Interfacial Ce-S bonds enhanced Mo-doped ZnIn2S4/oxygen-deficient CeO2 S-scheme heterojunction for efficient photocatalytic overall water splitting
Junchao Zhou and Sibi Liu contributed equally to this work.
Abstract
Photocatalytic overall water splitting (OWS) can convert solar energy into hydrogen (H2) and oxygen (O2), which is significant in reducing the reliance on fossil fuels. Constructing S-scheme heterojunctions is an effective method for facilitating charge transfer, but the huge interfacial charge transfer barrier poses a challenge to advance the efficiency of photocatalytic OWS. Here, a low-interfacial barrier Ce-S bond-enhanced Mo-doped ZnIn2S4/oxygen-deficient CeO2 (Mo-ZIS/OV-CeO2) S-scheme heterojunction photocatalyst was designed via a doping-defect coupling strategy. The abundant unsaturated S atoms generated by doping Mo atoms in ZnIn2S4 combine with the unpaired electrons on the Ce atom in OV-CeO2, forming the interfacial Ce-S bonds, which induce a 43% decrease in carrier transport activation energy and a 2.1-fold increase in build-in electric field intensity compared to ZIS/OV-CeO2. Reduced carrier transport activation energy and increased built-in electric field intensity provide a strong driving force for charge separation following the S-scheme pathway. Benefiting from the interfacial Ce-S bonds and the S-scheme transfer path, Mo-ZIS/OV-CeO2 exhibits H2 and O2 evolution rates of 512.7 and 256.3 μmol g−1 h−1, respectively, along with a solar-to-hydrogen efficiency of 0.14%. This study proposes an innovative insight into developing and constructing S-scheme heterojunction photocatalysts with efficient charge migration interfaces.
1 INTRODUCTION
Photocatalytic overall water splitting (OWS) technology harnesses solar energy to produce hydrogen (H2) and oxygen (O2) without pollution, presenting a viable solution to contemporary environmental and energy challenges.1-5 Recently, metal sulfides, metal oxides, metal-organic frameworks, covalent organic frameworks, etc. have been implemented for photocatalytic water splitting, and metal sulfides emerge as promising photocatalysts owing to their suitable energy band structure and outstanding photoelectric performance.6 Typical metal sulfides, including ZnIn2S4 (ZIS),6, 7 MoS2,8 CdS,9 and In2S3 10, 11 have been widely applied in photocatalytic water splitting applications. Among them, ZIS exhibits superior photocatalytic performance due to its abundant surficial active sites and strong capacity to reduce water for H2 generation.6, 12 However, the photocatalytic performance of individual ZIS remains constrained due to severe carrier recombination.13, 14
Constructing heterojunctions is a promising strategy to reduce carrier recombination and enhance the photocatalytic performance.15-17 Notably, constructing S-scheme heterojunctions can achieve directional charge migration and preserve the high redox capacity of the carriers, which is the most effective strategy for developing high-performance photocatalysts.18-21 For example, the PtS-ZnIn2S4/WO3-MnO2 S-scheme heterojunction was constructed through a two-step self-assembly process, achieving photocatalytic H2 and O2 evolution rates with a value of 14.8 and 5.6 μmol g−1 h−1, respectively.22 A InVO4@ZnIn2S4 S-scheme heterojunction was synthesized by epitaxially growing ZnIn2S4 nanosheets onto InVO4 nanosheets, exposing more active sites, which resulted in enhanced H2 and O2 evolution rates of 153.3 and 76.9 μmol g−1 h−1, respectively.23 In addition to constructing heterojunctions, interface regulation is another crucial strategy for suppressing charge recombination.11, 24 A tight heterojunction interface can reduce the interfacial charge transfer barrier and enhance carrier separation.18, 25, 26 By modulating the sulfur vacancy concentration of In2S3, the interfacial interaction in graphene oxide quantum dots/In2S3 heterojunction transitions from weak electrostatic adsorption to strong chemical interface bridging, obtaining an exceeding 2 times increase in interfacial built-in electric field and providing sufficient driving force for interfacial charge transfer.11 Nevertheless, constructing a ZIS-based photocatalyst simultaneously possessing a S-scheme charge transfer path and a tight heterojunction interface remains a significant challenge.
