ2 = 9.574; p = 0.008) with higher thresholds following anodal tDCS compared to sham tDCS (Z = −3.063; p = 0.002; Table 1, Fig. 2). In addition, there was a tendency for an effect on HPTs of tDCS of the right DLPFC during the stimulation (χ2 = 5.167; p = 0.076) and on CPTs of tDCS of the left DLPFC after the stimulation (χ2 = 4.667; p = 0.097) (Table 1a,b).
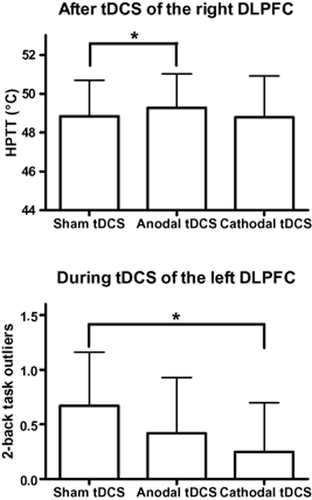
Upper graph: differences in heat pain tolerance thresholds (HPTTs) after transcranial direct current stimulation (tDCS) of the right dorsolateral prefrontal cortex (DLPFC) according to stimulation condition (sham, anodal and cathodal, tDCS). Lower graph: differences in outliers in the 2-back task after tDCS of the left DLPFC according to stimulation condition (sham, anodal and cathodal, tDCS). Data are given as mean + SD as error bar. *P ≤ 0.025.
3.2 Effects of tDCS of the right or left DLPFC on the 2-back task
There was a significant difference in the number of outliers at the 2-back task during tDCS of the left DLPFC, with less outliers during cathodal tDCS compared to sham tDCS (Z = −2.236; p = 0.025; Table 2). In addition, there was a tendency for a difference in the number of omissions during tDCS of the left DLPFC, with a reduction of omissions during cathodal tDCS compared to sham tDCS (Z = −1.611; p = 0.107). There was no difference for any other parameter of the 2-back task according to the condition of cortical stimulation (sham, anodal or cathodal tDCS of the right or left DLPFC).
4. Discussion
In this study, we investigated the effect of tDCS of the right or left DLPFC on pain perception and verbal WM in healthy subjects. Anodal tDCS of the right DLPFC increased HPTTs, whereas no significant effects were observed on thermal thresholds for tDCS of the left DLPFC. In contrast, reduced outliers in the 2-back task were detected during cathodal tDCS of the left DLPFC, whereas no significant effects were observed on the 2-back task for tDCS of the right DLPFC.
4.1 Effects of tDCS of the DLPFC on pain perception
The effects of tDCS of the right DLPFC on experimentally induced pain have never been studied, whereas the effects of tDCS of the left DLPFC have been previously assessed in only one study to date (Boggio et al., 2008). In this previous study, anodal tDCS of the left DLPFC was able to increase electrical pain threshold, whereas anodal tDCS delivered to M1 increased both electrical stimulus perception and pain thresholds. In the present study, anodal tDCS of the right DLPFC, but not of the left DLPFC, produced a significant increase of HPTTs.
Differences in stimulus characteristics at the origin of pain production (electrical vs. thermal pain) and therefore in the recruited pathways and cortical processing (Ploner et al., 2002) may contribute to the discrepancy between the two studies. In addition, in the study of Boggio et al. (2008), only anodal tDCS of the left DLPFC was assessed, without any comparison with the effects of cathodal tDCS or right DLPFC stimulation.
Three studies previously reported that rTMS delivered to the right or left DLPFC could reduce the sensitivity to thermal pain provoked in healthy subjects (Graff-Guerrero et al., 2005; Borckardt et al., 2007; Nahmias et al., 2009). Graff-Guerrero et al. (2005) showed an increased tolerance for cold-induced pain during low-frequency (1 Hz) rTMS of the right DLPFC without any effect of left DLPFC stimulation. Cold pain tolerance did not last beyond the time of cortical stimulation. Nahmias et al. (2009) showed a reduced sensitivity to cold pain (decreased CPTs) following high-frequency (10 Hz) rTMS of the right DLPFC, whereas left DLPFC stimulation was not tested. They observed a similar result following 10 Hz-rTMS delivered to M1. This research team reproduced their results in a subsequent study (de Andrade et al., 2011), further showing that the concomitant injection of an opioid receptor antagonist (naloxone) significantly decreased the analgesic effects of M1 stimulation, but did not change the effects of rTMS of the DLPFC. Finally, Borckardt et al. (2007) showed a reduced sensitivity to heat pain (increased HPTs) following high-frequency (10 Hz) rTMS of the left DLPFC, whereas right DLPFC stimulation was not tested. Besides these studies, in which provoked pain thresholds were measured, high-frequency (5 Hz) rTMS of the left DLPFC was also found to reduce capsaicin-induced tonic pain, whereas right DLPFC stimulation was ineffective (Fierro et al., 2010; Brighina et al., 2011).
