Diatom assemblages and limnological variables from 40 lakes and ponds on Bathurst Island and neighboring high Arctic islands†
Abstract
We examined the influence of catchment geology, specifically differences in buffering capacity, on the limnological characteristics and surface sediment diatom assemblages from lakes and ponds from Bathurst Island, High Arctic Canada. Differences in buffering capacity exist on Bathurst Island due to a geological gradient that spans from carbonate-bearing limestone in the east, to more stable quartz sandstone, siltstone, and shale in the west. We collected physical and chemical limnological data, as well as surface sediment diatom assemblages from nine ponds on the poorly buffered western portion of the island and combined these observations with a previously published dataset of 31 lakes and ponds, from the well-buffered eastern region. The addition of these nine ponds expanded the pH gradient of the existing Bathurst Island dataset (pH 8.0–8.6) to pH 6.8–8.6. A regional, weighted average diatom-inferred pH model was developed and showed strength similar to other Arctic calibration sets (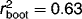 , root-mean-squared-error of prediction (RMSEP) = 0.298). Given the links between climate and pH shifts in the High Arctic, the ability to reconstruct pH should be a valuable tool for future paleolimnological studies.
, root-mean-squared-error of prediction (RMSEP) = 0.298). Given the links between climate and pH shifts in the High Arctic, the ability to reconstruct pH should be a valuable tool for future paleolimnological studies.
Introduction
The heightened sensitivity of Arctic environments to climatic change provides an excellent opportunity to study the limnological impacts of recent warming on otherwise relatively undisturbed lakes and ponds. While the majority of the sites studied in the High Arctic have shown some form of response to climate change (e.g., 1-3), not all freshwater ecosystems respond in a similar manner 4. For example, paleolimnological research on Arctic lakes has shown that poorly buffered waterbodies are particularly susceptible to climate-driven pH changes over Holocene timescales (e.g., 5-8).
As long-term monitoring data are not available in most Arctic regions, paleolimnological proxies, such as diatoms (e.g., 9), present us with a means to reconstruct past limnological and climatic shifts, thereby providing information about the natural variability of these ecosystems prior to the modern instrumental record. Given that lakewater pH is often the primary driver of diatom assemblages, as well as other lake sediment proxy bio-indicators, poorly buffered lakes may provide the most sensitive paleolimnological records with respect to past climatic fluctuations.
The duration and extent of lake ice cover is the primary mechanism used to explain shifts in Arctic freshwater diatom assemblages, mainly related to physical constraints on habitat availability and length of growing season 2. However, lake ice cover can also influence other factors including gas exchange with the atmosphere. During cool periods, Arctic lakes accumulate respired CO2, which becomes trapped under the ice, thereby lowering lakewater pH. Conversely, during warm periods, the reduction in ice cover allows more CO2 to escape to the atmosphere and there is a greater utilization of CO2 from enhanced algal photosynthesis, all of which lead to higher pH values. It is typically only poorly buffered lakes that experience these climate-driven pH shifts as high alkalinity lakes are buffered against such shifts in DIC speciation dynamics 5. For example, a paleolimnological study of a poorly buffered lake on Ellesmere Island showed a more varied and dynamic diatom response to Holocene climatic fluctuations compared to nearby well-buffered sites 6.
Bathurst Island in the Canadian High Arctic (Fig. 1) has a diverse geology with the eastern portion of the island containing more carbonate-rich rocks compared to the west (see geological map in Kerr 10). This makes it an ideal location to assess the influence of buffering capacity on lake water quality and modern diatom assemblages. Water quality characteristics, as well as modern diatom assemblages in the well-buffered, high pH waters on eastern Bathurst Island, have been previously documented 11, 12. However, limnological characteristics and modern diatoms from the poorly buffered western portion of the island have yet to be studied.
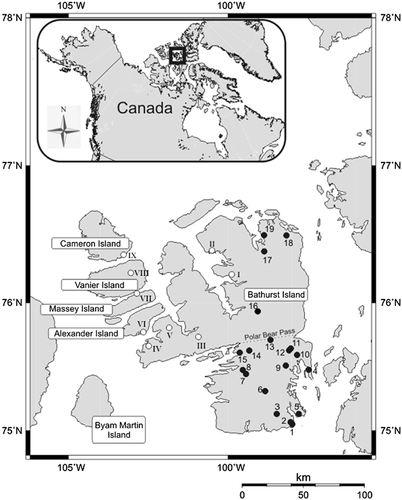
Map of Bathurst Island showing the study sites from both western (Roman numerals) and eastern (numbers) sites. Numbers on the map correspond to eastern sites as follows: BC = 1; BD, BE = 2; BF = 3; B-AO, B-AP = 4; BM, BN = 5; BP, BR = 6; BG = 7; BH = 8; BT = 9; BAD = 10; BV, BW BX = 11; BU = 12; BZ = 13; BJ = 14; BK = 15; BAC = 16; BAE, BAF, BAG, BAH, BAI = 17; BAM, BAN = 18; BAJ, BAK, BAL = 19; and to western sites: BAZ = I; BAY = II; BAS = III; BAT = IV; BAR = V; BAU = VI; BAV = VII; BAW = VIII; BAX = IX.
This paper expands the limnological gradient of freshwater ecosystems on Bathurst Island 11, 12 by adding limnological and surface-sediment diatom data for nine new sites that are located on the previously unstudied western region of the island and associated western flanking islands (Fig. 1). This research re-visits previous phycological work in this region, and provides a more comprehensive overview of the modern limnology from a sensitive ecological region. The addition of low alkalinity sites to the previously documented lakes and ponds from the eastern portion of Baffin Island 11, 12 have allowed us to create a diatom-based transfer function for reconstructing lakewater pH, which was otherwise not possible. This diatom-based pH model can now be applied downcore in poorly buffered lakes that will provide a sensitive record of past climate-driven pH dynamics (e.g., 5-8).
Study area
Bathurst Island (75°42′N, 97°21′W), located at the geographic center of the Canadian High Arctic, is home to the Polar Bear Pass National Wildlife area and supports a highly diverse wildlife population atypical of the Canadian Arctic Islands (Fig. 1). The geology of the island is primarily composed of Ordovician to Late Devonian shale, sandstones, limestone, and dolomites; however, the distribution of these rock types varies geographically across the island 10, 12 (Fig. 1). The eastern Bathurst geology is much more diverse and carbonate-rich than the west 10. The Eids and Bathurst Island formations in the north-east, Disappointment Bay, Stuart Bay, and Cape Phillips formations in the east and the Blue Fiord formation in the south-east all contain an abundant carbonate components thus leading to a geological contrast between eastern and western Bathurst Island, which is detailed in the geological map provided by Kerr 10. In comparison, the western half is comprised of Griper Bay, Helca Bay, and Bird Fiord formations, which are primarily quartz sandstone, siltstone, and shale with the only minor limestone component being in the Bird Fiord formation 10.
Bathurst Island has a relatively low topography across much of the island, particularly in the east. This has led to poor drainage in many areas, and as a result more developed soils. These soils support a greater abundance and diversity of vegetation than is typically seen on other High Arctic islands. Common species in these areas consist of a diverse assemblage of sedges (e.g., Carex aquatilis stans, Eriophorum angustifolium, Carex membranacea) as well as various other plants (e.g., Saxifraga oppositifolia), grasses (e.g., Dupontia fisheri), and shrubs (e.g., Salix arctica) 13, 14. The distinctive qualities of this island have led to the development of several protected regions on the island. Polar Bear Pass is a National Wildlife Area that bisects the island at its center, which consists of a wetland oasis surrounded by polar desert. To the north of Polar Bear Pass is the proposed site of Tuktusiuqvialuk National Park, which respresents a major calving area for Peary caribou, an endangered species.
The mean annual temperature of Bathurst Island in 2005 was approximately −14.7°C, which is typical for the High Arctic, with summer months (June–August) on average around 2.7°C but getting as warm as 14–18°C. A mean annual precipitation of 125.2 mm was recorded in nearby Resolute Bay (Climate station: Resolute CARS).
Materials and methods
Previous limnological research on Bathurst Island consisted of a survey by Lim et al. 12 of water chemistry and related surface sediment diatom assemblages 11 from sites on eastern Bathurst Island (full diatom and environmental data available in Hadley 15). To these sites, we have added nine new ponds (ponds defined as <2 m deep) sampled on July 9, 2005 by helicopter survey from previously unstudied regions on the western portion of the Bathurst Island chain (Fig. 1). This includes sites from western Bathurst Island as well as sites from neighboring Alexander, Massey, Vanier, and Cameron islands (Fig. 1). While adding more sites from western Bathurst Island would have been ideal, due to logistical constraints (i.e., severe weather and limited helicopter time) it was impossible to acquire additional sediments. Due to the prohibitive cost of this type of fieldwork we are unable to return for additional samples; however, we believe the addition of the new ponds used in this paper substantially increases the ecological and geological gradient of sampling sites from this island. The sampling protocols and “time window” of sampling (i.e., July) used in previous limnological surveys (e.g., 12, 16-18) were closely followed for this study in order to allow for the best possible comparisons of both inter- and intra-island variability.
Surface sediment samples
The nine new study ponds were all shallow (<50 cm depth) and therefore sediments were collected by hand sampling the top 1 cm of sediment, which was subsequently stored in 15 mL plastic scintillation vials. We collected surface sediments from near the center of each pond whenever possible; however, in some cases, collection from closer to shore was necessary where rocks or moss near the center covered the pond bottom. This procedure was identical to previous Arctic surveys (e.g., 12).
Diatoms
For diatom analysis, ∼0.3 g of wet sediments were digested with nitric acid using a CEM MarsX microwave digester 19, rinsed with deionized water until a neutral pH was achieved and permanently mounted on slides using Naphrax®. Diatoms were then enumerated at 1000× under oil immersion using a Leica DMR2 microscope with differential interference contrast. A minimum of 300–400 diatoms valves were counted for each sample and identified using similar taxonomic sources to Lim et al. 11 such as Krammer and Lange-Bertalot 20.
