Stimulus-responsive room temperature phosphorescence in purely organic luminogens
Funding information: the starting Grants of Tianjin University, Grant/Award Number: 001; Natural Science Foundation of Tianjin City; National Natural Science Foundation of China; Tianjin University
Abstract
Luminogens that exhibit stimulus-responsive room temperature phosphorescence (RTP) have attracted significant attention for their applications in a wide range of fields such as data storage, sensors, and bio-imaging. However, very few such materials are known, partly because of the unclear internal mechanism. In this review, we summarize recent advances in the field of stimulus-responsive RTP in purely organic luminogens, focusing on their unique emission behaviors and internal mechanisms governing the phenomena. We also attempt to identify the relationship between the mechanism, luminogens, and possible applications.
1 INTRODUCTION
Stimulus-responsive luminogens are a class of smart materials whose emission properties can be tuned by the application of an external stimulus such as mechanical force, heating, light, and pH.1-6 In view of their potential for practical applications, stimulus-responsive luminogens have attracted an increasing interest, especially purely organic materials with low toxicity and cost. Thus far, majority of organic stimulus-responsive systems have been based on fluorescent substances.7-9 Correspondingly, the change in emissive color and intensity under external stimulus could be monitored visually. The materials have been successfully implemented in data storage, sensors, and bio-imaging applications, among others.
In addition to color and intensity, the change in emission lifetime under an external stimulus is also a potential monitoring parameter. In order to realize this, emitting materials with visual afterglow are required. Afterglow phosphors have been typically limited to metal-containing inorganic materials, particularly rare-earth phosphors. The emission is governed by the slow liberation of trapped charge carriers from isolated traps of impurities, defects, or ions through thermal stimulation with low-luminescence efficiency.10 However, these materials can have intrinsic disadvantages, including high cost, potential toxicity, and instability in aqueous environments.
Recently, the study of purely organic luminogens that exhibit room temperature phosphorescence (RTP) has garnered attention and become a research hotspot.11-20 In contrast to common fluorescence luminogens, RTP arises from the excited triplet state with ultraslow radiation rate (kP), which was usually considered to be transition prohibitive (Figure 1). RTP emissions can have quite a long lifetime ranging from milliseconds to seconds; thus, if suitable stimulus-responsive RTP materials could be developed, the change of lifetime under external stimulus could act as an additional visual monitoring parameter. In other words, the number of visual monitoring parameters could increase from two to three in RTP materials, which would greatly facilitate their practical application. Furthermore, some other advantages could be expected based on the unique nature of RTP emissions. For example, the triplet state energy level should be lower than that of the corresponding singlet; the resultant larger Stokes shift of RTP could lead to a much enhanced contrast of emissive color under external stimulus.21-23 Also, triplet excitons are more sensitive to the surrounding environment, such as intermolecular interactions, oxygen, and solvent vapors, which could make them more sensitive to external stimuli compared to fluorescent luminogens.24-27 Thus, the increased number of visual parameters, and enhanced contrast and sensitivity in stimulus-responsive RTP materials would undoubtedly expand their application prospects. However, until now, very few stimulus-responsive RTP materials are known, partially because of the unclear inherent mechanism and lack of the rational molecular design strategy.
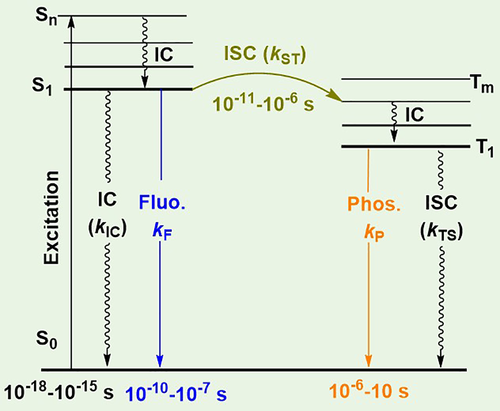
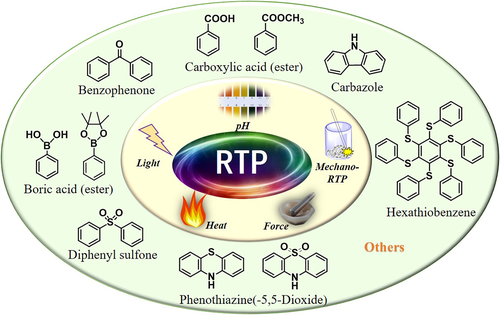
The internal mechanism of the common RTP effect was first studied in order to develop new stimulus-responsive materials. It was found that two main conditions had to be satisfied to realize RTP: (a) A molecular structure favoring intersystem crossing (ISC) transitions was required; (b) The particular environment in the solid state was necessary for the stabilization of the excited triplet state, such as a rigid matrix, regular molecular arrangement, and isolated oxygen. In recent years, a series of efficient RTP systems were successfully developed, where the frequently used constructing blocks included carbazole, phenothiazine-5,5-dioxide, benzophenone, hexathiobenzene, boric acid, and carboxylic acid derivatives (Figure 2).10-36 Besides these common aromatic systems, other nonaromatic RTP luminogens were also developed.37-40 As one of the most impressive examples, Li and co-workers reported a non-aromatic organic small molecule, cyanoacetic acid, that showed unexpected persistent RTP behavior with a lifetime as long as 862 ms for its regular layer-to-layer arrangement in the crystal state.37 This interesting phenomenon could be termed as a Molecular Uniting Set Identified Characteristic (MUSIC). Regardless of the relatively abundant RTP materials, very few showed stimulus-responsive effects upon changes in the molecular structure or external environment. These could be mainly divided into four types: heating-responsive, pH-responsive, light-responsive, and force-responsive RTP. In addition to these stimulus-responsive RTP effects in photoluminescence (PL), some special luminogens showed phosphorescence upon mechanical stimulus in the absence of ultraviolet (UV) irradiation, which could be termed as the “mechano-RTP effect”. In this short review, we have summarized the recent advances in the field of stimulus-responsive RTP in purely organic luminogens, with an attempt to construct the relationship between the mechanism, luminogens, and possible applications.
