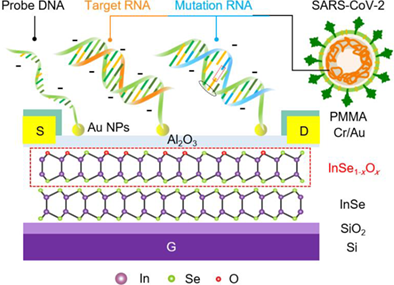
Fast and direct identification of SARS-CoV-2 variants via 2D InSe field-effect transistors
Duo Xu, Junji Li, and Yunhai Xiong contributed equally to this work.
Funding information: National Natural Science Foundation of China, Grant/Award Numbers: 92064007, 62001224, 52131304; Natural Science Foundation of Jiangsu Province, Grant/Award Numbers: BK20190476, BK20190457, BZ2020063; China Postdoctoral Science Foundation, Grant/Award Number: 2021M691600
Abstract
As the COVID-19 pandemic evolves and new variants emerge, the development of more efficient identification approaches of variants is urgent to prevent continuous outbreaks of SARS-CoV-2. Field-effect transistors (FETs) with two-dimensional (2D) materials are viable platforms for the detection of virus nucleic acids (NAs) but cannot yet provide accurate information on NA variations. Herein, 2D Indium selenide (InSe) FETs were used to identify SARS-CoV-2 variants. The device's mobility and stability were ensured by atomic layer deposition (ALD) of Al2O3. The resulting FETs exhibited sub-fM detection limits ranging from 10–14 M to 10–8 M. The recognition of single-nucleotide variations was achieved within 15 min to enable the fast and direct identification of two core mutations (L452R, R203M) in Delta genomes (p < 0.01). Such capability originated from the trap states in oxidized InSe (InSe1−xOx) after ALD, resulting in traps-involved carrier transport responsive to the negative charges of NAs. In sum, the proposed approach might highly provide epidemiological information for timely surveillance of the COVID pandemic.
1 INTRODUCTION
Throughout history, the human species has struggled against infectious diseases. Today, even with the great advances in modern medicine, the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) still spread worldwide to infect more than 600 million (confirmed cases) and kill 6.5 million (as of Oct. 2022).1-3 The frequent emergence of novel SARS-CoV-2 variants, including Beta, Gamma, Delta, and Omicron,4 has raised significant concerns about prevention and vaccination due to their increased transmissibility, virulence, or immune-evading ability.5, 6 For instance, the Delta variant of concern (VOC), also known as lineage B.1.617.2 emerging predominantly in many countries, is 40% more transmissible than the Alpha variant with an incubation period as short as 4 days for secondary transmission.7, 8 The Delta variant consists of mainly several crucial mutations of the variant's genomic domains encoding proteins of spike, nucleocapsid, and envelope, among others.9-11
Current benchmark detection methods, such as real-time quantitative reverse transcription PCR (RT-qPCR) and genome sequencing, are originally proposed in the laboratory as they strongly rely on nucleic-acid amplification with hour-level delay.12, 13 Alternatively, biosensing field-effect transistors with two-dimensional materials (2D bio-FETs) present advantages of short detection time (real-time), low noise, and compatibility with integrated circuits that can be utilized for that purpose.14, 15 These ultrasensitive sensors are based on in-plane homogeneities of 2D materials, such as graphene, black phosphorus (BP), and transition metal dichalcogenides (TMDCs).16-22 The FET structure of these sensors may also retain both real-time electrical characteristics and the unique sensitivity of the 2D materials to the target-gating effect. Until now, 2D bio-FETs have mainly been employed to detect spike proteins or nucleic acids (NAs). However, when multiple variants of SARS-CoV-2 dominate the epidemic spread, the protein strategies become limited since more time would be required to analyze the proteins, providing variant viruses with more time windows to escape quarantine and secondary transmission.23 Therefore, achieving a rapid and direct identification of SARS-CoV-2 NA variations on 2D bio-FETs is urgent for public health interventions.