Water chemistry
Water samples were collected and stored using Nalgene® plastic bottles and 125 mL glass bottles. Samples were measured in the field for specific conductivity using a YSI model 33 conductivity meter. Field measurements of pH were conducted using two-point calibrated Hanna pHep pH meters, and temperature was measured using three handheld thermometers. All other chemical (nutrient, major ions) analyses were performed at the National Water Research Institute (NWRI), following the same protocols used in previous Arctic surveys 21.
Water samples were analyzed by NWRI for both major cations (Ca2+, Mg2+, K+, Na+) and major anions (Cl−, SO42−), as well as for a variety of minor ions including barium (Ba), lithium (Li), and strontium (Sr). Nutrients measured included total phosphorus unfiltered (TPU), total phosphorus filtered (TPF), soluble reactive phosphorus (SRPF), nitrate (NO3), nitrate-nitrite (NO3-NO2), ammonia (NH3), total Kjeldahl nitrogen (TKN) and particulate organic nitrogen (PON), dissolved inorganic carbon (DIC), dissolved organic carbon (DOC), and particulate organic carbon (POC) as well as dissolved silica (SiO2) and chlorophyll a, both corrected (Chl a-C) and uncorrected (Chl a-UC) for pheophytin. Metal ions including Al, Be, Cd, Cr, Co, Cu, Fe, Pb, Mn, Mo, Ni, Ag, V, and Zn were also measured.
Statistical analysis
Water chemistry variables were eliminated from further statistical analysis if they were below the detection limit in >50% of the sites. In the very few cases where <50% of sites had values below the detection limit, a value of half the detection limit was used as an estimate to allow the sites to be included in the statistical analysis as has been done in previous limnological surveys 22-24. In this study, one value was lost entirely due to a broken bottle during shipment (TPF for pond B-AV) and was therefore replaced with an average value of all the other sites. CALIBRATE version 1.01 25 was used to assess the normality of all limnological variables. Variables that were not normally distributed were transformed to a normal distribution using square root, log(x) or log(x + 1) transformation, as appropriate. Variables that could not be transformed to achieve a normal distribution were eliminated from analysis. Variables eliminated during these procedures include NO3-NO2, Chl a corrected, Cd, Co, Cu, Cr, Pb, Be, V, and NO2, thus leaving 30 environmental variables to be included in our analysis. We used a Pearson correlation with Bonferroni-adjusted probabilities, run in Systat v. 11.0, to assess and eliminate pairs of significantly correlated environmental variables (p ≤ 0.002) prior to principal components analysis (PCA). PCA was run using the transformed environmental and diatom species data in Canoco v. 4.5 26 to determine the main directions of variation in the environmental data, as well as to determine any potential outliers with respect to both diatom species assemblages and environmental variables.
Confidence intervals were calculated for both diatom species and environmental data using site scores from PCA analysis, and sites were considered outliers if outside the 95% confidence intervals in both the diatom species and environmental datasets. Detrended correspondence analysis (DCA) was used to determine the gradient length for the individual datasets (east and west) and for the combined whole island dataset. Those environmental variables that significantly explained the variability in the diatom data were determined using a canonical correspondence analysis (CCA) with forward selection and Monte Carlo permutation tests (499 unrestricted permutations). These variables were then run in a series of individually constrained detrended canonical correspondence analyses (DCCA) to determine whether unimodal or linear reconstruction techniques would be appropriate.
Diatom-based pH models were constructed using weighted averaging (WA) and weighted averaging with tolerance downweighting (WAtol) with both classical and inverse deshrinking using C2 version 1.3 27 following the procedures outlined by Michelutti et al. 28. Models were constructed using 72 diatom taxa, whose abundance was at least 1% in at least one site. Species data were square root transformed to reduce the impact of dominant taxa and when necessary, environmental variables were transformed to normal distributions using either square root, log(x) or log(x + 1) transformation. The models were validated using bootstrapping and evaluated based upon bootstrapped coefficient of determination (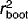 ) and root-mean-squared-error of prediction (RMSEP).
) and root-mean-squared-error of prediction (RMSEP).
Results and discussion
Physical variables
The elevation of the 40 ponds ranged from 3 to 183 m asl. Water temperature at the time of sampling ranged between 1.5 and 13.0°C (Table 1). There was no notable difference in mean elevation between east and west and as with the eastern half of the island 12 the Pearson's correlation matrix (Table 2) showed no significant relationship between elevation and temperature.
| Site ID | Ca (mg/L) | Mg (mg/L) | Na (mg/L) | K (mg/L) | SO4 (mg/L) | Cl (mg/L) | Al (mg/L) | Ba (mg/L) | Fe (mg/L) | Li (mg/L) | Mn (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eastern | |||||||||||
| B-C | 36.6 | 8.0 | 4.6 | 0.6 | 4.9 | 8.50 | 0.005 | 0.067 | 0.012 | 0.001 | 0.0013 |
| B-D | 43.2 | 18.9 | 3.9 | 0.6 | 2.1 | 7.14 | 0.005 | 0.075 | 0.239 | 0.004 | 0.0081 |
| B-E | 43.9 | 14.8 | 6.1 | 0.5 | 3.9 | 12.90 | 0.005 | 0.085 | 0.243 | 0.003 | 0.0044 |
| B-F | 9.2 | 4.1 | 0.7 | 0.1 | 0.6 | 1.16 | 0.005 | 0.005 | 0.015 | 0.001 | 0.0007 |
| B-G | 24.7 | 1.7 | 1.1 | 0.4 | 4.6 | 1.79 | 0.020 | 0.016 | 0.048 | 0.002 | 0.0031 |
| B-H | 19.4 | 2.4 | 1.4 | 0.5 | 6.7 | 1.48 | 0.005 | 0.007 | 0.018 | 0.002 | 0.0019 |
| B-J | 35.1 | 6.8 | 5.7 | 1.3 | 6.8 | 10.00 | 0.005 | 0.057 | 0.038 | 0.004 | 0.0059 |
| B-K | 53.3 | 10.5 | 6.2 | 1.3 | 15.8 | 10.50 | 0.940 | 0.119 | 2.420 | 0.008 | 0.0797 |
| B-M | 29.1 | 4.5 | 3.6 | 0.5 | 3.6 | 5.41 | 0.005 | 0.086 | 0.053 | 0.002 | 0.0038 |
| B-N | 28.8 | 4.7 | 3.2 | 0.5 | 4.8 | 5.63 | 0.020 | 0.074 | 0.064 | 0.002 | 0.0035 |