2 STIMULUS-RESPONSIVE RTP
2.1 Heating-responsive RTP
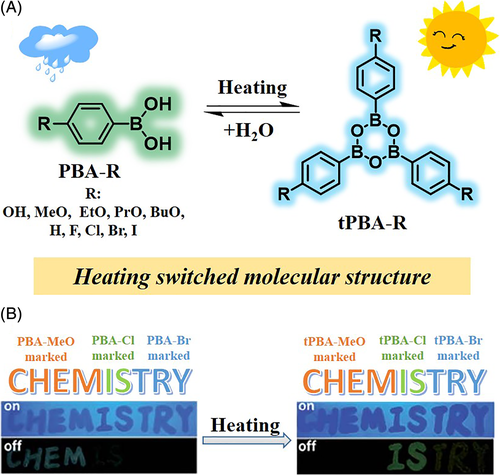
Heating-responsive luminescence refers to the emission behavior of luminogens responding to thermal stimulus.41-43 Heating-responsive materials have been widely applied as temperature sensors and fake detectors, among others, because of the easy access of this stimulus. However, most of these materials are based on fluorescence dyes, and very few phosphorescence-based materials are known. Li and co-workers attempted to fill this gap.44 They selected a series of commercially available phenylboronic acid derivatives for two main reasons: (a) the introduction of hydrogen bonds into boron-containing compounds might offer denser molecular packing and promote RTP emission; (b) phenyl boric acid (PBA) could be easily converted into triphenyl boroxine (tPBA) upon heating, thus yielding a heating-responsive emission. The experimental results convincingly validated this choice (Figure 3). Most of the PBA derivatives showed obviously ultralong RTP emission due to the existence of strong intermolecular interactions and dense molecular packing, and the phosphorescence lifetime was as long as 2.24 seconds (PBA-MeO). Furthermore, the transition between PBA-R and tPBA-R occurred upon heating, as confirmed by their corresponding single crystal structures. At the same time, very different RTP behaviors appeared when the molecular structure was modified resulting in different intermolecular interactions. With this unique heating-responsive RTP effect, a security application was successfully realized. As shown in Figure 3B, the letters of “CHEMISTRY” were made of three components, in which the RTP of PBA-MeO was thermally quenched, while those of PBA-Cl and PBA-Br were thermally activated. After heating at 80°C for 2 hours, the afterglow of “CHEM” (PBA-MeO) was much weakened, while those of “IS” (PBA-Cl) and “TRY” (PBA-Br) became clearer. Interestingly, no obvious change was observed in their fluorescence behaviors, including emission color and intensity. This work clearly demonstrates the advantage of stimulus-responsive RTP materials over traditional fluorescence materials.
