Rotifera Occurrence and Diversity in the Groundwater Wells of a Major European Wetland Complex
ABSTRACT
Rotifera are found in nearly all terrestrial and freshwater habitats, however, the knowledge about rotifers in groundwater is scarce. To enhance our understanding of Rotifera distribution in groundwater in the lowland regions of Central Europe, we investigated 101 wells throughout the Biebrza Valley—one of Europe's largest wetland complexes. Rotifers were found in most of the studied wells (97%); however, only 22 Monogononta species were identified. The majority of these were littoral species commonly associated with surface water macrophytes, suggesting passive transport from the river network rather than active colonization or specialization for subterranean life. No strictly stygobiontic (obligate groundwater) or stygophilic (preferring groundwater) Rotifera species were found. Instead, our results indicate a high prevalence of stygoxenic species—organisms that occur in groundwater environments only incidentally. Species richness was low compared to surface or transitional habitats such as springs and the hyporheic zone, which showed much greater rotifer diversity. These findings suggest that while rotifers can occur in groundwater, their ecological traits favor opportunistic colonization over groundwater specialization.
1 Introduction
Groundwater is important in providing good quality water for human consumption and many groundwater-dependent ecosystems. Although millions of people still rely on private wells as a source of drinking water, and quality of those waters is often monitored (Żurek and Bilek 2017), our knowledge about the organisms inhabiting well waters is still insufficient. According to Gibert and Culver (2009), the early 21st century brought a substantial number of reports indicating high species richness in groundwater organisms, particularly copepods (Galassi et al. 2009). Copepods outnumber other invertebrates in most groundwater communities, and over 1000 stygobiotic species (restricted to groundwater) are currently known. Furthermore, a high level of endemism among groundwater copepods has been reported, highlighting their ecological and evolutionary significance in subterranean habitats (Galassi 2001; Galassi et al. 2009; Gibert and Culver 2009; Rendoš et al. 2023).
Gibert and Culver (2009) noted that although Rotifera are found in nearly all terrestrial and freshwater habitats, they are almost absent from groundwater environments and can be considered a negligible component of groundwater fauna. Nevertheless, there are few reports on rotifers in groundwater, particularly in rural wells, and the available studies suggest relatively low species richness in these environments. Investigations of groundwater-dependent ecosystems in the Philippines recorded only four species of rotifers (Asplanchna sieboldi, Brachionus plicatilis, Lecane leontina, and Epiphanes senta) (Cavite et al. 2017). Similarly, a study conducted in 29 wells in Kilis Province (Turkey) identified just 12 species of rotifers (Bozkurt 2023). A higher diversity was observed in Hatay Province (Turkey) (Bozkurt and Bozça 2019), where seasonal studies conducted in 14 different water wells revealed 30 species of rotifers. Reports from urban wells also highlight the limited presence of rotifers in groundwater. For instance, rotifers were detected in only 4 out of 91 wells (4%) in Kraków, Poland (Dumnicka et al. 2025), and in 16 out of 70 wells (23%) in Prague, Czech Republic (Řehačkova 1953). Unfortunately, the studies from urban wells did not include detailed information on species composition or the precise number of rotifer species identified.
The rotifer fauna of springs can be considered to originate from groundwater. However, it seems to be much more diverse than the fauna of wells. Studies conducted in the springs of the Chihuahuan Desert in Mexico revealed the presence of 57 rotifer species, with Lepadella patella, Lepadella triptera, and Philodina megalotrocha being the most widespread across the sampled sites (Ríos-Arana et al. 2019). Similarly, research on 47 springs in the Knyszyn Forest (northeastern Poland) confirmed a high diversity of rotifers, with 101 species identified. Despite significant faunal differences among the springs, a core set of species, including Lepadella acuminata, Lepadella ovalis, L. patella, and Colurella adriatica, was consistently present across multiple sites (Jekatierynczuk-Rudczyk and Ejsmont-Karabin 2023). These findings suggest that rotifer diversity in groundwater-related habitats may be both substantial and largely unexplored. Moreover, the recent description of a new monogonont rotifer, Cephalodella binoculata, from a soil sample in Korea using an integrative taxonomy approach (Yang and Min 2023) further underscores the limited knowledge of groundwater-related habitats and their biodiversity.