| B-P | 33.0 | 4.4 | 1.3 | 0.2 | 1.7 | 2.38 | 0.010 | 0.018 | 0.052 | 0.002 | 0.0107 |
| B-R | 29.1 | 4.0 | 1.0 | 0.1 | 0.8 | 1.89 | 0.005 | 0.009 | 0.031 | 0.001 | 0.0009 |
| B-T | 25.6 | 2.1 | 0.9 | 0.1 | 0.8 | 1.82 | 0.005 | 0.013 | 0.034 | 0.002 | 0.0005 |
| B-U | 21.9 | 8.2 | 6.3 | 0.3 | 3.8 | 10.70 | 0.190 | 0.066 | 1.280 | 0.002 | 0.0107 |
| B-V | 23.1 | 8.4 | 6.5 | 0.2 | 6.9 | 10.10 | 0.020 | 0.044 | 0.361 | 0.002 | 0.0037 |
| B-W | 26.9 | 8.9 | 5.2 | 0.3 | 2.4 | 9.85 | 0.040 | 0.044 | 0.249 | 0.001 | 0.0035 |
| B-X | 26.4 | 7.4 | 6.5 | 0.3 | 3.7 | 10.20 | 0.005 | 0.051 | 0.103 | 0.002 | 0.0018 |
| B-Z | 33.8 | 8.6 | 7.9 | 0.9 | 5.0 | 19.10 | 0.290 | 0.175 | 0.774 | 0.004 | 0.0107 |
| B-AC | 41.8 | 5.2 | 1.5 | 0.4 | 18.6 | 2.83 | 0.010 | 0.131 | 0.040 | 0.001 | 0.0017 |
| B-AD | 19.9 | 5.2 | 2.9 | 0.5 | 2.5 | 4.66 | 0.090 | 0.020 | 1.410 | 0.002 | 0.0174 |
| B-AE | 32.0 | 2.9 | 1.5 | 0.2 | 7.0 | 2.87 | 0.005 | 0.039 | 0.029 | 0.001 | 0.0017 |
| B-AG | 28.3 | 2.6 | 1.5 | 0.2 | 7.0 | 2.88 | 0.005 | 0.034 | 0.042 | 0.001 | 0.0007 |
| B-AH | 33.8 | 6.1 | 1.6 | 0.3 | 16.2 | 1.97 | 0.040 | 0.025 | 0.066 | 0.003 | 0.0014 |
| B-AI | 38.0 | 6.9 | 1.8 | 0.4 | 22.0 | 2.83 | 0.005 | 0.032 | 0.043 | 0.002 | 0.0008 |
| B-AJ | 43.7 | 7.8 | 3.2 | 0.9 | 18.7 | 4.46 | 0.005 | 0.161 | 0.139 | 0.003 | 0.0043 |
| B-AK | 15.7 | 2.1 | 1.1 | 0.2 | 2.8 | 1.40 | 0.005 | 0.056 | 0.096 | 0.002 | 0.0033 |
| B-AL | 36.7 | 5.7 | 2.5 | 0.5 | 13.6 | 3.48 | 0.005 | 0.121 | 0.079 | 0.001 | 0.0021 |
| B-AM | 28.6 | 2.7 | 3.3 | 0.3 | 2.4 | 8.53 | 0.010 | 0.088 | 0.035 | 0.001 | 0.0017 |
| B-AN | 33.1 | 3.9 | 2.8 | 0.3 | 3.6 | 5.24 | 0.010 | 0.056 | 0.140 | 0.001 | 0.0017 |
| B-AO | 11.3 | 5.1 | 6.4 | 0.3 | 0.4 | 12.80 | 0.014 | 0.036 | 0.137 | 0.000 | 0.0021 |
| B-AP | 10.9 | 5.0 | 5.0 | 0.4 | 0.5 | 9.42 | 0.025 | 0.056 | 0.158 | 0.000 | 0.0027 |
| Mean | 29.6 | 6.1 | 3.5 | 0.4 | 6.3 | 6.26 | 0.0583 | 0.060 | 0.273 | 0.002 | 0.006 |
| Max | 53.3 | 18.9 | 7.9 | 1.3 | 22.0 | 19.10 | 0.9400 | 0.175 | 2.420 | 0.008 | 0.080 |
| Min | 9.2 | 1.7 | 0.7 | 0.1 | 0.4 | 1.16 | 0.0050 | 0.005 | 0.012 | 0.000 | 0.001 |
| Std | 10.4 | 3.7 | 2.2 | 0.3 | 6.0 | 4.45 | 0.1744 | 0.044 | 0.524 | 0.002 | 0.014 |
| Western | |||||||||||
| B-AR | 0.8 | 0.3 | 0.4 | 0.3 | 0.4 | 0.58 | 0.26 | 0.004 | 0.321 | 0.0003 | 0.0052 |
| B-AS | 0.2 | 0.1 | 0.4 | 0.1 | 0.5 | 0.71 | 0.04 | 0.003 | 0.066 | 0.0001 | 0.0068 |
| B-AT | 0.4 | 0.4 | 0.9 | 0.2 | 0.5 | 1.68 | 0.08 | 0.001 | 0.099 | 0.0002 | 0.0019 |
| B-AU | 10.9 | 15.8 | 104.0 | 9.3 | 4.8 | 184.00 | 0.04 | 0.011 | 0.077 | 0.0031 | 0.0071 |
| B-AV | 6.3 | 2.3 | 6.6 | 0.5 | 0.8 | 11.80 | 0.16 | 0.002 | 0.245 | 0.0005 | 0.0014 |
| B-AW | 0.3 | 0.3 | 0.4 | 0.2 | 0.4 | 0.65 | 0.18 | 0.001 | 0.298 | 0.0002 | 0.0048 |
| B-AX | 9.6 | 5.4 | 15.9 | 1.0 | 0.6 | 32.10 | 0.01 | 0.006 | 0.124 | 0.0021 | 0.0031 |
| B-AY | 23.9 | 5.8 | 5.9 | 1.1 | 3.5 | 7.33 | 0.13 | 0.122 | 0.527 | 0.0025 | 0.0143 |
| B-AZ | 1.6 | 0.5 | 0.3 | 0.2 | 0.4 | 0.55 | 0.17 | 0.003 | 0.301 | 0.0004 | 0.0046 |
| Mean | 6.0 | 3.4 | 15.0 | 1.4 | 1.3 | 26.60 | 0.12 | 0.017 | 0.229 | 0.001 | 0.0055 |
| Max | 23.9 | 15.8 | 104.0 | 9.3 | 4.8 | 184.00 | 0.26 | 0.122 | 0.527 | 0.003 | 0.0143 |
| Min | 0.2 | 0.1 | 0.3 | 0.1 | 0.4 | 0.55 | 0.01 | 0.001 | 0.066 | 0.000 | 0.0014 |
| Std | 7.9 | 5.2 | 33.8 | 3.0 | 1.6 | 59.91 | 0.08 | 0.039 | 0.152 | 0.001 | 0.0039 |
| Site ID | Mo (µg/L) | Ni (mg/L) | Sr (mg/L) | Zn (µg/L) | SiO2 (mg/L) | DOC (mg/L) | DIC (mg/L) | POC (µg/L) | SRPF (µg/L) | TPU (µg/L) | TPF (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eastern | |||||||||||
| B-C | 0.0010 | 0.001 | 0.077 | 0.005 | 0.15 | 3.3 | 26.2 | 347.9 | 1.1 | 7.2 | 5.9 |
| B-D | 0.0010 | 0.001 | 0.059 | 0.002 | 4.68 | 11.2 | 39.9 | 679.7 | 1.6 | 14.0 | 10.0 |
| B-E | 0.0010 | 0.001 | 0.069 | 0.001 | 3.36 | 10.7 | 35.8 | 695.7 | 0.9 | 11.6 | 10.0 |
| B-F | 0.0005 | 0.001 | 0.005 | 0.007 | 0.18 | 2.5 | 9.3 | 392.4 | 1.4 | 17.5 | 3.8 |
| B-G | 0.0020 | 0.002 | 0.097 | 0.002 | 0.23 | 1.5 | 14.3 | 357.8 | 1.0 | 5.5 | 4.3 |
| B-H | 0.0010 | 0.001 | 0.082 | 0.001 | 0.15 | 1.7 | 11.8 | 286.4 | 1.4 | 3.3 | 3.6 |
| B-J | 0.0010 | 0.002 | 0.179 | 0.008 | 0.40 | 5.8 | 23.7 | 473.3 | 1.0 | 7.0 | 5.4 |
| B-K | 0.0010 | 0.007 | 0.426 | 0.014 | 2.12 | 5.8 | 29.2 | 1100.0 | 1.1 | 64.0 | 8.8 |
| B-M | 0.0005 | 0.004 | 0.063 | 0.015 | 0.13 | 2.6 | 19.2 | 450.6 | 1.2 | 7.9 | 2.8 |
| B-N | 0.0010 | 0.001 | 0.056 | 0.006 | 0.49 | 1.9 | 18.9 | 366.1 | 0.9 | 4.8 | 3.1 |
| B-P | 0.0010 | 0.003 | 0.030 | 0.003 | 0.05 | 6.7 | 20.9 | 608.3 | 1.2 | 15.1 | 8.6 |
| B-R | 0.0030 | 0.001 | 0.041 | 0.007 | 0.06 | 5.9 | 20.1 | 705.0 | 1.8 | 17.3 | 11.5 |
| B-T | 0.0005 | 0.001 | 0.021 | 0.002 | 0.44 | 1.7 | 15.8 | 389.9 | 1.1 | 3.7 | 3.9 |
| B-U | 0.0010 | 0.009 | 0.032 | 0.006 | 0.85 | 6.2 | 18.8 | 1334.2 | 1.4 | 38.2 | 9.4 |
| B-V | 0.0010 | 0.001 | 0.027 | 0.001 | 0.91 | 6.5 | 18.8 | 925.7 | 1.5 | 14.0 | 6.9 |
| B-W | 0.0010 | 0.001 | 0.028 | 0.001 | 0.14 | 10.9 | 21.7 | 527.3 | 1.4 | 8.7 | 6.5 |
| B-X | 0.0010 | 0.001 | 0.035 | 0.001 | 0.62 | 4.9 | 19.7 | 595.8 | 4.3 | 9.8 | 5.6 |
| B-Z | 0.0020 | 0.003 | 0.095 | 0.004 | 0.50 | 3.7 | 22.1 | 1195.8 | 2.3 | 45.4 | 9.0 |
| B-AC | 0.0020 | 0.005 | 0.128 | 0.003 | 0.86 | 3.8 | 22.5 | 503.0 | 1.8 | 4.0 | 10.5 |
| B-AD | 0.0005 | 0.001 | 0.023 | 0.029 | 2.08 | 5.1 | 15.1 | 833.1 | 1.4 | 28.0 | 11.5 |
| B-AE | 0.0010 | 0.001 | 0.251 | 0.0005 | 0.21 | 1.8 | 18.3 | 469.0 | 1.4 | 4.8 | 4.3 |
| B-AG | 0.0005 | 0.001 | 0.215 | 0.002 | 0.15 | 1.6 | 15.9 | 447.2 | 1.4 | 5.8 | 5.4 |
| B-AH | 0.0005 | 0.001 | 0.283 | 0.001 | 0.55 | 3.2 | 19.7 | 473.3 | 1.4 | 15.1 | 4.4 |
| B-AI | 0.0010 | 0.001 | 0.351 | 0.004 | 0.89 | 3.8 | 21.2 | 586.5 | 1.4 | 7.6 | 4.5 |
| BAJ | 0.0030 | 0.003 | 0.153 | 0.017 | 1.12 | 6.9 | 26.6 | 640.3 | 1.4 | 16.0 | 8.8 |
| B-AK | 0.0010 | 0.004 | 0.035 | 0.004 | 0.15 | 2.2 | 10.1 | 515.8 | 1.4 | 9.7 | 4.3 |
| B-AL | 0.0030 | 0.003 | 0.106 | 0.005 | 0.66 | 4.8 | 22.2 | 630.9 | 1.4 | 9.6 | 4.6 |
| B-AM | 0.0010 | 0.003 | 0.041 | 0.005 | 0.20 | 1.8 | 16.4 | 474.3 | 1.4 | 7.3 | 1.7 |
| B-AN | 0.0005 | 0.004 | 0.032 | 0.002 | 0.76 | 2.7 | 20.2 | 725.3 | 1.4 | 12.3 | 3.6 |
| B-AO | 0.0003 | 0.000 | 0.017 | 0.001 | 0.12 | 6.3 | 10.4 | 810.6 | 0.4 | 15.4 | 5.9 |