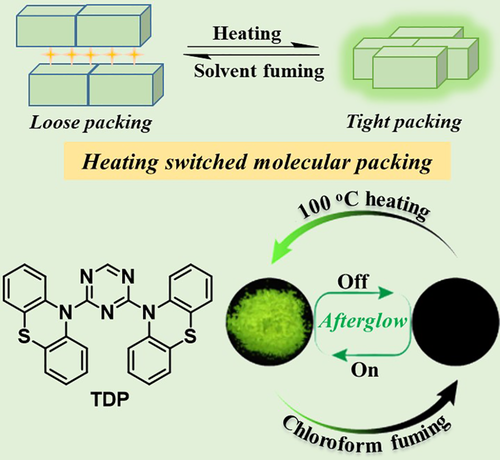
Besides the molecular structure, the RTP effect was also heavily related to molecular packing. Unique packing modes, such as H-aggregation, intermolecular n-π* electronic coupling, and π-π stacking, were found to promote the persistent RTP effect in suitable organic systems.31, 45-48 Further, even the same luminogen was reported to display different RTP effects in different polymorphic forms, highlighting the packing-dependent behavior.25, 49, 50 Thus, heating-switched molecular packing could be another route to realize the heating-responsive RTP effect. In 2018, Huang and co-workers reported a new RTP luminogen, TDP, with triazine substituted by two phenothiazine groups (Figure 4).51 Two single crystals of this material were cultured from different solvents. The crystal TDP obtained from ethyl acetate solution showed dense molecular packing and ultralong RTP emission with phosphorescence quantum yield and lifetime up to 6.8% and 56 ms, respectively. In TDP-CF, cultured from chloroform, the packing was not as dense, and chloroform molecules were involved in the crystal structure. This resulted in more active non-radiation motion, leading to a much weakened RTP with phosphorescence quantum yield and lifetime of just 1.2% and 1.5 ms, respectively. Intrigued by the different RTP effects in these two polymorphs, crystal TDP-CF was heated to explore a possible heating-responsive RTP effect. Heating at 100°C for about 1 hour yielded molecular packing and RTP effect similar to crystal TDP. Interestingly, fuming with chloroform vapor converted it back to TDP-CF, showing the reversible stimulus-responsive RTP effect.
2.2 pH-responsive RTP
pH is one of the most common laboratory measurements because so many chemical and biological processes are dependent on it. As a consequence, the development of pH-responsive materials and sensors is a fundamental and broad research field.52, 53 Owing to the great advantages of low toxicity, cost-effectiveness, and high sensitivity and specificity, organic luminogens have been widely applied as pH sensors.54, 55 However, majority of the pH-responsive materials are based on organic fluorophores, and materials based on phosphorescence are in the initial stages of development.
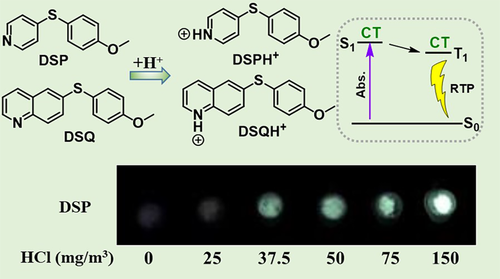
Before designing pH-responsive RTP materials, it is necessary to clarify the internal mechanism in a few appropriate RTP systems. In previous studies, Zhang and co-workers found that intra- or inter-molecular charge transfer from an electronic donor to acceptor in purely organic systems could significantly boost the efficiency of ISC and thus RTP.26, 56 Accordingly, they designed and synthesized two thioethers with proton-activated acceptor moieties (pyridine and quinoline) (Figure 5).57 It was anticipated that the addition of a proton to DSP or DSQ could result in efficient intramolecular charge transfer thus promoting the ISC transition as well as RTP, as validated by the experimental results. Initially, weak blue fluorescence or even no emission was observed for the DSQ and DSP solids. However, after treatment with protonic acid, both materials exhibited interesting turn-on RTP in the longer wavelength region. In DSP, for example, progressively enhanced yellow phosphorescence was easily observed after fuming with different concentrations of HCl gas (from 0 to 150 mg/m3), exhibiting the typical pH-responsive RTP effect (Figure 5). Linear fitting of RTP intensity at 507 nm revealed that the detection limit of HCl was as low as 8.3 mg/m3, which is half of the maximum allowable concentration (14.9 mg/m3) for human health, indicating the practical value of DSP. Thus, the modified molecular structure and corresponding intramolecular charge transfer upon acid stimulation could act as an efficient route for the development of pH-responsive RTP materials.