Here, we construct a low-interfacial barrier Ce-S bond-enhanced Mo-doped ZIS/oxygen-deficient CeO2 (Mo-ZIS/OV-CeO2) S-scheme heterojunction photocatalyst using a doping-defect coupling strategy. OV-CeO2 is selected as an O2 evolution photocatalyst due to its strong oxidation capability, and the presence of O vacancies plays a vital effect for enhancing the kinetics of O2 generation.27 The abundant unsaturated S atoms formed by doping Mo atoms into ZIS serve as high-energy unsaturated coordination centers, thereby promoting the in situ growth of OV-CeO2 and facilitating the formation of Ce-S bonds at the interface. The interfacial Ce-S bonds act as a pathway for charge transfer, resulting in a 43% decrease in carrier transport activation energy and a 2.1-fold increase in build-in electric field intensity in Mo-ZIS/OV-CeO2 compared to ZIS/OV-CeO2, which provides a driving force for charge separation along the S-scheme pathway. Consequently, Mo-ZIS/OV-CeO2 exhibits a solar-to-hydrogen (STH) efficiency of 0.14%, surpassing that of most reported ZnIn2S4-based heterojunctions.
1.1 Catalyst synthesis and characterization
Mo-ZIS/OV-CeO2 is constructed by incorporating Mo doping into ZnIn2S4 and combining oxygen-vacancy-rich CeO2 through a two-step hydrothermal method (Scheme 1). For comparison, pristine ZnIn2S4 (ZIS), ZnIn2S4 with Mo doping (Mo-ZIS), CeO2 with O vacancies (OV-CeO2), and ZIS/OV-CeO2 are also prepared. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), and high-resolution TEM (HRTEM) are employed to analyze the structures and morphologies of all catalysts. The results reveal that Mo-ZIS exhibits a dense flower-like morphology similar to that of ZIS, featuring a lattice fringe of 0.32 nm corresponding to the (102) plane of ZnIn2S4 (Figure 1A–C and Supporting Information S1: Figure S1).28 The OV-CeO2 nanorods have lengths of 100–500 nm and diameters of ∼10 nm, with an interlayer distance of 0.31 nm assigned to the (111) lattice plane of CeO2 (Figure 1D–F).29 OV-CeO2 nanorods are loaded onto the flower-like Mo-ZIS surface, and the coexistence of the (102) plane of ZnIn2S4 and the (111) plane of CeO2 demonstrates a tight contact at the heterogeneous interface in Mo-ZIS/OV-CeO2 (Figure 1G–I).29, 30 Energy dispersive X-ray (EDX) images of Mo-ZIS/OV-CeO2 show uniform distribution of Mo, S, Zn, In, Ce, and O atoms within the structure (Figure 1J and Supporting Information S1: Figure S2).
Schematic route for the synthesis of the Mo-ZIS/OV-CeO2 heterojunction. OV-CeO2, CeO2 with O vacancies; ZIS, ZnIn2S4.
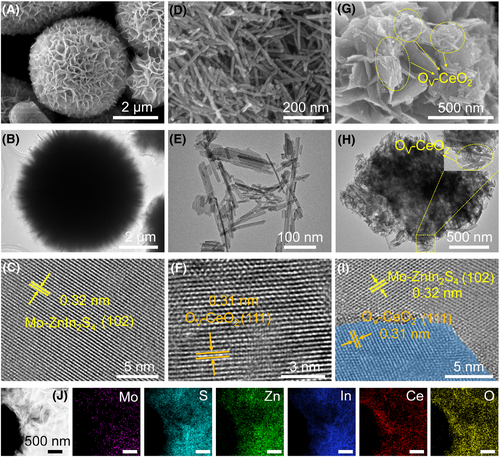
Morphologies and structures characterization of Mo-ZIS, OV-CeO2, and Mo-ZIS/OV-CeO2. (A) SEM image of Mo-ZIS; (B) TEM image of Mo-ZIS; (C) HRTEM image of Mo-ZIS; (D) SEM image of OV-CeO2; (E) TEM image of OV-CeO2; (F) HRTEM image of OV-CeO2; (G) SEM image of Mo-ZIS/OV-CeO2, the yellow dashed ellipse denotes OV-CeO2; (H) TEM image of Mo-ZIS/OV-CeO2, the yellow dashed ellipse in the enlarged image denotes OV-CeO2; (I) HRTEM image of Mo-ZIS/OV-CeO2, the blue area represents the lattice spacing of OV-CeO2; and (J) the corresponding EDX mapping images of Mo, S, Zn, In, Ce, and O for Mo-ZIS/OV-CeO2. EDX, energy dispersive X-ray; HRTEM, high-resolution TEM; OV-CeO2, CeO2 with O vacancies; SEM, scanning electron microscopy; TEM, transmission electron microscopy; ZIS, ZnIn2S4.