Thus, in the study of Graff-Guerrero et al. (2005) as in the present study, the stimulation of the right but not of the left DLPFC increased thermal pain tolerance. However, this result was obtained in the former study with a protocol of cortical stimulation known to reduce motor cortex excitability (low-frequency rTMS), whereas it was obtained in the present study with a protocol known to increase motor cortex excitability (anodal tDCS). This discrepancy illustrates that the effect of a protocol of cortical stimulation on a given, non-motor neural function, such as pain perception, cannot be predicted from the effect obtained on motor corticospinal output (expressed as the amplitude of motor-evoked potentials, MEPs) with the same protocol.
The hypothesis that a modulation of motor cortex excitability could account for the analgesic effects of cortical stimulation remains debated. (Lefaucheur et al. 2006) showed that high-frequency (10 Hz) rTMS delivered to M1 relieved pain in correlation with the restoration of defective intracortical GABAergic activity, as assessed by short intracortical inhibition (SICI). Antal et al. (2011) also showed that anodal tDCS of M1 ameliorated chronic pain and SICI in parallel. Finally, Fierro et al. (2010) showed that left DLPFC rTMS, together with the analgesic effect, was able to revert the effects of capsaicin-induced pain on motor cortex excitability, restoring normal MEP size and SICI level. Although the modulation of inhibitory circuits within M1 are likely involved in the analgesic effect of motor cortex stimulation, this does not imply a ‘motor effect’ through the recruitment of motor corticospinal pathways (Lefaucheur et al., 2006, 2010).
The two other aforementioned studies (Borckardt et al., 2007; Nahmias et al., 2009) also showed that the same type of rTMS protocol [so-called ‘excitatory’ high-frequency (10 Hz) rTMS] could reduce sensitivity to thermal pain whether the right or left DLPFC was stimulated. This is in contradiction with the results obtained in the treatment of depression, which are significant only for high-frequency rTMS of the left DLPFC and low-frequency rTMS of the right DLPFC (Rachid and Bertschy, 2006). This is evidence that the analgesic efficacy of DFLPC stimulation may be mediated by various neural processes, involving various neural circuits, but cannot be summarized only to an antidepressant effect. This is also the conclusion of the few reports of analgesic effects obtained by DLPFC stimulation in patients treated for depression or chronic pain (Sampson et al., 2006, 2011; Avery et al., 2007; Borckardt et al., 2009).
However, taken together, all these results may also have been influenced by a lateralization of pain perception and tolerance on the right hemisphere regarding the contribution of the DLPFC, the anterior cingulate cortex (ACC), and the inferior parietal cortex, as described in some imaging studies (Tolle et al., 1999; Coghill et al., 2001).
One last remark is that anodal tDCS of M1 (but not cathodal tDCS) was advocated as a promising method to treat chronic pain syndromes, such as fibromyalgia, neuropathic pain and back pain (Fregni et al., 2006a,b; Antal et al., 2011). In contrast to chronic pain syndromes, cathodal tDCS of M1 (but not anodal tDCS) was found to reduce acute pain induced by laser stimulation as well as the amplitude of the resulting laser-evoked potentials (LEPs; Antal et al., 2008; Csifcsak et al., 2009). Cathodal tDCS of S1 (but not anodal tDCS) could also reduce laser-induced pain (Antal et al., 2008). Finally, cathodal tDCS of a broad area of M1 (including face representation) was recently found to reduce the amplitude of trigeminal and extracranial pain-related evoked potentials (PREPs), whereas anodal tDCS increased it (Hansen et al., 2011). These tDCS effects were considered to reflect a preferential impact of cortical stimulation on the ACC origin of LEPs or PREPs, suggested a modulation of the medial pain pathways through the stimulation of sensorimotor cortex. Moreover, various imaging studies revealed that either invasive or non-invasive M1 stimulation led to regional cerebral blood flow changes in limbic cortical structures involved in the cognitive-emotional component of pain (Lorenz et al., 2003; Tamura et al., 2004). Therefore, DLPFC could be a suitable target to mediate the analgesic efficacy of tDCS, especially on the emotional component of pain.