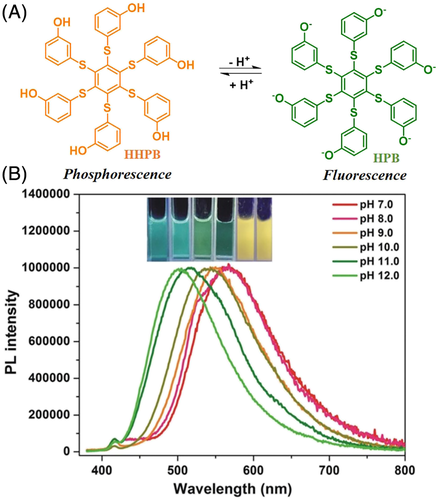
The transition of molecular states between aggregation and dissolution was also explored to realize the pH-responsive RTP effect. Inspired by the crystallization-induced phosphorescence (CIP) seen in hexathiobenzene derivatives, Qian and co-workers designed and synthesized a new organic luminogen, HHPB, by integrating a hexathiobenzene core along with alkali-responsive hydroxyl groups.58 Just like other hexathiobenzene derivatives, HHPB showed the typical CIP with blue fluorescence in solution and yellow phosphorescence when aggregated. The six hydroxyl groups enabled this hydrophobic molecule to dissolve in alkaline aqueous solution upon adjusting the pH value, and the HHPB molecules could thus be controlled between states of aggregation and dissolution. A possible pH-responsive RTP effect was tested by changing the pH of a HHPB suspension. In the pH range 7.0 to 12.0 (Figure 6), the yellow phosphorescence of HHPB progressively changed to a distinctly different green emission, accompanying the transition from a suspension to a dissolved state. Simultaneously, the emission lifetime also sharply decreased from 10.7 μs to 2.5 ns, confirming the transition from phosphorescence to fluorescence. Thus, CIP can serve as a universal basis for the design of pH-responsive RTP luminogens.
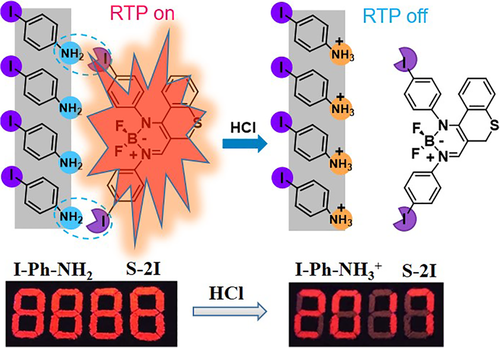
In 2018, Fu and co-workers realized pH-responsive RTP in a host-guest system by modifying the intermolecular interactions in the system.59 The rigid I-Ph-NH2 crystalline matrix was chosen as host to restrict the nonradiation motion, while trace amounts of S-2I acted as guest to form centers of luminescence. The intermolecular halogen bond (S-2I···NH2-Ph-I) enhanced the intermolecular heavy-atom effect and promote spin-orbit coupling between the singlet and triplet states, and the resultant system showed bright red RTP emission with a phosphorescence quantum yield and lifetime up to 15.96% and 0.83 ms, respectively. Upon treatment with HCl vapor, the proton could connect with ─NH2 to form ─NH3+, destroying the intermolecular halogen bond, resulting in a non-RTP state. With these unique properties, the application of antifake responding to HCl was realized (Figure 7).
2.3 Light-responsive RTP
Light-responsive materials have always been a research hotspot as they are safe, clean, and easy-to-use and control.60-62 Light-responsive organic luminogens are important components of these materials;63-65 however, their performance leaves a lot to be desired. This is likely due to the inferior sensitivity of traditional fluorescence luminogens toward low energy light, while high-powered lights could result in sample deterioration. Recent research has indicated that subtle changes in molecular conformation and packing could yield different RTP effects in purely organic luminogens.25, 66, 67 Thus, the development of light-responsive RTP luminogens could improve light sensitivity and the corresponding performance.
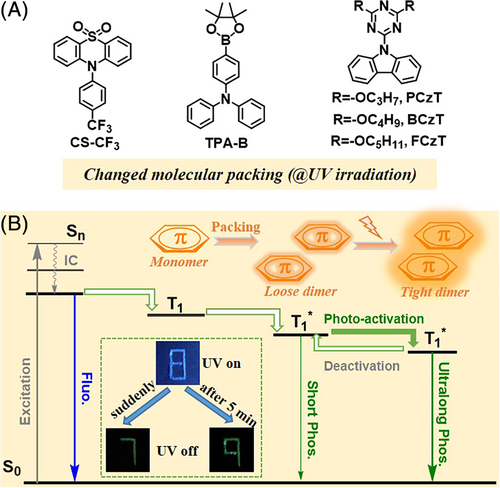
In 2017, Li and co-workers observed the first example of light-responsive RTP in a purely organic luminogen, CS-CF3 (Figure 8).48 In its crystal form, nearly no RTP emission was initially detected; however, highly enhanced ultralong phosphorescence appeared upon sustained irradiation by a hand-held UV lamp (0.35 mW/cm2). After about 5 minutes, the phosphorescence lifetime reached a maximum of 299 ms, showing the ultrahigh contrast light-responsive RTP on the lifetime. On the other hand, no obvious change could be observed in the fluorescence emission color and intensity. This is a demonstration of the advantage of light-responsive RTP materials over fluorescent materials, in both contrast and sensitivity. Furthermore, the material returned to the non-RTP state after about 2 hours, exhibiting reversibility. To elucidate the internal mechanism, single-crystal structures of CS-CF3 were determined before and after UV irradiation for 5 minutes. Molecular motion and the minor change in molecular packing from loose to a little tighter upon UV irradiation is likely responsible for this unique property. Based on a similar mechanism, another light-responsive RTP luminogen (TPA-B) was successfully developed later by Li et al, in which the phosphorescence lifetime increased from 5 to 211 ms after about 20 minutes of UV irradiation.68 Huang et al were also able to realize light-responsive RTP based on a similar mechanism in a series of organic molecules containing a triazine core, carbazole unit, and different alkoxy chains.69 Among them, BCzT bearing a butoxyl substituent demonstrated the best performance with RTP lifetime increasing from 1.8 to 1330 ms after 8 minutes UV irradiation. However, a high-powered UV lamp (80 mW/cm2) was used instead of the regular hand-held UV lamp (0.35 mW/cm2) generally used for detection in thin layer chromatography. Based on these examples, we can conclude that changes in the molecular packing under light-stimulus are an efficient route to develop light-responsive RTP luminogens with high sensitivity and contrast.