The primary aim of this study is to enhance our understanding of Rotifera distribution in groundwater across the lowland regions of Central Europe. To achieve this, we investigated 101 wells across the Biebrza Valley—one of Europe's largest and most ecologically significant wetland complexes. Due to the extensive number of samples distributed throughout the valley, we achieved comprehensive spatial coverage, allowing us to analyze spatial patterns of rotifer occurrence in groundwater. Our findings are presented in the context of Rotifera communities inhabiting surface waters in this region, covering a variety of habitats and microhabitats. By comparing rotifer communities from surface and groundwater environments, we aimed to identify species capable of penetrating the hyporheic zone and shallow groundwater, determining which can be classified as stygophilic (preferring but not restricted to groundwater) or stygoxenic (accidentally occurring in groundwater).
2 Study Area and Methods
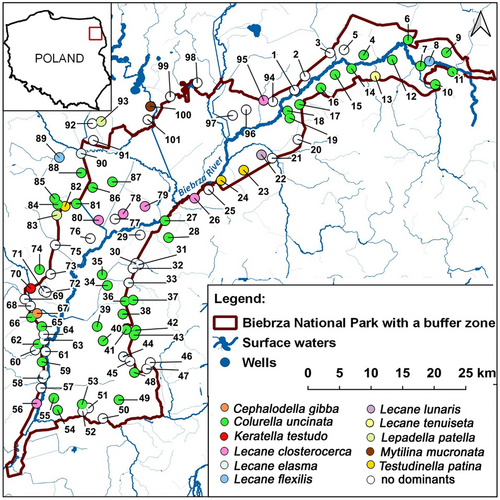
The research was conducted in 101 wells in the Biebrza River valley in 2021, from October 22 to November 15. The Biebrza River (Figure 1) is located in NE Poland within the area which has been protected as a national park since 1992 (Grygoruk et al. 2021; Grodzka-Łukaszewska et al. 2022). The Biebrza Valley is one of the largest wetland complexes in Europe. The river flows through boggy meadows and marshes, forming old riverbeds and floodplain water bodies in different stages of succession. The valley is underlain by diverse aquifer systems that influence the distribution and dynamics of groundwater (Grodzka-Łukaszewska et al. 2022). The peatland aquifers play an extremely important role in the Biebrza National Park by storing and slowly releasing water, thus maintaining the river flow in dry periods (Anibas et al. 2012).
Water samples from the wells were collected using a 10 L calibrated vessel, with volumes ranging from 20 to 100 L, being filtered through a 50 µm plankton net, and fixed with 96% ethanol. All studied wells have a top cover that isolates them from external weather conditions. The field measurements included the depth of the wells and water level (with a hydrological whistle) as well as temperature, dissolved oxygen, and electrical conductivity using an HQ40D Multi Meter (Hach-Lange GmbH, Germany).
The surveyed wells depths ranged from 1.0 to 11.0 m, with a mean depth of 3.7 ± 2.3 m. The water column thickness varied between 0.2 and 6.5 m, averaging 1.4 ± 1.2 m. Water temperature remained relatively constant, with an average of 10.3 ± 1.1°C. Dissolved oxygen levels fluctuated between 1.5 and 10.1 mg L−1, with a mean concentration of 6.8 ± 2.2 mg L−1. Electrical conductivity values ranged from 190 to 1711 μS cm−1, with an average of 837 ± 321 μS cm−1.
Rotifers were analyzed under an optical microscope NIKON Eclipse Ni-U. In each sample, all rotifers, both Bdelloidea and Monogononta were counted. Monogononts were identified to species. As regards Bdelloidea, such identification was impossible in preserved samples.