| B-AP | 0.0002 | 0.000 | 0.015 | 0.001 | 0.17 | 4.7 | 10.7 | 915.4 | 0.4 | 14.8 | 3.8 |
| Mean | 0.0011 | 0.002 | 0.099 | 0.005 | 0.75 | 4.6 | 19.9 | 627.6 | 1.4 | 14.4 | 6.2 |
| Max | 0.0030 | 0.009 | 0.426 | 0.029 | 4.68 | 11.2 | 39.9 | 1334.2 | 4.3 | 64.0 | 11.5 |
| Min | 0.0002 | 0.000 | 0.005 | 0.001 | 0.05 | 1.5 | 9.3 | 286.4 | 0.4 | 3.3 | 1.7 |
| Std | 0.0008 | 0.002 | 0.105 | 0.006 | 1.03 | 2.7 | 6.9 | 255.9 | 0.7 | 13.2 | 2.8 |
| Western | |||||||||||
| B-AR | 0.0025 | 0.67 | 0.002 | 1.54 | 0.67 | 4.5 | 1.5 | 560.4 | 1.1 | 9.3 | 5.5 |
| B-AS | 0.0025 | 0.17 | 0.001 | 0.47 | 0.19 | 1.6 | 0.6 | 713.4 | 0.2 | 6.8 | 1.1 |
| B-AT | 0.0025 | 0.31 | 0.002 | 0.52 | 0.05 | 1.9 | 1.1 | 494.0 | 0.2 | 11.7 | 1.6 |
| B-AU | 0.0910 | 0.31 | 0.097 | 0.43 | 0.15 | 4.4 | 14.2 | 485.8 | 0.7 | 10.2 | 6.1 |
| B-AV | 0.0270 | 0.68 | 0.025 | 0.89 | 0.33 | 8.2 | 5.3 | 544.1 | 0.4 | 6.5 | 3.8 |
| B-AW | 0.0050 | 0.57 | 0.001 | 1.37 | 0.21 | 2.4 | 1 | 505.0 | 0.2 | 9.1 | 3.2 |
| B-AX | 0.0790 | 0.41 | 0.042 | 0.48 | 0.27 | 6.5 | 8.7 | 658.0 | 0.5 | 9.0 | 5.0 |
| B-AY | 1.1900 | 1.35 | 0.063 | 3.60 | 0.07 | 15.1 | 18.8 | 1679.3 | 1.0 | 26.9 | 7.7 |
| B-AZ | 0.0050 | 0.55 | 0.002 | 0.96 | 0.17 | 3.4 | 1.9 | 629.3 | 0.2 | 9.7 | 2.9 |
| Mean | 0.156 | 0.558 | 0.026 | 1.140 | 0.23 | 5.3 | 5.9 | 696.6 | 0.5 | 11.0 | 4.1 |
| Max | 1.190 | 1.350 | 0.097 | 3.600 | 0.67 | 15.1 | 18.8 | 1679.3 | 1.1 | 26.9 | 7.7 |
| Min | 0.003 | 0.170 | 0.001 | 0.430 | 0.05 | 1.6 | 0.6 | 485.8 | 0.2 | 6.5 | 1.1 |
| Std | 0.389 | 0.345 | 0.035 | 1.008 | 0.19 | 4.3 | 6.7 | 376.8 | 0.4 | 6.2 | 2.2 |
| Site ID | NO2 (mg/L) | NO3-NO2 (mg/L) | NH3 (mg/L) | TKN (mg/L) | PON (µg/L) | TN (µg/L) | Chl a U (µg/L) | Temp (°C) | pH | Cond (µs/cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Eastern | ||||||||||
| B-C | 0.001 | 0.010 | 0.0025 | 0.158 | 13.0 | 420.49 | 0.2 | 11.0 | 8.2 | 211.0 |
| B-D | 0.001 | 0.005 | 0.0060 | 0.898 | 39.0 | 991.63 | 0.9 | 19.5 | 8.4 | 282.0 |
| B-E | 0.001 | 0.005 | 0.0200 | 0.948 | 42.0 | 1020.65 | 0.5 | 19.5 | 8.3 | 276.0 |
| B-F | 0.001 | 0.005 | 0.0025 | 0.099 | 9.0 | 328.64 | 0.9 | 16.5 | 8.3 | 69.0 |
| B-G | 0.001 | 0.005 | 0.0025 | 0.123 | 7.0 | 362.71 | 0.4 | 4.0 | 8.1 | 111.0 |
| B-H | 0.001 | 0.005 | 0.0025 | 0.065 | 8.0 | 267.95 | 0.1 | 10.0 | 8.0 | 109.0 |
| B-J | 0.001 | 0.005 | 0.0060 | 0.473 | 22.0 | 714.75 | 1.3 | 18.0 | 8.4 | 211.0 |
| B-K | 0.001 | 0.005 | 0.0025 | 0.389 | 117.0 | 745.70 | 0.1 | 16.0 | 8.1 | 266.0 |
| B-M | 0.001 | 0.005 | 0.0050 | 0.206 | 19.0 | 477.87 | 0.1 | 3.5 | 8.2 | 139.0 |
| B-N | 0.001 | 0.005 | 0.0025 | 0.164 | 11.0 | 420.97 | 0.1 | 5.0 | 8.1 | 131.0 |
| B-P | 0.001 | 0.005 | 0.0130 | 0.681 | 44.0 | 874.23 | 0.1 | 9.0 | 8.3 | 137.0 |
| B-R | 0.002 | 0.005 | 0.0110 | 0.504 | 54.0 | 768.93 | 0.4 | 8.5 | 8.3 | 131.0 |
| B-T | 0.002 | 0.005 | 0.0025 | 0.075 | 18.0 | 296.86 | 0.7 | 6.0 | 8.2 | 112.0 |
| B-U | 0.001 | 0.005 | 0.0050 | 0.543 | 196.0 | 937.89 | 3.4 | 7.5 | 8.1 | 152.0 |
| B-V | 0.002 | 0.005 | 0.0070 | 0.627 | 92.0 | 888.83 | 0.8 | 9.0 | 8.2 | 160.0 |
| B-W | 0.0005 | 0.005 | 0.0170 | 1.080 | 26.0 | 1070.23 | 0.2 | 13.0 | 8.3 | 176.0 |
| B-X | 0.0005 | 0.019 | 0.0025 | 0.517 | 45.0 | 783.03 | 0.8 | 9.5 | 8.3 | 168.0 |
| B-Z | 0.001 | 0.005 | 0.0025 | 0.258 | 134.0 | 646.94 | 1.6 | 7.0 | 8.2 | 215.0 |
| B-AC | 0.001 | 0.005 | 0.006 | 0.339 | 18.0 | 605.24 | 1.1 | 8.0 | 8.3 | 200.0 |
| B-AD | 0.001 | 0.005 | 0.005 | 0.450 | 72.0 | 747.82 | 2.0 | 9.0 | 8.3 | 128.0 |
| B-AE | 0.0005 | 0.005 | 0.013 | 0.107 | 23.0 | 355.11 | 1.3 | 4.0 | 8.3 | 140.0 |
| B-AG | 0.001 | 0.005 | 0.005 | 0.094 | 21.0 | 332.59 | 1.7 | 8.0 | 8.4 | 135.0 |
| B-AH | 0.001 | 0.005 | 0.011 | 0.317 | 13.0 | 581.03 | 1.6 | 9.0 | 8.5 | 110.0 |
| B-AI | 0.002 | 0.005 | 0.009 | 0.324 | 31.0 | 605.21 | 2.5 | 10.5 | 8.5 | 196.0 |
| BAJ | 0.003 | 0.005 | 0.035 | 0.752 | 43.0 | 915.18 | 1.6 | 12.0 | 8.6 | 216.0 |
| B-AK | 0.001 | 0.005 | 0.007 | 0.144 | 30.0 | 414.47 | 0.1 | 5.0 | 8.3 | 88.0 |
| B-AL | 0.003 | 0.005 | 0.007 | 0.488 | 40.0 | 743.57 | 0.8 | 12.0 | 8.5 | 173.0 |
| B-AM | 0.001 | 0.005 | 0.005 | 0.129 | 16.0 | 380.17 | 0.1 | 5.0 | 8.3 | 149.0 |
| B-AN | 0.002 | 0.005 | 0.006 | 0.264 | 54.0 | 572.81 | 0.3 | 8.0 | 8.5 | 169.0 |
| B-AO | 0.003 | 0.010 | 0.006 | 0.485 | 71.0 | 777.42 | 1.1 | 7.0 | 8.1 | 85.0 |
| B-AP | 0.003 | 0.009 | 0.008 | 0.410 | 72.0 | 721.31 | 1.1 | 7.0 | 7.8 | 81.0 |
| Mean | 0.001 | 0.006 | 0.008 | 0.391 | 45.2 | 637.75 | 0.9 | 9.6 | 8.3 | 158.9 |
| Max | 0.003 | 0.019 | 0.035 | 1.080 | 196.0 | 1070.23 | 3.4 | 19.5 | 8.6 | 282.0 |
| Min | 0.001 | 0.005 | 0.003 | 0.065 | 7.0 | 267.95 | 0.1 | 3.5 | 7.8 | 69.0 |
| Std | 0.001 | 0.003 | 0.007 | 0.273 | 42.1 | 237.58 | 0.8 | 4.4 | 0.2 | 56.1 |
| Western | ||||||||||
| B-AR | 0.003 | 0.007 | 0.007 | 0.139 | 9.0 | 388.83 | 1.00 | 6.0 | 7.0 | 10.0 |
| B-AS | 0.003 | 0.013 | 0.021 | 0.059 | 51.0 | 306.90 | 2.00 | 6.0 | 6.9 | 5.0 |
| B-AT | 0.002 | 0.016 | 0.010 | 0.060 | 13.0 | 273.95 | 1.50 | 1.5 | 6.8 | 10.0 |
| B-AU | 0.002 | 0.008 | 0.019 | 0.425 | 13.0 | 672.92 | 0.05 | 9.0 | 8.3 | 510.0 |
| B-AV | 0.002 | 0.008 | 0.014 | 0.315 | 17.0 | 586.25 | 0.05 | 9.5 | 7.8 | 60.0 |
| B-AW | 0.001 | 0.006 | 0.007 | 0.059 | 16.0 | 264.90 | 0.90 | 9.0 | 7.3 | 11.0 |
| B-AX | 0.002 | 0.007 | 0.011 | 0.383 | 41.0 | 666.87 | 0.50 | 7.5 | 8.1 | 125.0 |
| B-AY | 0.003 | 0.008 | 0.027 | 0.970 | 281.0 | 1273.89 | 7.00 | 13.0 | 8.4 | 135.0 |
| B-AZ | 0.002 | 0.009 | 0.008 | 0.103 | 45.0 | 374.94 | 1.40 | 2.0 | 7.3 | 13.0 |
| Mean | 0.002 | 0.009 | 0.014 | 0.279 | 54.0 | 534.38 | 1.6 | 7.1 | 7.5 | 97.7 |
| Max | 0.003 | 0.016 | 0.027 | 0.970 | 281.0 | 1273.89 | 7.0 | 13.0 | 8.4 | 510.0 |
| Min | 0.001 | 0.006 | 0.007 | 0.059 | 9.0 | 264.90 | 0.1 | 1.5 | 6.8 | 5.0 |
| Std | 0.001 | 0.003 | 0.007 | 0.297 | 86.6 | 321.15 | 2.1 | 3.7 | 0.6 | 162.9 |
| Site ID | Elev m asl | PON: POP | POC: Chl a-U | TN : TPU | Lake or pond | Lat. °N | Long. °W |
|---|---|---|---|---|---|---|---|
| Eastern | |||||||
| B-C | 91 | 10 | 1739 | 58 | L | 75°03′74″ | 97°59′74″ |
| B-D | 30 | 10 | 755 | 70 | P | 75°04′63″ | 98°02′96″ |
| B-E | 30 | 26 | 1391 | 88 | P | 75°04′63″ | 98°02′96″ |
| B-F | 21 | 1 | 436 | 18 | P | 75°08′25″ | 98°28′94″ |
| B-G | 21 | 6 | 894 | 65 | L | 75°27′43″ | 99°26′67″ |