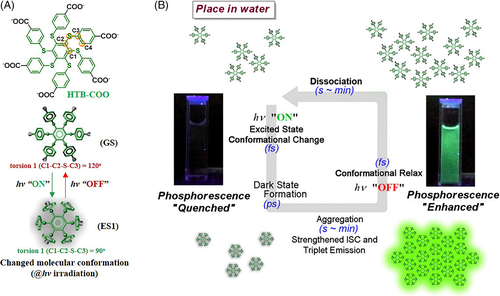
In all the examples mentioned above, the molecular motions under light stimulus occurred in the solid state, and would surely be restricted to some extent. If the molecular motions could be achieved in solution state, the resultant light-responsive RTP would be more sensitive. Zhu and co-workers successfully achieved this by utilizing the significantly different molecular conformations between the ground and excited states.70 The schematic diagram of the light-responsive RTP of HTB-COO is illustrated in Figure 9. Due to the hydrophilia of the six carboxylate groups, HTB-COO dissolved easily in aqueous solution and no emission could be observed due to the negligible oscillator strength in first excited state (ES1). Under continuous irradiation by a hand-held 365 nm UV lamp, the molecules tended to aggregate in the more regular molecular conformation in the ES1 state. Accordingly, the ISC transition was much enhanced, leading to the appearance of RTP emission. Because of the enhanced freedom of molecular motion in solution, the activation process was complete within 1 minute, showing much more sensitive light-responsive RTP.
Besides the promotion of ISC transition, the suppression of molecular motion is another method to achieve efficient RTP. In 2018, Zhao and co-workers developed a new light-responsive RTP system based on the photo-crosslinking strategy (Figure 10), in which a guest molecule G was doped into a polymer matrix polyviny alcohol (PVA) with photo-crosslinking potential.71 Initially, it showed a moderate RTP effect with phosphorescence lifetime and quantum yield of 0.28 second and 2.85%, respectively. After continuous 254 nm UV irradiation, photo-crosslinking occurred leading to the formation of covalent cross-linked bonds between PVA polymers. After about 65 minutes irradiation under a 254 nm UV lamp, the RTP lifetime and quantum yield increased to 0.71 second and 11.23%, respectively, as the result of the more rigid environment and restricted nonradiation motion; these were about 2.5 and 3.9 times higher than that of G-doped PVA films without cross-linking. It is believed that even more light-responsive RTP systems could be designed based on photo-crosslinking, which is an elegant molecular design strategy.

Generally, molecular oxygen presents a triplet ground state, which could interact with the triplet excitons of luminogens and quench their phosphorescence emission. Thus, most instances of RTP were realized in the absence of oxygen. Inspired by this aspect, Reineke and coworkers developed a new type of light-responsive RTP material based on the oxygen consumption mechanism (Figure 11A).72 In their system, a thin emitting layer (900 nm thick) was constructed using poly(methyl methacrylate) as host and 2% (wt%) NPB as guest molecules, while a 600-nm-thick oxygen-barrier layer was deposited on top of the sample to prevent exposure of the emitting layer to surrounding oxygen. After excitation by 365 nm UV light, the NPB molecules reached their excited singlet state S1 from which some jumped to the excited triplet state T1 through ISC. In the presence of molecular oxygen, triplet excitons in the T1 state depopulated via triplet-triplet interactions with molecular oxygen, and the RTP afterglow was not observed initially. However, after UV irradiation (365 nm) for a while, the triplet molecular oxygen in the emitting layer was consumed and the triplet excitons of NPB decayed as long-lasting green phosphorescence with a lifetime of 406 ms, in another manifestation of light-responsive RTP. Also, it could return to the initial state upon refilling of oxygen through imperfections in the oxygen barrier, which could be expedited by heating the sample via IR or a simple hotplate. Thus, reversible UV light-responsive RTP effect was achieved for this system upon light irradiation and heating, and has been successfully utilized for applications in information storage and security.