In this study, a 2D indium selenide (InSe) bio-FET was developed through a two-step atomic layer deposition (ALD) of Al2O3. The resulting sensor showed a fast response and stable performance toward direct NAs detection of SARS-CoV-2 variants at the single-nucleotide level. The sensor also exhibited a detection range as wide as 10−14–10−8 M with a limit of detection (LOD) as low as 0.71 fM for DNA and 0.79 fM for RNA. The superior resolution enabled the constructed devices to quickly identify precise information about SARS-CoV-2 single-nucleotide variations (SNVs) within 15 min, including different mutation types and positions. To verify the identification capacity, two signature mutations of the Delta variant (L452R and R203M) were selected and the results revealed outstanding ability originating from the shallow oxidation of InSe (InSe1−xOx) observed after ALD. The density functional theory (DFT) calculations confirmed the O-doping can result in localized energy states as carrier traps between the conduction band and valence band of InSe, as well as in dramatically enhancing the carrier transport in response to negative charges in NAs.
2 RESULTS AND DISCUSSION
2.1 Fast and ultra-sensitive detection of SARS-CoV-2 via 2D InSe FETs
2D InSe is a promising FET channel material with low effective electron mass (me* = 0.143 m0), adjustable band gap (1.26–2.11 eV), and high intrinsic mobility (>103 cm2 V−1 s−1).24-31 However, the deficient chemical stability of 2D InSe has greatly limited its potential applications in biosensing. In this work, an environmentally stable 2D InSe FET with real-time monitoring, high sensitivity, and sufficient stability was developed for the selective recognition of SARS-CoV-2 nucleic acids (Figure 1A).
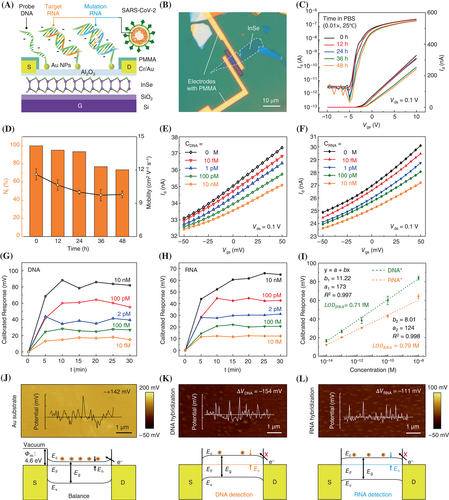
The fabrication of 2D InSe FETs was achieved through a controlled two-step ALD of Al2O3 on 2D InSe flakes. As shown in Figures S1 and S2, the 2D InSe flakes with a thickness of 6–10 nm were obtained from bulk single crystals by mechanical exfoliation. The final structure of 2D InSe FETs (Figures 1B and S3) revealed a channel region exposed for the probe incubation and detection of the target NAs. The regular performances of FETs depicted in Figure S4B and S4C revealed mobility reaching 15.7 cm2 V−1 s−1 at 25°C. The stability of devices greatly improved after Al2O3 encapsulation (Figure 1C). The Id–Vgs curves measured every 12 h in phosphate-buffered saline (0.01× PBS) illustrated preserved on-state current (Vgs = 10 V) as high as 73.5% (NI = I/I0) of the original current, without significant decline in mobility after soaking in PBS for 48 h. These features would fully meet the device stability requirements for probe immobilization (36 h) and sensing in PBS (Figure 1D). Meanwhile, the electrical performance and stability of devices are also tested with different Al2O3 layers, of which the deposit cycle number should consider the balance of InSe passivation and oxidation (Figure S5). Afterward, high-density Au nanoparticles (Au NPs) were fabricated for efficient probe immobilization (Figure S6). The obtained Al2O3 layer prevented the devices from the common p-type doping effect and the positive shift in the threshold voltage (Vth) induced by Au NPs.32, 33
During the detection of NAs by FET, a decrease in Id was observed due to the phosphate skeleton of NAs generating one negative charge per base in PBS. The induced negative charges may ignore the electrical double layer shield within Debye length (λD) of 7.5 nm in PBS (0.01×).34 Meanwhile, the thermal stability of target-probe duplexes influenced the proportion of target NAs hybridized and immobilized on FETs, resulting in variable equivalent negative gate voltages on channels (target-gating effect). After hybridization, Fermi energy (EF) shifted downward, reducing the mobility and density of electrons, thereby resulting in different current drops (Figure 1 E,F, Table S1).