Raman and X-ray photoelectron spectroscopy (XPS) are also effective methods for characterizing the structures of all samples. In the Raman spectrum of Mo-ZIS, excepted for the LO1, TO2, and A1g characteristic peaks of ZIS, a vibration peak attributed to the Mo-S bonds is detected at 405 cm−1, confirming that the Mo atom is successfully doped and bonds with the S atom (Figure 2A and Supporting Information S1: Figure S3).24 Meanwhile, the Mo 3d spectrum of Mo-ZIS exhibits two XPS peaks corresponding to Mo 3d5/2 and Mo 3d3/2, associated with Mo5+, further demonstrating successful Mo doping in the ZIS structure (Supporting Information S1: Figure S4).31 In the S 2p XPS spectra of ZIS, Mo-ZIS, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2, the S2− and S22− peaks are observed (Figure 2B).28 The positive shift in the S 2p peak of Mo-ZIS compared to ZIS is ascribed to the presence of S vacancies which arise from the loss of S atoms due to the disruption of the original S-metal bonding by doping Mo atoms.32 As for OV-CeO2, the Raman peaks at approximately 457 and 605 cm−1 correspond to the F2g Raman mode of cubic CeO2 and the presence of Ce3+ coupled with O vacancies, respectively, which demonstrate the successful introduction of O vacancies into CeO2 (Supporting Information S1: Figure S3).33 Additionally, the O 1s spectrum of OV-CeO2 features two XPS peaks at 529.5 and 531.3 eV, corresponding to oxygen atoms within the OV-CeO2 lattice (Olatt) and oxygen adsorbed on its surface (Oads), and the higher ratio of Oads to Olatt (Oads/Olatt) in the samples indicates the higher content of O vacancies (Figure 2C).34, 35
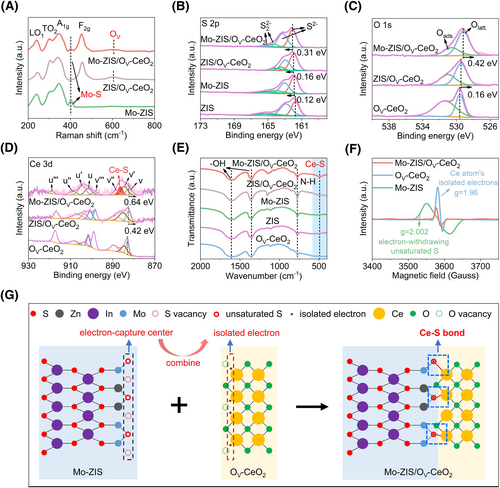
The transformation of electronic structure and interfacial binding. (A) Raman spectra of Mo-ZIS, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2, OV represents the presence of O vacancies; (B) S 2p XPS spectra of ZIS, Mo-ZIS, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2; (C) O 1s XPS spectra of OV-CeO2, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2; (D) Ce 3d XPS spectra of OV-CeO2, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2, the v and u correspond to Ce 3d3/2 and Ce 3d5/2, respectively, the red area represents the Ce-S XPS peak; (E) FTIR spectra of ZIS, Mo-ZIS, OV-CeO2, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2, the blue box represents the Ce-S stretching vibration; (F) EPR spectra of Mo-ZIS, OV-CeO2, and Mo-ZIS/OV-CeO2; and (G) mechanism diagram illustration of the formation of Ce-S bonds at the Mo-ZIS/OV-CeO2 interface, the red, black, purple, blue, yellow, and green balls are S, Zn, In, Mo, Ce, and O atoms, the red hollow balls are unsaturated S atoms, the brown balls are isolated electrons, the red hollow and green dashed balls are S and O vacancies, the black dashed box represents the unsaturated S atoms that serve as electron-capture centers, the red dashed box represents the isolated electron around Ce atoms, and the blue dashed boxes represent the Ce-S bonds. FTIR, fourier transform infrared; OV-CeO2, CeO2 with O vacancies; XPS, X-ray photoelectron spectroscopy; ZIS, ZnIn2S4.