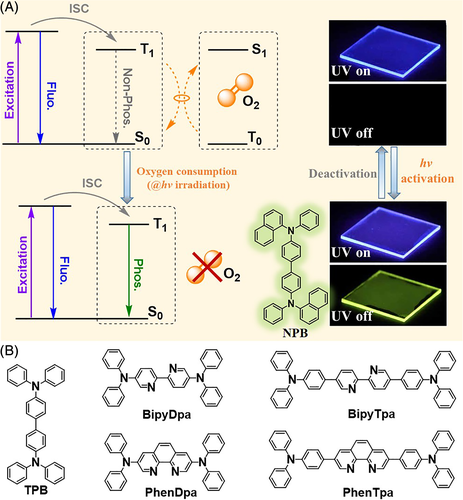
Another visible light-responsive RTP system was developed by Reineke et al based on a similar internal mechanism (Figure 11B).73 In this system, an electron acceptor (A), bipyridine or phenanthroline group, was chosen as the core with an electron donor (D), diphenylamine or triphenylamine, as the sides. With such a molecular structure, two main purposes could be achieved: first, the D-A structure could lower the energy level of target compounds and realize the excitation of visible light; second, the additional nitrogen atoms in the bipyridine or phenanthroline core could enhance the spin-orbit coupling and ISC transitions, thus promoting the formation of triplet excitons. Among them, the design strategy was well identified in PhenDpa and PhenTpa with phenanthroline groups because of their better conjugation and more rigid conformation. After a period of 420 nm light-emitting diode (LED) irradiation, the RTP lifetimes and quantum yields for PhenDpa and PhenTpa increased to 330 ms and 5.7%, and 680 ms and 1.2%, respectively. Thus, oxygen consumption under light (UV as well as visible) could alter the internal mechanism and corresponding application of light-responsive RTP materials.
2.4 Force-responsive RTP
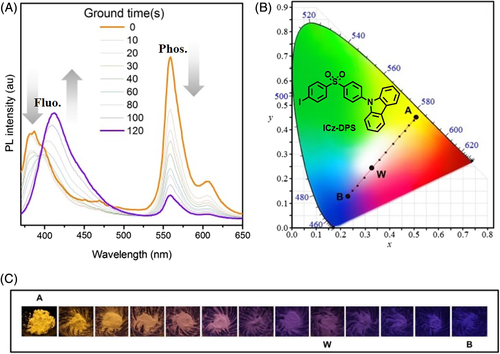
Force-responsive RTP refers to luminogens that respond to the stimulus of mechanical force. In reality, this was frequently observed in purely organic RTP luminogens due to the well-known CIP effect.74-76 For instance, ICz-DPS crystals exhibited bright yellow phosphorescence associated with deep blue fluorescence, as a consequence of the restricted thermal motion and isolated oxygen in the crystal matrix.77 The phosphorescence peak at 559 nm reduced upon grinding, due to the transition from crystal to amorphous state, which was monitored by powder X-ray diffraction (XRD). At last, only blue fluorescence emission remained for ICz-DPS, showing the blue-shifted force-responsive emission (Figure 12).
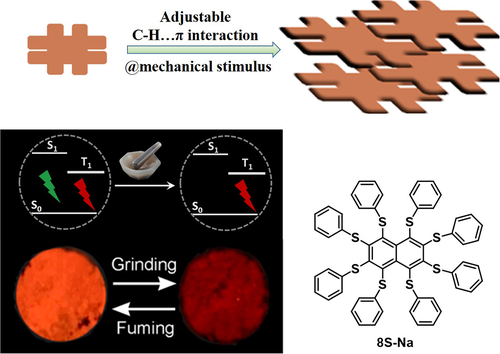
Although most of the purely organic RTP luminogens displayed a blue-shifted emission under force stimulus as a result of the sharply decreased phosphorescence, there were also some exceptions. In the RTP luminogen 8S-Na, Zhu et al observed abnormal red-shifted emission under mechanical stimulus; a fluorescence band in the visible region and phosphorescence band in near infrared ray (NIR) were observed for the film fabricated from ethanol solution, while only the NIR phosphorescence peak existed after heavy grinding.78 Careful analysis of its single crystal structure revealed that the adjustable C─H⋯π interaction-assisted self-assembly is likely responsible for this interesting behavior. Strong C─H⋯π interactions have been reported to promote the RTP emission in hexathiobenzene derivatives.79, 80 In the initial state, two different molecular conformations with different C─H⋯π interactions existed in the 8S-Na film, of which one was relatively weak while the other was strong. After grinding, all the C─H⋯π interactions were converted into strong interactions, resulting in exclusively NIR phosphorescence that exemplified red-shifted force-responsive emissions (Figure 13).