To obtain an accurate relationship between the concentration and the decrease in current, independent NA concentration measurements were conducted and the results are given in Figures S7 and S8. The bio-FET response was defined as a calibrated response (∆Vcal): that can diminish the device-to-device variations.35, 36 Here, ∆Vcal reflects the equivalent negative gate voltage induced by DNA or RNA (target-gating effect), with the value tending to stability and consistency after 10–15 min incubation (Figure 1G,H). The bio-FET exhibited a wide linear work range (10−14–10−8 M), covering the detection of both ultra-low and medium-low concentrations (Figure 1I). From the linear fitting data, the LOD was determined as 0.71 fM for DNA and 0.79 fM for RNA based on signal/noise = 3 (Figure S9). Although the long-term stability and repeatability need to be improved due to the strict requirement of the Al2O3 thickness (Figure S10), the bio-FET shows enough stability and robustness in a single test.
To confirm the target-gating effect of NAs and the target-probe hybridization efficiency, the surface potentials (Figure 1J–L, top) with or without DNA and RNA hybridization were directly detected through Kelvin probe force microscopy (KPFM) and the resulting band bendings are depicted in Figure 1J–L (bottom). The balanced state corresponded to the surface potential of Au substrate without duplex formation. During NAs detection, the formed negative potential served as the negative voltage to reduce the gate field effect. Accordingly, Fermi energy moved downward, reducing both the electron mobility and density. The DNA induced a larger potential difference (∆VDNA) of −154 mV than that of the RNA (∆VRNA = −111 mV, Figure 1K,L). Thus, the double-stranded DNA (dsDNA) generated a more distinct target-gating effect and significant responses than DNA–RNA duplexes due to the different thermodynamic stability of the duplex.
Compared to other diagnostic methods, such as RT-qPCR, genome sequencing, and electrochemical biosensors,37 the proposed 2D InSe bio-FETs showed comprehensive advantages in terms of fast detection (10–15 min) and wide detection range (106). These features may potentially facilitate the application of 2D bio-FETs in SARS-CoV-2 nucleic acids testing to distinguish asymptomatic cases and even subclinical infections in early-stage diagnosis.38, 39
2.2 Direct identification of single-nucleotide variations
SNVs with multiple types of mutation are common in SARS-CoV-2 variants. For example, the R203M and R203K in Figure 2A represent in-situ mutations of Delta and Gamma variants, respectively. These variants have different nucleotide mismatched types but are in the same position. In this regard, the proposed 2D InSe FETs can be used to identify SNVs. As shown in Figure S11, FETs were employed to distinguish DNA sequences with different mismatch types in the same 6th position, and the device responses were compared with (non-)complementary sequences. As can be seen in Figure 2B, the ∆Vcal of complementary DNA far exceeded that of noncomplementary and mismatched sequences. All the responses became stable after 15 min, with the signal of C–C mismatch lower than those of C–T and C–A mismatch.

The reliability of the sensing results was verified by analyzing the (thermal) stability of mismatched DNA using high-resolution melting (HRM) analysis. The HRM technology can be employed to monitor the process of dsDNA separation (fluorescence signal attenuation) as a function of the temperature rise. In Figure 2C, the normalized reporter (Rn) of fluorescence intensity represents the dsDNA proportion in the solution, and Tm is the temperature corresponding to the melting curve for Rn = 0.5, considered a crucial indicator for evaluating sequences' thermal stability. The Tm of the complementary sequence was found 11°C higher than that of the C–C mismatch type. By comparison, the Tm values of C–A (55.7°C) and C–T (55.2°C) mismatch sequences were very close, which were highly consistent with the ∆Vcal of 2D bio-FETs (Figure 2D).