| B-H | 122 | 27 | 1640 | 80 | L | 75°27′16″ | 99°32′12″ |
| B-J | 0 | 14 | 364 | 101 | P | 75°37′64″ | 99°38′30″ |
| B-K | 91 | 2 | 11 000 | 12 | L | 75°78′63″ | 99°20′20″ |
| B-M | 0 | 4 | 4060 | 60 | L | 75°08′26″ | 97°47′42″ |
| B-N | 0 | 6 | 2680 | 87 | L | 75°08′26″ | 97°47′42″ |
| B-P | 335 | 7 | 7400 | 58 | P | 75°19′02″ | 98°50′58″ |
| B-R | 335 | 9 | 1762 | 44 | P | 75°19′02″ | 98°50′58″ |
| B-T | 61 | 90 | 557 | 79 | P | 75°31′42″ | 98°11′90″ |
| B-U | 30 | 7 | 392 | 24 | P | 75°38′79″ | 98°05′65″ |
| B-V | 152 | 13 | 1157 | 63 | P | 75°39′14″ | 98°02′59″ |
| B-W | 152 | 12 | 2636 | 122 | P | 75°39′14″ | 98°02′59″ |
| B-X | 152 | 11 | 745 | 80 | P | 75°39′14″ | 98°02′59″ |
| B-Z | 0 | 4 | 747 | 14 | L | 75°43′25″ | 98°40′68″ |
| B-AC | 122 | 3 | 457 | 150 | P | 75°56′66″ | 99°04′76″ |
| B-AD | 0 | 3 | 417 | 27 | P | 75°36′01″ | 97°50′99″ |
| B-AE | 61 | 46 | 361 | 73 | L | 76°23′18″ | 98°52′05″ |
| B-AG | 61 | 53 | 263 | 56 | P | 76°23′18″ | 98°52′05″ |
| B-AH | 61 | 1 | 296 | 38 | P | 76°23′18″ | 98°52′05″ |
| B-AI | 61 | 10 | 235 | 79 | P | 76°23′18″ | 98°52′05″ |
| BAJ | 122 | 6 | 400 | 57 | L | 76°39′52″ | 98°52′82″ |
| B-AK | 122 | 6 | 5320 | 42 | P | 76°39′52″ | 98°52′82″ |
| B-AL | 122 | 8 | 789 | 77 | P | 76°39′52″ | 98°52′82″ |
| B-AM | 61 | 3 | 4500 | 51 | P | 76°30′14″ | 98°10′05″ |
| B-AN | 61 | 6 | 2418 | 46 | P | 76°30′14″ | 98°10′05″ |
| B-AO | 22 | 7 | 737 | 50 | P | 75°29′047″ | 97°28′150″ |
| B-AP | 23 | 7 | 832 | 49 | P | 75°29′073″ | 97°28′138″ |
| Mean | 88 | 13 | 1755 | 64 | N/A | N/A | N/A |
| Max | 335 | 90 | 11 000 | 150 | N/A | N/A | N/A |
| Min | 0 | 1 | 235 | 12 | N/A | N/A | N/A |
| Std | 89 | N/A | N/A | N/A | N/A | N/A | N/A |
| Western | |||||||
| B-AR | 152 | 2 | 560 | 42 | P | 75°47′953″ | 101°50′300″ |
| B-AS | 122 | 9 | 357 | 45 | P | 75°44′249″ | 100°55′285″ |
| B-AT | 122 | 1 | 329 | 23 | P | 75°40′130″ | 102°29′006″ |
| B-AU | 3 | 3 | 4720 | 66 | P | 75°46′360″ | 102°38′733″ |
| B-AV | 15 | 6 | 5920 | 90 | P | 76°04′127″ | 102°50′749″ |
| B-AW | 137 | 3 | 561 | 29 | P | 76°12′861″ | 103°01′865″ |
| B-AX | 30 | 10 | 1316 | 74 | P | 76°21′316″ | 103°16′055″ |
| B-AY | 61 | 15 | 240 | 47 | P | 76°22′868″ | 100°29′540″ |
| B-AZ | 183 | 7 | 449 | 39 | P | 76°12′483″ | 99°52′542″ |
| Mean | 92 | 6 | 1606 | 51 | N/A | N/A | N/A |
| Max | 183 | 15 | 5920 | 90 | N/A | N/A | N/A |
| Min | 3 | 1 | 240 | 23 | N/A | N/A | N/A |
| Std | 65 | N/A | N/A | N/A | N/A | N/A | N/A |
- A complete table of all water chemistry variables measured can be found in Hadley 15. Italics indicate sites below detection limit.
| Ca | 1.00 | |||||||||||||||||||||||||||||
| Mg | 0.57 | 1.00 | ||||||||||||||||||||||||||||
| Na | 0.18 | 0.76 | 1.00 | |||||||||||||||||||||||||||
| K | 0.23 | 0.61 | 0.79 | 1.00 | ||||||||||||||||||||||||||
| SO4 | 0.77 | 0.50 | 0.27 | 0.41 | 1.00 | |||||||||||||||||||||||||
| Cl | 0.19 | 0.74 | 0.99 | 0.74 | 0.22 | 1.00 | ||||||||||||||||||||||||
| Al | −0.36 | −0.11 | 0.08 | 0.21 | −0.23 | 0.06 | 1.00 | |||||||||||||||||||||||
| Ba | 0.67 | 0.59 | 0.45 | 0.43 | 0.62 | 0.45 | −0.05 | 1.00 | ||||||||||||||||||||||
| Fe | −0.10 | 0.25 | 0.27 | 0.29 | −0.06 | 0.24 | 0.78 | 0.26 | 1.00 | |||||||||||||||||||||
| Li | 0.64 | 0.59 | 0.40 | 0.55 | 0.55 | 0.36 | 0.15 | 0.47 | 0.31 | 1.00 | ||||||||||||||||||||
| Mn | 0.04 | 0.27 | 0.30 | 0.45 | 0.04 | 0.25 | 0.64 | 0.29 | 0.76 | 0.52 | 1.00 | |||||||||||||||||||
| Mo | 0.64 | 0.25 | −0.07 | −0.02 | 0.53 | −0.07 | −0.28 | 0.51 | −0.09 | 0.28 | −0.05 | 1.00 | ||||||||||||||||||
| Ni | 0.54 | 0.18 | 0.01 | 0.08 | 0.47 | 0.00 | 0.10 | 0.51 | 0.20 | 0.45 | 0.32 | 0.52 | 1.00 | |||||||||||||||||
| Sr | 0.79 | 0.54 | 0.36 | 0.41 | 0.86 | 0.35 | −0.36 | 0.52 | −0.21 | 0.57 | −0.10 | 0.46 | 0.35 | 1.00 | ||||||||||||||||
| Zn | 0.34 | 0.14 | 0.00 | 0.09 | 0.20 | −0.01 | 0.03 | 0.28 | 0.11 | 0.34 | 0.23 | 0.50 | 0.51 | 0.13 | 1.00 | |||||||||||||||
| SiO2 | 0.52 | 0.43 | 0.14 | 0.22 | 0.45 | 0.14 | −0.02 | 0.36 | 0.26 | 0.48 | 0.14 | 0.17 | 0.23 | 0.37 | 0.13 | 1.00 | ||||||||||||||
| DOC | 0.16 | 0.59 | 0.47 | 0.37 | 0.00 | 0.42 | 0.28 | 0.28 | 0.58 | 0.32 | 0.47 | 0.10 | 0.08 | −0.01 | 0.16 | 0.15 | 1.00 | |||||||||||||
| DIC | 0.93 | 0.77 | 0.35 | 0.32 | 0.65 | 0.35 | −0.34 | 0.67 | 0.00 | 0.65 | 0.13 | 0.55 | 0.43 | 0.69 | 0.28 | 0.54 | 0.37 | 1.00 | ||||||||||||
| SRPF | 0.59 | 0.39 | 0.17 | 0.06 | 0.55 | 0.16 | −0.27 | 0.46 | −0.05 | 0.34 | −0.06 | 0.58 | 0.46 | 0.54 | 0.24 | 0.33 | 0.06 | 0.55 | 1.00 | |||||||||||
| NH3 | −0.15 | 0.08 | 0.09 | 0.10 | −0.05 | 0.07 | 0.05 | −0.07 | 0.14 | −0.17 | 0.05 | −0.08 | −0.22 | −0.09 | −0.09 | −0.17 | 0.43 | −0.08 | −0.35 | 1.00 | ||||||||||
| TKN | 0.37 | 0.75 | 0.56 | 0.41 | 0.26 | 0.52 | 0.09 | 0.46 | 0.49 | 0.38 | 0.39 | 0.29 | 0.19 | 0.25 | 0.22 | 0.22 | 0.91 | 0.55 | 0.23 | 0.41 | 1.00 | |||||||||
| TPU | 0.07 | 0.41 | 0.30 | 0.24 | 0.01 | 0.27 | 0.59 | 0.34 | 0.77 | 0.42 | 0.65 | 0.14 | 0.28 | −0.07 | 0.30 | 0.11 | 0.57 | 0.17 | 0.08 | 0.09 | 0.55 | 1.00 | ||||||||
| TPF | 0.48 | 0.56 | 0.26 | 0.25 | 0.26 | 0.26 | 0.15 | 0.38 | 0.44 | 0.46 | 0.44 | 0.49 | 0.38 | 0.26 | 0.40 | 0.35 | 0.64 | 0.58 | 0.46 | 0.08 | 0.65 | 0.52 | 1.00 | |||||||
| Chl a-U | −0.05 | −0.05 | −0.24 | −0.19 | −0.02 | −0.23 | 0.16 | 0.05 | 0.27 | −0.11 | −0.03 | 0.12 | −0.13 | −0.12 | 0.02 | 0.15 | 0.18 | −0.04 | −0.04 | 0.24 | 0.16 | 0.30 | 0.21 | 1.00 | ||||||
| POC | 0.02 | 0.32 | 0.34 | 0.23 | 0.00 | 0.31 | 0.56 | 0.42 | 0.75 | 0.29 | 0.60 | 0.04 | 0.24 | −0.06 | 0.11 | 0.08 | 0.60 | 0.12 | 0.01 | 0.22 | 0.54 | 0.79 | 0.45 | 0.41 | 1.00 | |||||
| PON | 0.14 | 0.34 | 0.29 | 0.13 | 0.05 | 0.27 | 0.39 | 0.42 | 0.72 | 0.32 | 0.60 | 0.15 | 0.28 | −0.01 | 0.16 | 0.11 | 0.61 | 0.23 | 0.10 | 0.19 | 0.60 | 0.75 | 0.52 | 0.38 | 0.89 | 1.00 | ||||
| Temp | 0.41 | 0.65 | 0.32 | 0.35 | 0.26 | 0.28 | −0.07 | 0.31 | 0.22 | 0.51 | 0.24 | 0.19 | 0.08 | 0.22 | 0.20 | 0.39 | 0.64 | 0.58 | 0.19 | 0.15 | 0.60 | 0.34 | 0.47 | 0.13 | 0.23 | 0.23 | 1.00 | |||
| pH | 0.74 | 0.64 | 0.39 | 0.27 | 0.66 | 0.38 | −0.49 | 0.54 | −0.16 | 0.45 | −0.15 | 0.58 | 0.37 | 0.78 | 0.31 | 0.28 | 0.22 | 0.73 | 0.65 | −0.07 | 0.48 | 0.07 | 0.40 | −0.05 | 0.03 | 0.14 | 0.40 | 1.00 | ||