2.5 Mechano-RTP
In recent years, light emission induced by mechanical stimuli in the absence of UV irradiation (mechanoluminescence [ML]) has attracted a lot of attention for its great potential in applications such as displays, lighting, bioimaging, and stress-sensing.6, 81-85 Regardless of the long history (the first report was on sugar by Francis Bacon in 1605), the weak emission limited the exploration of the ML process and the related excited state. In 2015, the marriage of aggregation induced emission (AIE) and the ML process renewed interest in ML materials.5, 86 Based on this guideline, a series of efficient ML systems were developed but their ML spectra were very similar to fluorescence spectra in the PL process; there were no reports of AIE luminogens exhibiting mechanophosphorescence or fluorescence-phosphorescence dual ML, although in principle there should be.87-92
In 2016, Li and co-workers reported fluorescence-phosphorescence dual ML for the first time in a novel AIE luminogen, DPP-BO.93 With this result, the existence of some inherent energies like singlet (S) state and triplet (T) state in the ML process could be confirmed. Unfortunately, phosphorescence was not observed at room temperature in the PL process, even though strong and long-lifetime phosphorescence was achieved at low temperature (77 K). Later, in 2017, they developed another ML system based on phenothiazine derivatives exhibiting both RTP and mechanophosphorescence.94 Careful analyses of the single-crystal structures coupled with theoretical calculations showed that efficient intermolecular interactions in the solid state are largely responsible for the unique mechano-RTP effect in both the systems (Figure 14).
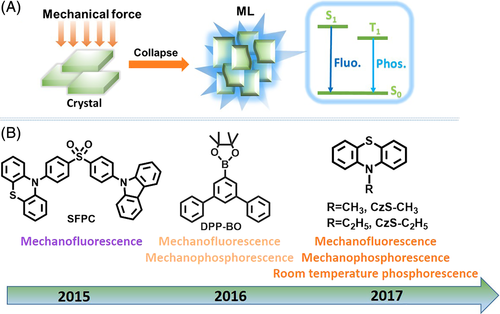
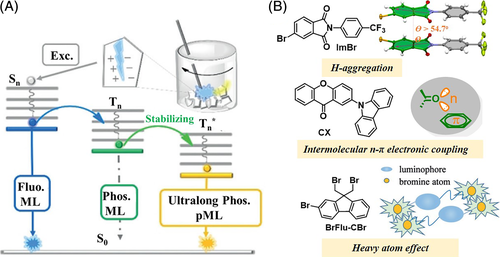
Following this, purely organic luminogens with persistent ML were explored in 2018. In these, the mechano-RTP effect was confirmed for the long lifetime of the triplet excitons. In previous reports, it was found that persistent RTP in the PL process was more likely to be realized in crystals with specific molecular packing features, such as H-aggregation, intermolecular n-π* electronic coupling, and π-π stacking. Based on these successes, several purely organic luminogens with ultralong mechano-RTP were developed. For example, Shi and Xu et al successfully observed a yellow “afterglow ribbon” that lasted for ~1 second in both the PL and ML processes for the ImBr crystal; here the H-aggregation was considered to be mainly responsible for the stabilized triplet excitons and resultant afterglow.95 Zhang and co-workers reported ultralong mechano-RTP arising from intermolecular electronic coupling of the stacked n- and π units in the CX crystal, which had different T1 state conformations with (n, π*) and (π, π*).96 Li and co-workers opened a new avenue to develop ultralong mechano-RTP luminogens through adopting strong halogen interactions with external heavy atom effect.97 All these examples clearly document the mechano-RTP effect (Figure 15).
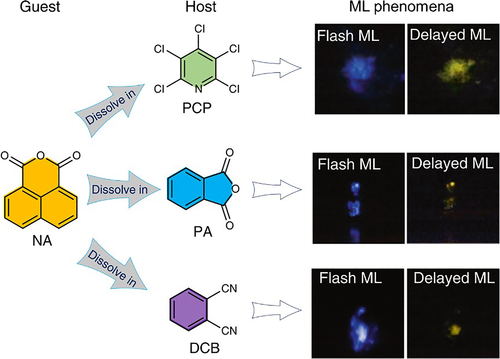
Apart from these one-component ultralong mechano-RTP luminogens, others based on host-guest systems were developed by Tang et al83 In these systems, the hosts (PCP, PA, and DCB) presented normal transient ML emission, while the guest (NA) showed potential for persistent RTP emission in the PL process upon doping into the rigid matrix. When solid NA/PCP, NA/PA, or NA/DCB obtained via melt-casting was crushed, a flash of blue light along with delayed ultralong yellow emission lasting for seconds was visible to the naked eye (Figure 16). The flash and delayed ML were strong enough to be seen under soft room lighting. The ML spectra for these systems were found to correspond well to the PL spectra. Femtosecond transient absorption studies revealed that the ISC of the host was accelerated substantially in the presence of a trace amount of 1,8-naphthalic anhydride, which is likely responsible for the ultralong mechano-RTP.