For comparative purposes, unpublished data on the occurrence of rotifer species in different ecosystems and habitats were elaborated and used. These data were obtained in studies described in publications listed in Tables 1 and 2. To estimate true rotifer species diversity in the studied set of wells and different analyzed habitats, we calculated the bias-corrected Chao2 and Jackknife2 estimators (Gotelli and Colwell 2011).
| Species | Frequency (%) | Maximum density (ind. L−1) |
|---|---|---|
| Cephalodella gibba (Ehrenberg 1832) | 8 | 3.90 |
| Cephalodella sp. | 2 | 0.08 |
| Colurella obtusa (Gosse 1886) | 3 | 0.53 |
| Colurella uncinata (Müller 1773) | 55 | 105.60 |
| Dicranophorus rostratus (Dixon-Nuttall and Freeman 1902) | 1 | 0.98 |
| Keratella cochlearis (Gosse 1851) | 2 | 0.06 |
| Keratella quadrata (Müller 1786) | 1 | 0.01 |
| Keratella testudo (Ehrenberg 1832) | 1 | 7.25 |
| Lecane closterocerca (Schmarda 1859) | 12 | 11.84 |
| Lecane crenata (Harring 1913) | 1 | 0.01 |
| Lecane elasma (Harring and Myers 1926) | 1 | 0.41 |
| Lecane flexilis (Gosse 1886) | 7 | 10.12 |
| Lecane hamata (Stokes 1896) | 5 | 0.26 |
| Lecane lunaris (Ehrenberg 1832) | 1 | 0.36 |
| Lecane tenuiseta (Harring 1914) | 2 | 13.53 |
| Lepadella ovalis (Müller 1786) | 2 | 0.05 |
| Lepadella patella (Müller 1773) | 13 | 0.65 |
| Mytilina mucronata (Müller 1773) | 2 | 1.26 |
| Notommata aurita (Müller 1786) | 3 | 1.80 |
| Squatinella rostrum (Schmarda 1846) | 1 | 0.01 |
| Testudinella incisa (Ternetz 1892) | 1 | 0.01 |
| Testudinella patina (Hermann 1783) | 14 | 21.57 |
| Species | Frequency (%) | Number of samples | Habitat | Source of data |
|---|---|---|---|---|
| Colurella uncinata | 55 | 101 | Groundwater (wells) | This study |
| 56 | 32 | River current | 3, 5 | |
| 81 | 32 | River epiphyton | 3, 5 | |
| 21 | 33 | Peat bogs | 2, ud | |
| 53 | 36 | Hydroarenal of lakes | 4 | |
| 90 | 42 | Lake epiphyton | 4 | |
| 29 | 42 | Springs | 6 | |
| 11 | 35 | Quarry lakes (pelagial) | 1 | |
| Testudinella patina | 14 | 101 | Groundwater (wells) | This study |
| 31 | 32 | River current | 3, 5 | |
| 75 | 32 | River epiphyton | 3, 5 | |
| 9 | 33 | Peat bogs | 2, ud | |
| 6 | 36 | Hydroarenal of lakes | 4 | |
| 48 | 42 | Lake epiphyton | 4 | |
| 0 | 42 | Springs | 6 | |
| 0 | 35 | Quarry lakes (pelagial) | 1 | |
| Lepadella patella | 13 | 101 | Groundwater (wells) | This study |
| 50 | 32 | River current | 3, 5 | |
| 94 | 32 | River epiphyton | 3, 5 | |
| 58 | 33 | Peat bogs | 2, ud | |
| 69 | 36 | Hydroarenal of lakes | 4 | |
| 67 | 42 | Lake epiphyton | 4 | |
| 45 | 42 | Springs | 6 | |
| 14 | 35 | Quarry lakes (pelagial) | 1 | |
| Lecane closterocerca | 12 | 101 | Groundwater (wells) | This study |
| 66 | 32 | River current | 3, 5 | |
| 100 | 32 | River epiphyton | 3, 5 | |
| 58 | 33 | Peat bogs | 2, ud | |
| 97 | 36 | Hydroarenal of lakes | 4 | |
| 100 | 42 | Lake epiphyton | 4 | |
| 52 | 42 | Springs | 6 | |
| 17 | 35 | Quarry lakes (pelagial) | 1 |
- Note: Description of research methods may be found in: 1—Ejsmont-Karabin (1995); 2—Ejsmont-Karabin (1999); 3—Ejsmont-Karabin and Karabin (2004); 4—Ejsmont-Karabin and Karpowicz (2021); 5—Ejsmont-Karabin and Zieliński (2017); 6—Jekatierynczuk-Rudczyk and Ejsmont-Karabin (2023).