| Cond | 0.74 | 0.86 | 0.69 | 0.64 | 0.68 | 0.69 | −0.28 | 0.59 | −0.02 | 0.64 | 0.13 | 0.40 | 0.33 | 0.76 | 0.20 | 0.40 | 0.31 | 0.82 | 0.53 | −0.04 | 0.53 | 0.14 | 0.50 | −0.21 | 0.09 | 0.15 | 0.46 | 0.78 | 1.00 | |
| Elev. | −0.02 | −0.27 | −0.49 | −0.46 | −0.09 | −0.50 | −0.09 | −0.32 | −0.14 | −0.24 | −0.24 | 0.07 | 0.04 | −0.14 | −0.22 | −0.17 | 0.03 | −0.09 | −0.05 | 0.31 | −0.03 | −0.12 | −0.01 | 0.16 | −0.08 | −0.02 | −0.03 | −0.13 | −0.23 | 1.00 |
| Ca | Mg | Na | K | SO4 | Cl | Al | Ba | Fe | Li | Mn | Mo | Ni | Sr | Zn | SiO2 | DOC | DIC | SRPF | NH3 | TKN | TPU | TPF | Chl a-U | POC | PON | Temp | pH | Cond | Elev. |
pH, specific conductivity, and major ions
Contrary to what was found on the eastern half of Bathurst Island, sites on the western portion of the island show considerably more variability in the range of pH measurements. A very narrow pH gradient (8.0–8.6), attributable to carbonate-rich geological deposits and the resultant high buffering capacity of the lakes and ponds on the eastern half of Bathurst Island was not observed in the western sites. On western Bathurst Island pH varied from 6.8 to 8.4, with 66% of the sites falling outside the pH range recorded by Lim et al. 12. The lack of extensive carbonate-bearing bedrock, described by Kerr 10, has resulted in a weakened buffering capacity of the sites on western Bathurst Island. This reduction in CaCO3 input may render these sites more susceptible to climate-driven pH changes similar to those observed in other poorly buffered High Arctic regions (e.g., 5-8).
Specific conductivity measurements from the western study sites (mean = 97.7 μS/cm) were much lower than those recorded in the east by Lim et al. (2001b; mean = 150.8 μS/cm). The weak negative correlation (r = −0.32) noted between elevation and specific conductivity recorded for the eastern Bathurst Island dataset 12 weakens further (r = −0.23) when sites from the west are included in the statistical analysis. While a strong positive correlation between pH and specific conductivity is now evident (r = 0.78, p = 0.01), this is likely an indication that conductivity is more the result of terrestrial weathering of calcareous bedrock and evaporite deposits than marine influence, at least for the majority of the study sites (Table 1). One exception was pond B-AU, a low elevation site located close to the ocean that has elevated Na and Cl and a high specific conductivity of 510 μS/cm (Table 1).
Calcium levels in the western sites are greatly reduced (range = 0.2–23.9 mg/L, mean = 6.0 mg/L) compared with those observed from the eastern sites (range = 9.2–43.9 mg/L, mean = 30.8; Table 1) and in other High Arctic regions (e.g., 16, 18, 29). The lack of substantial carbonate deposits on western Bathurst explains the reduced Ca2+ concentrations in the ponds. An increase in the correlation between Ca2+ and pH from 0.26 12 to 0.736 (Table 2) in the entire dataset further demonstrates the importance of calcium concentrations in the east versus west pH gradient.
As with eastern Bathurst Island, both Na+ and Cl− concentrations showed negative correlation to the site's distance from the ocean, as measured by elevation (Na+ = −0.49 and Cl− = −0.50). Mean Na+ and Cl− concentrations for the western ponds were greatly elevated due primarily to one site (B-AU), which was likely heavily influenced by its proximity to the ocean (Table 1). When this site was removed from our analyses, the mean Na+ for the western sites dropped from 15.0 to 3.4 mg/L and Cl− from 26.6 to 6.16 mg/L, which is similar to what was recorded by Lim et al. (12; mean Na = 3.3 mg/L and mean Cl = 6.22 mg/L). Furthermore, by removing pond B-AU from the analyses, the major ions follow the same Ca2+>Mg2+>Na+>K+ sequence of relative concentration observed on the eastern half of Bathurst Island, as well as studies on many other High Arctic islands (e.g., 30, 31). Relative concentrations of significant water chemistry variables in the western ponds were, on average, Cl−>DIC>SO42−, and do not follow the same pattern recorded in the eastern sites. Low DIC concentrations observed on western Bathurst Island are likely related to reduced terrestrial input from limestone deposits similar to the effect on calcium concentrations. Several of the western ponds (e.g., B-AU, B-AY) were near the sea, which would account for the relatively high concentrations of both Cl− and SO42− in these ponds compared to the rest of the western sites.
Variability in K+ concentrations has previously been attributed to varying abundances of vascular plants in catchments, with those sites that have the highest plant cover showing proportionally higher K+ concentration 12, 32. This is supported by our data, with the highest K+ concentrations (e.g., 1.0 and 1.1 mg/L; Table 1) occurring in sites with considerable plant growth (B-AX and B-AY, respectively), as noted in our detailed field notes.
Nutrients
In general, major nutrients (nitrogen and phosphorus) are found in similar concentrations in our sites on western Bathurst to what was reported by Lim et al. 12 (Table 1). Sites on Bathurst Island generally follow the pattern found elsewhere in the Arctic (e.g., 18) whereby ponds surrounded by dense vegetation (i.e., ponds in the low-lying sedge dominated wet meadows) contain relatively elevated concentrations of nitrogen, phosphorus, carbon and, in many cases, chlorophyll a. The link between nutrient levels and vegetation is likely amplified on Bathurst Island because these vegetated regions also support more diverse and abundant wildlife, leading to further nutrient enrichment from animal scat. As with the eastern half of the island, TKN is the most variable (0.059–0.970 mg/L, mean = 0.279 mg/L) and is typical of oligotrophic Arctic ponds. Without exception, NO2, NO3-NO2, and NH3 were all near or below detection levels on western Bathurst Island similar to what was described by Lim et al. 12.
Like nitrogen, phosphorus variables did not differ greatly between the eastern and western sites. TPU and TPF values ranged between 6.5–26.9 and 1.1–7.7 μg/L, with means of 11.3 and 4.1 μg/L, respectively. SRPF is lower on the western half of Bathurst Island, with all nine ponds having values (0.1–1.1 μg/L, mean = 0.5 μg/L) below the mean value of 1.4 μg/L found in the eastern sites 12.