The mismatched nucleotide position is also crucial mutation information requiring determination. For example, the core mutation of D614G (Figure 2A) has been detected in Delta, Beta, and Gamma variants, meaning that the location of D614G can be used as a universal signature for identifying VOCs. Similarly, the states of duplexes formed by sequences with different mismatched positions were analyzed through HRM (Table 1). In Figure 2E, the HRM data illustrated the proportion of position-mismatched dsDNA remaining high at 40°C. However, the melting curves showed noticeable differences at 60°C. Note that Td is the temperature at which the derivative value of −Rn reached a maximum, revealing the most intense separation under the random thermal motion. Hence, Td was more suitable for revealing the difference in the slighter thermodynamics of mutations with the same mismatched type but various positions. As shown in Figure 2F, the Td values of the 21st, 13th, and 6th mismatched sequences were determined as 65.3°C, 60.9°C, and 59.3°C, respectively.
| ssDNA/ssRNA | Modification on Au NPs | Sequences of DNA (22-mer) | Mismatch position | Mismatch type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Probe DNA (ORF1ab) | HS(C6)– | GCA | GAC | GCC | ATA | CAC | CTT | TCCA | / | / |
| Complementary DNA | CGT | CTG | CGG | TAT | GTG | GAA | AGGT | / | / | |
| Mismatch 1–3 | CGT | CTX | CGG | TAT | GTG | GAA | AGGT | 6 | C–X X = A, T, C |
|
| Mismatch 4 | CGT | CTG | CGG | TAT | TTG | GAA | AGGT | 13 | C–T | |
| Mismatch 5 | CGT | CTG | CGG | TAT | GTG | GAA | AGTT | 21 | C–T | |
| Probe DNA (N) | HS(C6)– | GAG | AAG | TTC | CCC | TAC | TGC | TGCC | / | / |
| Complementary RNA | CUC | UUC | AAG | GGG | AUG | ACG | ACGG | / | / | |
| Mutant RNA (Delta, N) | CUC | UUC | AAG | GGA | AUG | ACG | ACGG | 12 | C–A | |
| Probe DNA (S) | HS(C6)– | ACA | ATC | TAT | ACA | GGT | AAT | TATA | / | / |
| Complementary RNA | UGU | UAG | AUA | UGU | CCA | UUA | AUAU | / | / | |
| Mutant RNA (Delta, S) | UGU | UAG | AUA | UGG | CCA | UUA | AUAU | 12 | A–G | |
- Note: The target DNA (ORF1ab) with different mismatch types (C–A, C–T, C–C) at the 6th position, and C–T mismatched sequences at different positions (6th, 13th, 21st). Sequences of the target RNA (Delta, N) and RNA (Delta, S). The direction of the probe sequence is 3' → 5', while the sequence direction of the target is 5' → 3'.
Accordingly, the normalized responses of bio-FETs to mismatched DNA sequences were measured in different positions with the same C–T mismatch (Figure S12). At both incubation temperatures of 40°C and 60°C, the ∆Vcal to mismatched sequences in different positions were significantly lower than those of complementary sequences (Figure 2G). However, the average responses of sequences with different SNVs positions at 40°C overlapped when compared to the ∆Vcal measured at 60°C and cannot be identified in the range of 25%–30%. In line with the HRM curves, the exposure of FETs at 60°C resulted in a much more obvious difference in ∆Vcal. Especially, the ∆Vcal of 21st and 6th mismatched sequences presented significant differences (**p < 0.01, †p < 0.05), suggesting the hybridization difference of targets harboring different mismatch positions. Herein, the ∆Vcal of 2D InSe FET was quite consistent with the Td obtained from HRM analysis.
The as-prepared bio-FETs presented temperature-resolved detection properties, and can directly distinguish SNVs with different types and positions from their (non-)complementary sequences. Since the stability of various target-probe duplexes can be affected by many factors, such as helix conformation and C–G base-pair proportion, the HRM for different target sequences was employed to guide the temperature setting modulation.