The coexistence of the Raman characteristic peaks of ZIS and OV-CeO2, along with the presence of Mo-S stretching vibration peaks, confirms the successful formation of the Mo-ZIS/OV-CeO2 heterojunction (Figure 2A). Meanwhile, the X-ray diffraction patterns indicate that Mo-ZIS/OV-CeO2 exhibits diffraction peaks that correspond to ZnIn2S4 (JCPDS card No. 65-2023) and CeO2 (JCPDS card No. 34-0394), confirming that the Mo-ZIS/OV-CeO2 heterojunction was successfully synthesized, consistent with the Raman results (Supporting Information S1: Figure S5).29, 36 By loading OV-CeO2 on the surface of Mo-ZIS, the Raman vibrational peaks and XPS peaks of Mo-ZIS/OV-CeO2 exhibit significant shifts compared to OV-CeO2, implying the intense interfacial interaction between Mo-ZIS and OV-CeO2 (Figure 2B–D, Supporting Information S1: Figures S6, and S7).28, 37 Moreover, the XPS peaks exhibit more significant shifts in Mo-ZIS/OV-CeO2 (S 2p (0.31 eV shift) and Ce 3d (0.64 eV shift)) compared to ZIS/OV-CeO2 (S 2p (0.16 eV shift) and Ce 3d (0.42 eV shift)), which suggests the stronger interfacial interaction intensity and more substantial electron transfer in Mo-ZIS/OV-CeO2. Notably, in addition to the XPS peaks attributed to Ce 3d3/2 and Ce 3d5/2, Mo-ZIS/OV-CeO2 exhibits an additional peak at 886.4 eV associated with the formation of Ce-S bonds, absent in ZIS/OV-CeO2, indicating that strong substantial electron transfer induces the formation of a robust interfacial Ce-S bond between Mo-ZIS and OV-CeO2 (Figure 2D).38 Additionally, the Fourier transform infrared (FTIR) spectrum of Mo-ZIS/OV-CeO2 also exhibits an extra Ce-S peak at 493 cm−1, further confirming the successful construction of Ce-S bonds at the heterogeneous interface (Figure 2E).39 These results indicate that Mo atom doping into ZIS can facilitate the construction of the Ce-S bonds between Mo-ZIS and OV-CeO2.
To elucidate the Ce-S bond formation mechanism, we utilize electron paramagnetic resonance spectra (EPR) to analyze the electron structures within the samples. After doping Mo atom into ZIS, an EPR signal at g = 2.002 appears in Mo-ZIS, demonstrating that the Mo atom incorporation introduces a significant number of unsaturated S atoms with strong electron-withdrawing capability (Figure 2F,G, and Supporting Information S1: Figure S8).11, 24, 26 OV-CeO2 displays the strongest EPR signal at g = 1.96, attributed to the presence of abundant isolated electrons in OV-CeO2.40, 41 The abundance of O vacancies creates an electron-rich environment for Ce atoms which can readily combine with unsaturated S atoms. Notably, along with interaction between Mo-ZIS and OV-CeO2, the EPR signal corresponding to the isolated electron of OV-CeO2 decreases and the Oads/Olatt ratio associated with the O vacancies reduces from 1.85 to 0.43, indicating depletion of isolated electrons in the Mo-ZIS/OV-CeO2 structure (Figure 2C,F and Supporting Information S1: Note 1).35 Based on the XPS, FTIR, and EPR results, we demonstrate that the unsaturated S atoms introduced by Mo doping in Mo-ZIS readily capture isolated electrons on the Ce atom in OV-CeO2, leading to the formation of Ce-S bonds, as depicted in Figure 2G.