Carbon
DOC values across Bathurst Island measured slightly higher (1.1–15.1 mg/L, mean = 4.3 mg/L) than what is commonly reported in many high Arctic environments (e.g., 17, 29). Elevated DOC is probably a result of the abundance of vegetation on some parts of the island, such as at Polar Bear Pass.
Dissolved inorganic carbon (DIC) values vary greatly across Bathurst Island following a similar pattern to what was described above with respect to calcium concentrations. DIC in the west was lower (0.6–18.8 μg/L) than what was observed at the eastern sites (9.3–39.9 μg/L) and in previous Canadian Arctic research (e.g., 16-18). As with calcium, this is undoubtably the result of differences in underlying geology 10.
Chlorophyll a
As is common in most Arctic studies, corrected Chl a measurements were below the detection limit in nearly all sites, and therefore only Chl a values uncorrected for pheophytin (hereafter Chl a) will be discussed. Chl a values ranged between below detection (0.1 μg/L) to a maximum of 7.0 μg/L, with a mean of 1.6 μg/L. B-AY, the pond with the highest Chl a concentration, was a small, shallow pond surrounded by dense vegetation and which had a small herd of muskox present near the pond at the time of sampling. Numerous scats were also observed in the catchment during sampling. Similar outlier sites with atypical Chl a levels have been documented on Banks Island 16 and Melville Island 18, where increased nutrients were also linked to higher concentrations of vegetation and/or wildlife activity.
PCA analysis
PCA analysis highlights the importance of the pH gradient in the variability between eastern and western sites (Fig. 2), with the majority of the western sites plotting in the top right quadrant (low pH, Ca, Cond). The 31 highly buffered eastern sites plot largely along vectors associated with major nutrients (TPU, TN) and DOC, as was observed by Lim et al. 12. Together, PCA axes 1 and 2 explained 65.7% of the total variation within the data.
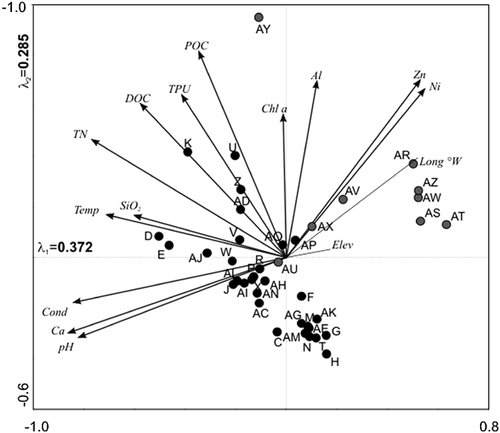
Principal components analysis (PCA) biplot of environmental variables (arrows) and sampling sites (circles). Sampling sites are separated into eastern Bathurst Island (gray) and western Bathurst Island (black) sites. Environmental variables that were plotted passively are indicated by thin lines without arrowhead.
Surface sediment diatom flora
The diatom flora of eastern Bathurst Island (Table 3) can broadly be divided into two primary groups. Several benthic taxa, primarily species from the genera Achnanthes sensu lato, Fragilaria sensu lato, and Nitzschia sensu lato, are typical of the Canadian High Arctic and are found in ponds throughout the Arctic archipelago 34. Ecologically these taxa are often considered generalists and tend to have wide environmental tolerances. Cymbella spp., Amphora spp. and Denticula spp. comprise the second group of diatom taxa that dominates lakes and ponds on eastern Bathurst Island (Fig. 4). In general, these taxonomic groups are epiphytic and have often been found associated with submerged aquatic macrophytes and mosses. A combination of typical Arctic diatom flora and taxa more indicative of higher nutrient concentration and lush aquatic vegetation (submerged mosses and grasses) is not surprising given the variability of the region 12.
| Species name | No. of occurrences | Maximum abundance (%) | Hill's N2 | pH WA optimum |
|---|---|---|---|---|
| Achnanthes childanos Hohn & Hellerman | 19 | 4.75 | 17.2 | 8.3 |
| Achnanthes hostii (Cleve) | 2 | 8.78 | 1.7 | 7 |
| Achnanthes flexella (Kütz.) Brun | 25 | 21.05 | 18.8 | 8.3 |
| Achnanthes kryophila J. B. Petersen | 10 | 7.84 | 8 | 8.2 |
| Achnanthes laevis Østrup | 14 | 3.61 | 12.5 | 8.3 |
| Achnanthes lanceolata (Bréb. ex Kütz.) Grun. | 1 | 8.22 | 1 | 8.1 |
| Achnanthes marginulata Grunow | 21 | 9.09 | 16.5 | 7.9 |
| Achnanthes minutissima Kütz. | 36 | 48.87 | 28.4 | 8.1 |
| Achnanthes oestrupii (H. Bachm. & A. Cleve) Hust. | 8 | 14.85 | 4.9 | 8.2 |
| Achnanthes petersenii Hustedt | 10 | 15.5 | 6.6 | 7.5 |
| Achnanthes sp. 2 | 13 | 4.8 | 11.4 | 7.9 |
| Achnanthes sp. 1 | 7 | 3.27 | 6.1 | 8.3 |
| Achnanthes subatomoides (Hust.) Lange-Bertalot & Archibald | 8 | 5.57 | 6.8 | 8.2 |
| Achnanthes ventralis (Krasske) Lange-Bertalot | 13 | 6.96 | 11 | 8.2 |
| Amphora aequalis Krammer | 11 | 2.05 | 10.5 | 8.3 |
| Amphora inariensis Krammer | 11 | 8.09 | 8.9 | 8.3 |
| Amphora libyca Ehrenberg | 10 | 4.02 | 8.9 | 8.3 |
| Amphora veneta Kützing | 4 | 3.33 | 3.9 | 8.3 |
| Brachysira cf. neoexilis Lange-Bertalot | 1 | 9.04 | 1 | 6.9 |
| Caloneis cf. silicula Ehrenberg | 5 | 10.29 | 3.8 | 8.3 |
| Caloneis schumanniana (Grunow) Cleve | 13 | 3.77 | 12 | 8.3 |
| Caloneis sp. 1 | 15 | 3.19 | 13.6 | 8.4 |
| Cymbella angustata (W. Smith) Cleve | 24 | 15.72 | 18.6 | 8.3 |
| Cymbella arctica (Lagerstedt) Schmidt | 31 | 11.01 | 26.9 | 8.2 |
| Cymbella cf. arctica (Lagerstedt) Schmidt | 12 | 19.94 | 8.3 | 8.4 |
| Cymbella cesatii (Rabenhorst) Grunow | 19 | 4.79 | 16.9 | 8.3 |
| Cymbella designata Krammer | 20 | 9.8 | 16.4 | 8.3 |
| Cymbella latens Krasske | 24 | 4.38 | 20.8 | 8.2 |
| Cymbella microcephala Grunow | 21 | 16.04 | 14.6 | 8.3 |
| Cymbella minuta Hilse | 24 | 31.82 | 17.4 | 8.1 |
| Cymbella silesiaca Bleisch | 24 | 9.09 | 19.4 | 8.1 |
| Cymbella similis Krasske | 15 | 2.93 | 13.3 | 8.3 |
| Cymbella subaequalis Grunow | 5 | 5.66 | 3.9 | 8.3 |
| Cymbella tumidula Grunow | 13 | 9.15 | 8.7 | 8.3 |
| Denticula elegans Kützing | 11 | 22.19 | 7.6 | 8.4 |
| Denticula kuetzingii Grunow | 17 | 37.94 | 12.6 | 8.3 |
| Diadesmis sp. 1 | 15 | 23.44 | 11 | 8.3 |
| Diatoma moniliformis Kütz. | 8 | 6.56 | 6.7 | 8.2 |
| Diatoma oculata (Bréb.) Cleve | 16 | 4.43 | 14.5 | 8.3 |
| Diatoma tenuis Agardh | 3 | 5.01 | 2.3 | 8 |
| Eunotia arcus Ehrenberg | 9 | 6.83 | 7.8 | 8.4 |
| Fragilaria cf. construens (Ehrenberg) Grunow | 7 | 7.29 | 5.7 | 8.2 |
| Fragilaria capucina var. capucina Desmazières | 30 | 32.91 | 21.6 | 8 |
| Fragilaria capucina var. gracilis (Øestrup) Hustedt | 2 | 5.14 | 1.4 | 7.8 |
| Fragilaria capucina var. vaucheriae (Kützing) Lange-Bertalot | 8 | 20.35 | 6 | 7.6 |
| Fragilaria pinnata Ehrenberg | 18 | 92.82 | 11.7 | 8.3 |
| Frustulia rhomboides var. crassinervia (Brébisson) Ross | 1 | 23.67 | 1 | 6.9 |
| Navicula cf. bacilllum Ehrenberg | 2 | 1.68 | 2 | 8.3 |
| Navicula bryophila Petersen | 9 | 4.4 | 7.8 | 8.3 |
| Navicula cf. gallica (W. Smith) Lagerstedt | 8 | 48.65 | 4.5 | 7.5 |
| Navicula cryptocephala Kützing | 15 | 7.03 | 11.7 | 8 |
| Navicula cryptotenella Lange-Bertalot | 5 | 6.9 | 4.3 | 7.5 |
| Navicula jaernefeltii Hustedt | 5 | 5 | 3.9 | 8.1 |
| Navicula pseudoscutiformis Hustedt | 10 | 13.44 | 6.8 | 8.2 |
| Navicula pupula var. pupula Kützing | 5 | 1.86 | 4.2 | 8 |
| Navicula salinarum Grunow | 11 | 2.39 | 10 | 8.3 |
| Navicula soehrensis Krasske | 7 | 5.38 | 5.6 | 8.4 |
| Navicula sp. 2 | 5 | 2.61 | 4.7 | 8.3 |
| Navicula vulpina Kützing | 21 | 10.96 | 16.2 | 8.2 |
| Neidium umiatense Foged. | 7 | 9.09 | 5.5 | 8.2 |
| Nitzschia alpina Hustedt | 14 | 9.09 | 12.1 | 8.2 |
| Nitzschia frustulum (Kützing) Grunow | 35 | 36.9 | 26.8 | 8 |
| Nitzschia inconspicua Grunow | 19 | 20.72 | 12.9 | 8 |
| Nitzschia palea (Kützing) W. Smith | 17 | 4.1 | 14.6 | 8.1 |
| Nitzschia perminuta (Grunow) M. Peragallo | 35 | 26.05 | 27.3 | 8.1 |
| Nitzschia perminuta T1 | 23 | 15.09 | 18.8 | 8.3 |
| Pinnularia balfouriana Grunow | 16 | 58.5 | 12.2 | 8.3 |
| Pinnularia digerntissima Gregory | 5 | 1.68 | 4.5 | 7.7 |
| Pinnularia interrupta W. Smith | 2 | 3.72 | 1.8 | 7 |
| Pinnularia subrostrata (A. Cleve) Cleve-Euler | 13 | 9.09 | 9.8 | 8.2 |
| Stauroneis anceps Ehrenberg | 9 | 1.71 | 8.7 | 8.4 |
| Tabellaria flocculosa strain IV (Roth) Kütz. (str. IV sensu Koppen) | 3 | 12.06 | 2.8 | 7.1 |
However, our CCA indicates that, when considering lakes and ponds across all of Bathurst Island, lakewater pH becomes the primary environmental gradient driving diatom assemblages (Fig. 3). This is in marked contrast to when only eastern sites were considered 11 and diatoms followed a gradient of total nitrogen. Acidiphilous to circumneutral taxa, such as Brachysira cf. neoexilis, Frustulia rhomboides var. crassinervia, and Tabellaria flocculosa, are found exclusively on western Bathurst in one of the most acidic ponds (pH <7) and account for as much as 40% of the diatom relative abundance (Fig. 4).