Based on the excellent performance of the proposed sensor for identifying SNVs of DNA sequences, it can be concluded that the 2D InSe FETs may be useful for detecting core mutations of the Delta variant. The first mutation L452R (S) accounts for virus escape from antibody LY-CoV555, making the Delta variant more transmissible.10 The R203M (N) is a signature mutation of the Delta variant, with in-situ mutations with different single-nucleotide types (R203K) found in the Gamma variant.
The good consistency between HRM analysis and device response indicated that HRM can be used to optimize the sensor temperature for the detection of the thermodynamic mismatch of specific sequences. For mutant RNA (R203M, N), the melting curves of complementary and mismatched sequences may be distinguished at 60°C, while RNA (L452R, S) curves looked different at 40°C (Figure 2H). Therefore, the bio-FETs were exposed to PBS (0.01×) containing two target RNA sequences at corresponding temperatures (Figure S13). The mean response of the RNA (R203M, N) sequence (30.2 mV) can clearly be distinguished from the mutant sequence (16.3 mV) at 60°C (Figure 2I). For the duplex of mutant RNA (L452R, S), the stability looked significantly different from that of a complementary duplex at 40°C. In Figure 2J, the average ∆Vcal of L452R (S) was estimated to be 43.2 mV, revealing a significant difference (†††p < 0.001) between complementary (68.0 mV) and noncomplementary (7.0 mV) sequences.
The temperature (T)-resolved identification ability could improve the viability of 2D InSe bio-FETs, thereby preventing complex probe design and interference of targets' spatial structures. The trinity of T-resolved, time (t)-resolved, and electrical (∆Vcal) response in our bio-FETs can also realize the variations typing without strictly limiting the mutation positions in the target sequence region. Hence, 2D InSe FET with good feasibility can be utilized to acquire multi-dimensional SARS-CoV-2 variants information for a viable prediction of COVID-19 and prevention of the hidden transmission of VOCs (Table S2).
2.3 Mechanism of realizing variants identification
After a step-wise ALD process (Figure 3A), an Al2O3 thin film was constructed as the protective layer on FET. The Raman spectroscopy analysis revealed the InSe channel exhibiting Ag mode at 309 cm−1 corresponding to the In-O vibration (Figure 3B), indicating the formation of InSe1−xOx after ALD Al2O3.40 The x-ray photoelectron spectroscopy (XPS) results further confirmed the presence of InSe1−xOx (Figure 3C). Evident broadening and downshift were observed in Se 3d and In 3d binding energy profiles, originating from the electron transfer from the unoxidized (InSe) layers to the oxygen-substituted (InSe1−xOx) layers.
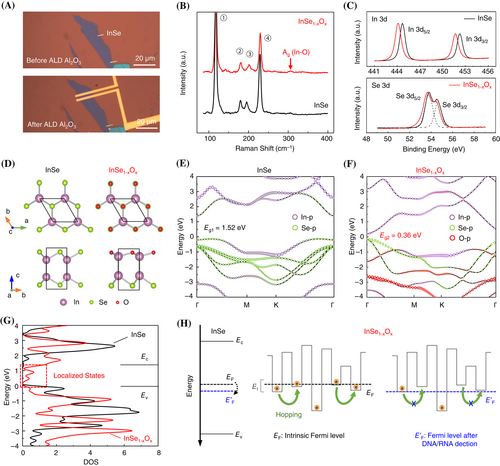
To verify the influence of channel oxidation and subsequent improvement in sensing ability, the band structure and density of states (DOS) of pristine 2D InSe (black) and 2D InSe1−xOx (red) were calculated within the framework of DFT. In Figure 3D, the O atoms were used to replace Se atoms since Se vacancies (VSe) may result in excess electrons from exposed In atoms to reduce the dissociation barrier of O (~0.2 eV) and enable the oxidation process.41 The corresponding oxidations were also directly reflected in the band structure (Figure 3 E,F). The replaced O atom effected the p-orbital of In atom and revealed significant changes in the InSe conduction band, specifically manifested as the band gap narrowing (1.52–0.36 eV) and transition from indirect band gap to direct band gap. After the replacement of the O atom, the DOS curves (Figure 3G) illustrated localized states of InSe1−xOx between Ec and Ev of InSe, where the corresponding energy levels acted as trap states to capture carriers.42
Since the DNA and RNA sequences are negatively charged, the hybridization would create stable surface potential on the channel. For 2D InSe, the negative surface potential induced a slight downward shift in Fermi energy. The perturbation energy relationship was determined as ∆EF ≪ Ec − EF, so that n0 can be considered constant. Thus, the dynamic changes of NAs with SNVs were too slight to influence the channels under the band transport mechanism.