1.2 Photocatalytic performance of Mo-ZIS/OV-CeO2
We evaluated the photocatalytic OWS performance of the samples in pure water under Xe lamp (AM 1.5 G) irradiation, using Pt and CoOx as H2 and O2 evolution co-catalysts, respectively (Supporting Information S1: Figure S9). The Mo-ZIS/OV-CeO2 photocatalyst can split water into H2 and O2 at a molar ratio of 2:1 (Figure 3A). After the doping of Mo atoms, Mo-ZIS exhibits H2 and O2 evolution rates with a value of 16.6 and 7.9 μmol g−1 h−1, which is 2.1 times higher than ZIS (7.7 μmol g−1 h−1 of H2 and 3.3 μmol g−1 h−1 of O2). Notably, by introducing OV-CeO2 and optimizing the mass ratio between Mo-ZIS and OV-CeO2, Mo-ZIS/OV-CeO2 exhibited the highest photocatalytic OWS activity, achieving H2 and O2 evolution rates of 512.7 and 256.3 μmol g−1 h−1, respectively (Figure 3A,B, and Supporting Information S1: S10). The apparent quantum yield (AQY) and STH efficiency are important parameters for evaluating photocatalytic performance. The AQY values of Mo-ZIS/OV-CeO2 obtained at various wavelengths align with its absorption spectrum, confirming that the photocatalytic process is driven by photons (Figure 3C and Supporting Information S1: Table S1). Additionally, Mo-ZIS/OV-CeO2 exhibited the highest AQY at 380 nm, achieving a value of 3.01%. The STH average value of Mo-ZIS/OV-CeO2 is approximately 0.14%, surpassing that of most ZnIn2S4-based heterojunctions (Figure 3D, Supporting Information S1: Figure S11, and Table S2).15, 16, 22, 23, 42-46
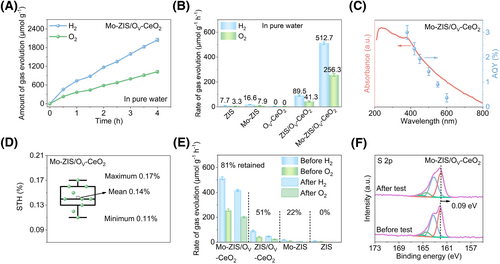
Photocatalysis OWS performance characterization. (A) Time-dependent photocatalytic OWS performance of Mo-ZIS/OV-CeO2 (15 mg photocatalysts; 0.33% Pt and 20% CoOx as co-catalysts; 50.0 mL water, under standard AM 1.5 illumination (100 mW cm−2), all reactions are carried out at 278 K and 3 kPa), the optimal mass ratio of Mo-ZIS to OV-CeO2 is 6:4, error bars represent the standard deviations from the statistic results of three sets of experiments; (B) gas evolution rates of ZIS, Mo-ZIS, OV-CeO2, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2 over OWS, error bars represent the standard deviations from the statistic results of three sets of experiments; (C) the wavelength-dependent AQY for photocatalytic OWS of Mo-ZIS/OV-CeO2, the red arrow indicates the absorption curve, the blue arrow indicates the AQY values, error bars represent the standard deviations from the statistic results of three sets of experiments; (D) the STH value of Mo-ZIS/OV-CeO2 in 10 separate photocatalytic tests, the center line represents the median, the top and bottom box limits represent the upper and lower quartile, respectively, the small rectangle represents the mean value and the maximum/minimum values are indicated by the top/bottom bars; (E) gas evolution rates of ZIS, Mo-ZIS, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2 before and after 16 h of photocatalytic OWS tests, error bars represent the standard deviations from the statistic results of three sets of experiments; and (F) the S 2p XPS spectra of Mo-ZIS/OV-CeO2 before and after 16 h of photocatalytic OWS tests. AQY, apparent quantum yield; OV-CeO2, CeO2 with O vacancies; OWS, overall water splitting; STH, solar-to-hydrogen; XPS, X-ray photoelectron spectroscopy; ZIS, ZnIn2S4.