While most tDCS and rTMS studies, as our study, have focused on immediate effects of the stimulation on pain perception, we cannot exclude that other types of effects could be built up with more time or occur with a delay as suggested by some rTMS studies in experimentally induced pain and chronic pain (Lefaucheur et al., 2001; Yoo et al., 2006; Nahmias et al., 2009).
4.2 Effects of tDCS on working memory and attention
The 2-back task performed in this study showed a significant reduction of outliers (i.e., disproportionally slow reactions) and a tendency towards a reduction of omissions during cathodal tDCS of the left DLPFC. These results argue for enhanced executive control processes, possibly associated with reduced attentional distraction or increased ability to shield the information held in WM from interference by potentially distracting information (Corbetta and Shulman, 2002; Marklund et al., 2007). Alternatively, it seems conceivable that cathodal tDCS may have improved visual attention when applied to the left DLPFC by altering inter-hemispheric balance (inhibition of the left hemisphere) leading to a relative increase of visuospatial attention in the right dominant hemisphere leading to faster motor reactions (Perez et al., 2009). Indeed, a reduced number of outliers as observed in this study may have been due to an effect of tDCS on motor reaction time and not on WM itself. A change in the number of errors might have offered a more specific estimate of the effects of tDCS on WM.
Contrary to our results, previous studies using a letter-based 3-back task of a higher degree of difficulty but a similar technique of cortical stimulation showed a time-dependent improvement of WM induced by anodal tDCS of the left DLPFC in healthy subjects as well as in patients with Parkinson's disease or Alzheimer's dementia (Fregni et al., 2005; Boggio et al., 2006, 2009; Ohn et al., 2008). Between these studies and our, the main difference were the retest study design and the reduced difficulty of the 2-back task, leading to a ceiling effect in the present study. In addition, we have limited the evaluation to the period immediately following the stimulation, whereas some of the previous results on WM were observed at a distance from the time of tDCS (Fregni et al., 2005; Ohn et al., 2008).
5. Conclusion
In this study, the effects of cathodal versus anodal tDCS were compared in the same subjects, controlled by a sham tDCS condition in all cases. However, the facts that two different populations underwent right and left DLPFC stimulations, with a relatively small number of subjects recruited for these two experiments, and that tDCS effects were assessed during and immediately after the time of stimulation are clear limitations of this study.
Our main observation was that significant modulations of pain perception and WM were obtained for different conditions of DLPFC stimulation applied to different hemispheres. Therefore, these changes do not appear correlated, and this does not support the involvement of cognitive changes in the analgesic efficacy of cortical stimulation. In contrast, a recent study of anodal tDCS of the motor cortex in chronic neuropathic pain suggested that a cognitive process, such as visual illusion, could interact with cortical stimulation to enhance tDCS analgesic effects (Soler et al., 2010). In fact, we cannot completely exclude a cognitive contribution to the observed analgesic effects, since the task used for WM assessment was perhaps not sufficiently selective to show substantial changes after treatment nor sufficiently specific to study neural mechanisms involved in pain processing. The influence of DLPFC stimulation on more specifically pain-related cognitive functions (pain memory or pain-related attention) would be interesting to study. The question of the modulation of short-term memory of pain by the stimulation of S1 would also be addressed in a future study.
Author contributions
V.M. contributed to conception, performance, analyses, preparation and revision of the manuscript. M.J. contributed to conception, performance and preparation of the manuscript. K.M. contributed to conception and performance. A.H. contributed to conception and analyses. P.K., W.H.O., F.R. and J.P.L. contributed to preparation and revision of the manuscript.