However, the value of ∆EF for 2D InSe1−xOx was not negligible when compared to Et − EF in Equation (2) since the location of Et was much closer to EF than Ec. Consequently, the negative potential could limit electrons from filling the shallow traps and suppress electron hopping transport. The effect of trap state filling restraint was further amplified through Equation (1) to yield a more detectable current drop. The results suggested significantly enhanced sensitivity of FETs with trap state-dominated channels, enabling the bio-FET to identify signals of SARS-CoV-2 mutations at a single -nucleotide level.
In short, the existence of trap states in InSe1−xOx during the ALD process was demonstrated, and the mechanism was explained.45 These traps were generally known as a factor impairing the electronic conductivity, thereby should be avoided when fabricating devices. However, the negative factor possessed a unique positive side to biosensing since the carrier transport via trap filling and detrapping behavior exhibited robust and energetic sensitivity to ∆EF caused by NA-induced equivalent gate bias.46, 47 Compared to other strategy for the protection and improvement of the sensor performance like 2D metal–organic framework (MOF),48 the Al2O3 layer did not actively have the functions to enhance selectivity and sensitivity but effectively modulate the sensing property of 2D InSe channels. And the proposed strategy may free 2D materials from the strict fabrication requirement of perfect surface-states, and provide a scalable research strategy for expanding the functional diversity of 2D bio-FETs.
3 CONCLUSIONS
In summary, a 2D InSe FET was successfully developed for fast and direct identification of SARS-CoV-2 variants, with remarkable stability and sensitivity ensured by the two-step ALD process. The obtained 2D bio-FETs presented a wide detection range (10−14–10−8 M) and an ultra-low LOD to both target DNA (0.71 fM) and RNA (0.79 fM). Moreover, the devices were capable of recognizing SNVs of SARS-CoV-2 (ORF1ab), including different mismatched types in three independent positions (p < 0.05). The two core mutations of the Delta variant (L452R and R203M) were also directly identified according to the significant differences in calibrated responses (p < 0.01) between the mutations and (non-)complementary sequences. The capability contributed to the shallowly-oxidized 2D InSe during ALD, where trap states played a positive role in ultra-sensitive biosensing instead of resulting in mobility degradation negatively. Compared to normal band transport, the carrier trapping and hopping behaviors were more responsive to the target-gating effect (ΔEF) originating from the negative charges in NA sequences. In sum, the proposed 2D InSe FETs sensor provided a novel strategy for fast and direct identification of SARS-CoV-2 VOCs, promising for efficient forewarning of cryptic transmissions for directing public health interventions.
AUTHOR CONTRIBUTIONS
Duo Xu, Junji Li, and Yunhai Xiong equally conceived and conducted the experiments. Duo Xu performed device fabrication and measurement, and wrote the manuscript. Junji Li, Han Li, and Dan Shan contributed to probe immobilization, target selection, and HRM analysis. Yunhai Xiong, Kairui Qu, Tong Zhao, and Xinyu Shi worked on material characterization. Jialin Yang, Wenqiang Liu, and Shengli Zhang performed DFT calculation and data analysis. Lianfu Jiang provided InSe single crystal. Xiang Chen and Haibo Zeng supervised the project and contributed to result discussions. All the authors discussed the results and commented on the manuscript.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (92064007, 62001224, 52131304), the Natural Science Foundation of Jiangsu Province (BK20190476, BK20190457, BZ2020063), and the 69th batch of China Postdoctoral Science Foundation (2021M691600). We thank Prof. Jianfa Zhang and Dr. Wenhao Ge for the assistance of HRM test.
CONFLICT OF INTEREST
The authors declare no conflict of interest.