- Abbreviation: ud, unpublished data.
The effect of environmental parameters (temperature, oxygen, and electrical conductivity) on the total abundance of Monogononta was tested using linear regression, while the influence of aquifer type (alluvial vs. peatland) was assessed with a two-way analysis of variance (ANOVA) using Type III sum of squares (SS). Statistical analyses were performed using XLSTAT Ecology (Addinsoft, USA).
3 Results
We did not find significant differences in Monogononta abundance between alluvial and peatland aquifers (F = 2.4; p = 0.12). There was no significant impact of environmental parameters, like oxygen (F = 1.1; p = 0.30), temperature (F = 0.03; p = 0.87), or electrical conductivity (F = 0.12; p = 0.73) on the abundance of Monogononta.
Rotifers were present in nearly all wells, with their absence recorded in only 3% of the sites (wells no. 61, 99, and 101). Bdelloids were found in 95 wells, and their average density was 8.6 ± 25.7 ind. L−1. Their maximum density of 194.1 ind. L−1 was recorded in the well no. 76. The exclusive presence of Bdelloidea was recorded in 22 wells. Their density in those wells ranged from 0.2 to 97.1 ind. L−1 with an average of 12.6 ± 14.8 ind. L−1. Monogononta occurred in 76% of the wells. Their density ranged from 0.01 to 89.3 ind. L−1 with an average of 4.1 ± 13.7 ind. L−1. The exclusive presence of Monogononta was recorded only in two cases (wells no. 9 and 94).
In total, 22 taxa of Rotifera were recorded in the studied wells (Table 1). However, as many as 8 were found only once, and 5—twice. The mean number of species recorded in the individual wells was 1.4 ± 1.2, and the largest number of eight species was found in well no. 52.
The most frequent species was Colurella uncinata, which occurred in more than half of the studied wells, whereas Testudinella patina, L. patella, and Lecane closterocerca were recorded in 14, 13, and 12 wells (respectively) (Tables 1 and 2). These species with the highest frequency also reached the highest densities, usually dominating the other species in the studied wells (Figure 1). Thus, C. uncinata dominated in 39 wells, and L. closterocerca in five wells. There were 19 species of rotifers characteristic for littoral habitats. Only three pelagic species (Keratella cochlearis, K. quadrata, and Keratella testudo) were noted, however, they occurred sporadically. Of these, only K. testudo reached significant abundance (7.2 ind. L−1) in well no. 7.
Apart from the four most common species, which frequently dominated Rotifera communities (Table 2), occasional dominance of Cephalodella gibba, K. testudo, Lecane elasma, L. flexilis, L. lunaris, L. tenuiseta, and Mytilina mucronata was also observed (Figure 1). Aside from the clear dominance of C. uncinata, which was present in groundwater across the entire Biebrza Valley, no distinct spatial patterns were detected in the distribution of Rotifera species (Figure 1). This suggests a patchy distribution of Rotifera in groundwater.