From the examples of mechano-RTP described above, it is seen that ML and PL processes are similar, but not quite the same. While the excited singlet state and triplet state exist in the PL process and ML emissions, ML was heavily related to the molecular structure and packing or external environment. The RTP emissions in both ML and PL processes often presented different lifetimes, indicating that their excited state processes were not exactly the same. Thus, more studies are required to gain a deep understanding of the ML process, especially those related to ISC transition and excited triplet emission.
3 CONCLUSIONS AND PERSPECTIVES
In this review, the recent developments in organic stimulus-responsive RTP were summarized, focusing on their unique emission behaviors and the internal mechanisms behind the exciting phenomena. Based on the different stimuli, these luminogens were mainly divided into four categories: heating-responsive RTP, pH-responsive RTP, light-responsive RTP, and force-responsive RTP in the PL process. By classifying their internal mechanisms, it was found that changes in the molecular structure, packing mode, and surrounding environment were mainly responsible for the stimulus-responsive RTP, regardless of the nature of the stimulus. Following these, another unique emission process, namely, mechano-RTP, was introduced; here, the RTP emission could be achieved by a mechanical stimulus in the absence of light-irradiation. This was an important extension of stimulus-responsive RTP materials, from the perspective of mechanism and applications. It was anticipated that the information gained from these examples could help scientists to gain a deep understanding of stimulus-responsive RTP, thus aiding its further progress.
As stimulus-responsive RTP is a new research field, materials that exhibit these properties are rather scarce, and those exhibiting excellent performance are even rarer. Thus, the first requirement is the development of new stimulus-responsive RTP materials. According to internal mechanisms classified above, at least three strategies could be considered: (a) to decorate the classical RTP cores with new functional groups that can undergo molecular structure or conformation transitions under external stimuli; (b) to select RTP cores with free rotation or vibration units, which could drive the regular motion of whole molecules under an external stimulus, resulting in changes in the molecular packing; (c) to develop host-guest systems with RTP emission, in which the surrounding environment of the RTP emitters can be easily adjusted under an external stimulus.
Secondly, more elaborate and detailed characterization of stimulus-responsive RTP is necessary.98-101 A comprehensive understanding thus obtained could accelerate practical applications. Taking the mechano-RTP effect as example, although RTP emissions have been clearly observed under mechanical stimulus for some organic luminogens, there are still no efficient instruments to measure the lifetime of ML emissions. Also, a clear relationship could not be established between ML intensity and mechanical stimulus intensity or angle. All of this contributes to an uncertainty regarding mechano-RTP, limiting its further research and applications.
The final aspect is application. All materials are developed with the intention of practical application. In comparison with the traditional stimulus-responsive fluorescence luminogens, RTP luminogens show advantages in terms of contrast and sensitivity, which would surely promote their practical application. Furthermore, the change in RTP lifetime under external stimulus could act as a visual monitoring parameter in addition to emission color and intensity. This could enable their application in new fields such as time-resolved bioimaging and time-resolved security.
With the abundance of materials, elaboration of characterization, and extension of applications, the research into stimulus-responsive RTP materials would develop rapidly. Correspondingly, the related theories could be further developed. It is hoped that this short review will provide some guidance on the way forward and contribute toward further development of this research field.
ACKNOWLEDGEMENTS
We are grateful to the starting Grants of Tianjin University and Tianjin Government, National Natural Science Foundation of China (No. 51903188), Natural Science Foundation of Tianjin City (No. 19JCQNJC04500) for financial support.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Biographies

Jie Yang received his bachelor and PhD degrees from Wuhan University in China in 2013 and 2018, respectively, under the supervision of Prof Zhen Li. He is now a lecturer at Tianjin University (TJU) and his research interests mainly focus on the design and synthesis of new organic luminescent materials.

Manman Fang received her PhD degree from Wuhan University in China in 2018 under the supervision of Prof Zhen Li. She is now a lecturer at Tianjin University (TJU) and her research interests mainly focus on the design and synthesis of new optoelectronic functional materials.

Prof Zhen Li received his bachelor and PhD degrees from Wuhan University (WHU) in China in 1997 and 2002, respectively, under the supervision of Prof Jingui Qin. In 2003-2004, he worked in the HKUST as a research associate in the group of Prof Ben Zhong Tang. In 2010, he worked at the Georgia group of Prof Seth Marder. He has been a full professor at WHU since 2006 and the chair professor at TJU from 2018. His research interests are in the development of organic molecules and polymers with new structures and new functions for organic electronics and photonics.