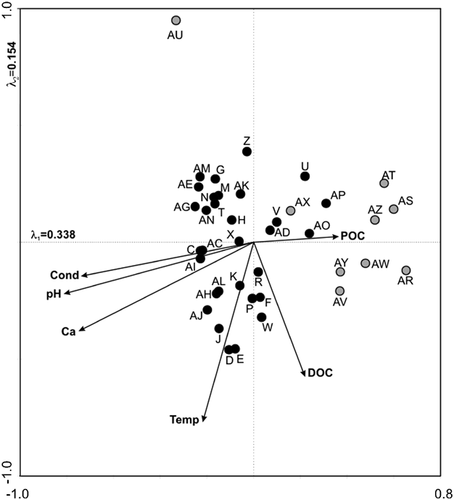
Canonical correspondence analysis (CCA) biplot showing six forward-selected environmental variables (arrows) and study sites (circles). Sites are split into western Bathurst Island (gray) and eastern Bathurst Island (black).
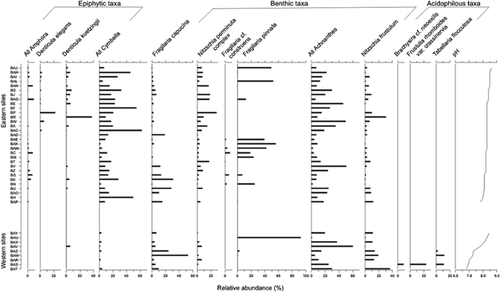
Diatom profile showing the dominant diatom taxa from all 40 Bathurst Island sites. Eastern and western sites are plotted separately in order of declining pH.
Climate-driven changes in the duration and extent of ice cover can influence habitat availability and the length of growing season in Arctic freshwaters (e.g., 1, 2), and may also alter key limnological variables including nutrient levels and lakewater pH 34. In poorly buffered Arctic lakes, lakewater pH changes are primarily controlled by dissolved inorganic carbon (DIC) speciation (i.e., CO2(+H2CO3), HCO3−, and CO32−) driven by photosynthesis and respiration, which is largely controlled by the duration and extent of ice cover 5, 6.
Our data suggests two distinct ecological gradients exist on Bathurst Island, with highly buffered eastern sites more closely tracking limiting nutrients such as nitrogen and phosphorus, while poorly buffered ponds on western Bathurst Island would likely be more susceptible to climate driven pH changes 5, 6. The expansion of the previous survey to include poorly buffered sites on the western half of Bathurst Island has allowed us the opportunity to build a diatom-inferred pH transfer function for Bathurst Island.
Transfer function development
Of the nine western and 31 eastern sites in our dataset, no sites were identified as outliers in both the Bathurst Island diatom and environmental datasets on either axis 1 or axis 2 of the PCA. DCA analysis revealed that the addition of the nine western sites expanded the gradient length from 2.0 to 3.0 SD; therefore, unimodal ordination methods were deemed appropriate 26. Based on the CCA with forward selection, the environmental variables pH, DOC, temperature, specific conductivity, calcium, and POC explained significant (p ≤ 0.05) and independent amounts of variation in the diatom data set, accounting for 27.3% of the variance along the first four ordination axes. The CCA biplot (Fig. 3) identifies pH as the primary gradient represented by axis 1, with temperature and DOC (correlated with other nutrients; i.e., nitrogen and phosphorus) primarily influencing axis 2.
Of the six measured environmental variables we identified as significant during the forward selection of the first CCA analysis, pH accounted for the greatest proportion of variance explained (21.3%) and therefore was judged an appropriate candidate for inference model development. When analyzed as a single constraining variable in a DCCA, pH had a gradient length of 2.3 SD, suggesting that a unimodal model would be appropriate 35. Multiple WA models were constructed, both with and without tolerance downweighting, and using both classical and inverse deshrinking. All models were found to be very similar when compared based upon 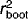 and RMSEP criteria, and therefore we have only demonstrated the one model (WA(cla)) that had a slightly higher
and RMSEP criteria, and therefore we have only demonstrated the one model (WA(cla)) that had a slightly higher 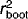 compared to the other three (Fig. 5). This is consistent with previous limnological studies that have shown WA(cla) to be the most robust inference model for diatom-based reconstruction in the Canadian Arctic (e.g., 28, 33, 36). The expanded diatom dataset and weighted average pH diatom-inferred pH model on Bathurst Island showed similar strength (
compared to the other three (Fig. 5). This is consistent with previous limnological studies that have shown WA(cla) to be the most robust inference model for diatom-based reconstruction in the Canadian Arctic (e.g., 28, 33, 36). The expanded diatom dataset and weighted average pH diatom-inferred pH model on Bathurst Island showed similar strength (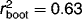 , RMSEP = 0.298) to transfer functions from other studies in the High Arctic 5, 33. Worth noting is the trend observed in residual values relative to observed values. This trend represents a tendency to over-estimate variables at low pH (pH ∼6.5), and under-estimate values at high pH (pH ∼8.5) (Fig. 5). Residual trends such as those observed here are not uncommon in WA models and may result from “edge effects” at each end of the environmental gradient 37.
, RMSEP = 0.298) to transfer functions from other studies in the High Arctic 5, 33. Worth noting is the trend observed in residual values relative to observed values. This trend represents a tendency to over-estimate variables at low pH (pH ∼6.5), and under-estimate values at high pH (pH ∼8.5) (Fig. 5). Residual trends such as those observed here are not uncommon in WA models and may result from “edge effects” at each end of the environmental gradient 37.
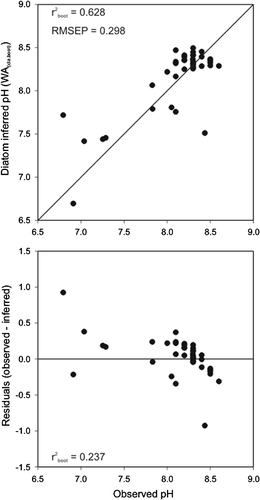
Diatom-inferred versus observed pH and the corresponding residuals for the WA(cla,boot) model.
Conclusions
Major differences in the pH, specific conductivity and major ions, which can be linked to the underlying geology, were recorded when we compared ponds from western to eastern Bathurst Island. Perhaps the most striking limnological differences were those noted for calcium and DIC concentrations on western Bathurst Island, which were lower than those commonly recorded from eastern Bathurst Island, as well as from many other Arctic sites. Measurements of pH also clearly tracked the differences in geology from the eastern (high pH) and western (low pH) parts of the island. Diatom responses to pH have been well established in other Arctic regions (e.g., 33, 34) and, not surprisingly, similar relationships were noted on Bathurst Island. By expanding the previous survey to include poorly buffered sites on the western half of Bathurst Island, and thereby expanding the pH gradient from 0.5 to 2.0 pH units, we were able to develop a reasonably strong pH model for the Bathurst Island dataset. This model has similar robustness to those developed for other Arctic regions (e.g., 28, 33). Although we acknowledge the relatively small number of low pH sites in this study, cross-validation of the inference model via bootstrapping appeared promising and no other pH model is available for this region. Given the increased sensitivity of Arctic environments to climatic change, and previous findings suggesting a first order relationship between climate and pH (e.g., 5-8), such diatom-inferred pH models may be useful in future paleolimnological studies in this important region.
Acknowledgments
The authors wish to thanks Catherine Crawley, Bronwyn Keatley, John Glew, and Wes Blake Jr. for assistance in the field. Two anonymous reviewers provided many useful comments. We also thank Neal Michelutti for his assistance in improving the manuscript. Logistical support in the field was provided by the Polar Continental Shelf Program (PCSP). This work has been made possible by the Natural Sciences and Engineering Research Council (NSERC) grants of John P. Smol and Marianne S.V. Douglas, as well as the Northern Scientific Training Program grant (NSTP) of Kris Hadley.