Despite the large number of wells examined, only 22 species of Monogononta were identified, with the estimated species richness reaching just 25 (Chao2) or 34 species (Jackknife2) (Table 3). The limited species diversity in groundwater (wells) contrasts with the high number of species recorded in groundwater outflows (springs), where 79 species were identified, and the potential species count could be twice as high (Table 3). An even greater species richness was observed in the hydroarenal zone (Table 3), a transitional layer between surface waters and the phreatic zone groundwater. Similarly, higher species numbers were recorded in various surface water habitats (Table 3).
| Ecosystems | Habitat | Number of samples | Observed | Chao2 | Jackknife2 | Source of data |
|---|---|---|---|---|---|---|
| Groundwater | Wells | 101 | 22 | 25 | 34 | This study |
| Springs | Current | 42 | 79 | 150 | 152 | 5 |
| Small river | Current | 20 | 89 | 127 | 140 | 4 |
| Small river | Macrophytes | 20 | 119 | 188 | 190 | 4 |
| Quarry lakes | Pelagial | 35 | 60 | 86 | 86 | 1 |
| Lakes | Pelagial | 30 | 48 | 53 | 58 | 3 |
| Lakes | Macrophytes | 30 | 148 | 206 | 217 | 3 |
| Lakes | Hydroarenal | 69 | 104 | 173 | 182 | 2 |
4 Discussion
Although the overall number of Rotifera species identified from the surveyed wells was low, rotifers were present in the vast majority of these ecosystems. Despite not being found in every individual well, their widespread occurrence highlights the ability of rotifers to colonize groundwater habitats.
Rotifers were found in most of the studied wells (97%); however, only 22 Monogononta species were identified, with the estimated actual species number being only slightly higher. Similar results were obtained in studies of Rotifera in Turkish wells, where they were present in 86% of the analyzed sites, with a comparable number of species recorded (Bozkurt 2023). The high occurrence of rotifers in the wells in our study may have been influenced by the large volume of the sample, as up to 100 L of water were filtered from each well. In contrast, rotifers were much less frequent in urban wells of Kraków, southern Poland, where only 4% of the 91 examined wells contained Rotifera (Dumnicka et al. 2025). It is difficult to explain these differences, because the cited publication provides information only on the occurrence of rotifers without more precise taxonomic data. It is worth noting that the methods used in the study by Dumnicka et al. (2025) were very similar. The values of temperature and electrical conductivity also differed little.
The source of rotifers in groundwater in our study is most likely river waters enriched with littoral species of rotifers washed out from among the plants by a strong current. This is indicated by the strong dominance of littoral species in the rotifer community. The species most frequently found in the wells, that is, C. uncinata, T. patina, L. patella, and L. closterocerca, are also most frequently observed among river macrophytes (Table 2). All four species are commonly found worldwide in various habitats, mainly among aquatic vegetation (Bielańska-Grajner et al. 2015). Similarly, in the study of zooplankton communities inhabiting wells in Turkey (Bozkurt 2023), all observed rotifer species can be described as known worldwide and rather common littoral ones. L. closterocerca was the most frequent there, whereas T. patina was not found at all. However, earlier studies conducted in 14 wells in Hatay Province, Turkey, reported a different composition of rotifer species, with pelagic taxa such as K. cochlearis and Trichocerca similis occurring most frequently. Nevertheless, littoral species C. uncinata, L. closterocerca, L. patella, and T. patina were observed as well, but in less than 40% of the studied wells (Bozkurt and Bozça 2019). Notably, none of the dominant species in the studied wells were among the 42 Monogononta species recorded in the bed sediments of the mountain gravel stream Oberer Seebach (Schmid-Araya 1993).
The transfer of river organisms to groundwater is likely a slow process, considering that the first meters of soil passage remove most microorganisms (Medema and Stuyfzand 2002). However, in the case of rotifers that reproduce primarily (Monogononta) or exclusively (Bdelloidea) asexually and create resting stages that are extremely resistant to environmental conditions, even single individuals can develop into large population (Nogrady et al. 1993). Despite this, few rotifer species managed to develop larger populations in the wells under this study. These are littoral, ubiquitous, and detritus-algae-eating species. In wells devoid of vegetation, they are probably more or less associated with the sediments or the well wall, only appearing periodically in the water column. This may explain their extremely low numbers observed in this study and reported from Turkish wells (Bozkurt 2023). We do not observe any spatial pattern of distribution of Rotifera in the groundwater of the Biebrza Valley, suggesting a rather patchy distribution. A similar distribution pattern of copepods in the groundwater of this area was reported by Smolska et al. (2024).
In the same area (northeastern Poland), a significantly higher species diversity of Rotifera was observed in the transition zone between groundwater and surface water, specifically in springs (Jekatierynczuk-Rudczyk and Ejsmont-Karabin 2023). A similarly high number of Rotifera species was reported in the springs of the Chihuahuan Desert in Mexico (Ríos-Arana et al. 2019). The increased species richness in transitional zones suggests that many rotifer species may be capable of penetrating the shallower layers of the hyporheic (phreatic) zone, where dynamic hydrological conditions and resource availability likely support diverse assemblages.
While rotifers have been observed in groundwater environments, no strictly stygobiontic (obligate groundwater-dwelling) rotifer species have been identified to date (Galassi 2001; Gibert and Culver 2009). Clark et al. (2021) during their research of the Robe Valley subterranean fauna, recognized some unidentified bdelloids of uncertain status. Turbanov et al. (2016) studying cave fauna in countries of the former Soviet Union listed five stygophile rotifer species, namely: Anuraeopsis fissa, Brachionus urceolaris, Ascomorpha ecaudis, Dicranophorus forcipatus, and C. gibba. However, all the species are very common world-wide in surface freshwaters (Bielańska-Grajner et al. 2015). Thus, they are rather stygoxenes, accidental taxa in groundwater that are temporarily imported from surface waters. Species from genera such as Cephalodella, Colurella, Lecane, Lepadella, Philodina, and Proales have been recorded in interstitial waters, aquifers, and hyporheic zones, but they are also widespread in surface waters (Ríos-Arana et al. 2019; Ejsmont-Karabin 2023). Their occurrence in both surface and subsurface waters suggests they are not obligate groundwater dwellers. Our research results indicate an absence of stygophilic Rotifera species that prefer groundwater habitats. Instead, our findings suggest a high prevalence of stygoxenic Rotifera, which occur in groundwater only incidentally. Therefore, rotifers can inhabit groundwater environments, however, their ecological and physiological characteristics contribute to their absence as strictly stygobiontic (or stygophilic) species. The absence of strictly stygobiontic rotifers may be linked to their reproductive strategies and life history traits. Rotifers typically have short generation times, high reproductive rates, and the ability to undergo cryptobiosis (a dormant state enabling survival in extreme conditions), which allows them to disperse between aquatic habitats rather than specialize in stable groundwater environments (Stelzer 2005; Ricci and Caprioli 2005; Wallace et al. 2006). In contrast, stygobiontic crustaceans often exhibit life history traits such as long lifespans, low fecundity, and morphological adaptations to subterranean life (e.g., depigmentation and reduced eyesight) (Galassi 2001; Brancelj and Dumont 2007; Galassi et al. 2009). These differences suggest that while rotifers may occasionally occur in groundwater, their ecological and evolutionary characteristics do not favor exclusive groundwater specialization. However, one should wonder why, out of the numerous littoral species (Ejsmont-Karabin and Karpowicz 2021), only four were able to create relatively larger populations. If this indicates their ability to tolerate conditions encountered in the groundwater habitat, then perhaps we are dealing here with yet another category, that is, stygotolerants—species with moderate frequency and intermediate or variable density, which tolerate subterranean conditions but also occur in other surface habitats.
In conclusion, the findings indicate that rotifers lack specialization for groundwater environments, yet some littoral species are capable of temporarily inhabiting groundwater, mainly due to infiltration and passive transport, with certain populations even reaching high abundances.
Acknowledgments
The authors thank Elżbieta Jekatierynczuk-Rudczyk for her contribution to sample collection and fieldwork. The authors are grateful to the editor and reviewers for their time and energy in providing helpful comments that have improved this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Most of the data supporting the findings of this study are provided within the article. Raw data on species abundances at individual sampling sites are available from the corresponding author upon reasonable request.