We also evaluated the photocatalytic stability of the samples. After 16 h of photocatalytic tests, Mo-ZIS/OV-CeO2 retains 81% of its initial performance, whereas ZIS/OV-CeO2 retains only 51% (Figure 3E and Supporting Information S1: Figure S12). To investigate the reason for the improved stability, the S 2p XPS spectrum and structure of Mo-ZIS/OV-CeO2 after photocatalytic testing were analyzed. For metal sulfide photocatalysts, the S atoms in the structure are highly susceptible to oxidation by photogenerated holes, resulting in photo-corrosion and destroying its structure.6 Benefitted from the robust interfacial Ce-S bond, the S 2p binding energy shift of Mo-ZIS/OV-CeO2 after photocatalytic tests is only 0.09 eV, indicating that the oxidation of S atoms is significantly suppressed (Figure 3F). Meanwhile, the structure of Mo-ZIS/OV-CeO2 remained mostly unchanged after photocatalytic tests (Supporting Information S1: Figure S13). Based on this, we conclude that the improved stability results from the construction of robust interfacial Ce-S bonds, which accelerate the extraction of photogenerated holes and prevent the oxidation of Mo-ZIS.
1.3 Kinetics of charge separation and transport
The optical and electrical properties of photocatalysts are crucial in determining the photocatalytic performance. Ultraviolet-visible (UV-vis) spectroscopy was conducted to assess the light absorption capabilities of all catalysts.47 With the introduction of Mo doping and OV-CeO2, light absorption gradually extends into the infrared region and exhibits enhanced absorption intensity along, and Mo-ZIS/OV-CeO2 exhibits the highest light absorption capability (Figure 4A). The electrochemical impedance spectroscopy (EIS) results reveal that Mo-ZIS/OV-CeO2 exhibits the smallest Nyquist radius size, indicating that the successful construction of the interfacial Ce-S bonds can reduce the interface carrier transport barrier (Supporting Information S1: Figure S14 and Table S3).28 Moreover, we calculate the carrier transport activation energy of various samples using the Arrhenius equation by analyzing EIS plots obtained at different temperatures.48 After the construction of interfacial Ce-S bonds, Mo-ZIS/OV-CeO2 exhibits a lower carrier transport activation energy (0.17 eV), which is reduced by 43% compared to ZIS/OV-CeO2 and beneficial for carrier separation and transfer (Figure 4B). Meanwhile, we calculated the built-in electric field intensity via the surface photovoltage and surface photoelectron density (Supporting Information S1: Figure S15).49 Notably, the built-in electric field intensity of Mo-ZIS/OV-CeO2 demonstrated a substantial increase, being 2.1-time greater than that of ZIS/OV-CeO2 (Figure 4C). This indicates that the introduction of interfacial Ce-S bonds enhances the built-in electric field of Mo-ZIS/OV-CeO2 providing a superior driving force for carrier transport.
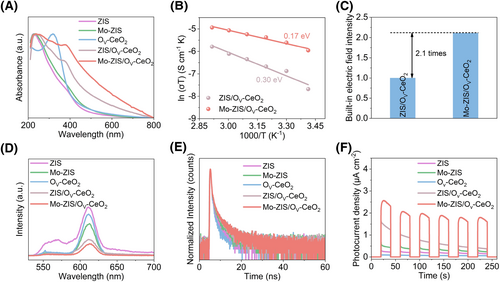
The kinetics of photogenerated carrier generation and separation. (A) UV–Vis spectra of ZIS, Mo-ZIS, OV-CeO2, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2; (B) carrier transport activation energy of ZIS/OV-CeO2 and Mo-ZIS/OV-CeO2; (C) built-in electrical field intensity of ZIS/OV-CeO2 and Mo-ZIS/OV-CeO2 (assuming the intensity of ZIS/OV-CeO2 to be “1”); (D) PL spectra, (E) time-resolved photoluminescence decay spectra, and (F) photocurrent densities of ZIS, Mo-ZIS, OV-CeO2, ZIS/OV-CeO2, and Mo-ZIS/OV-CeO2. OV-CeO2, CeO2 with O vacancies; PL, photoluminescence; UV-Vis, ultraviolet-visible; ZIS, ZnIn2S4.
Photoluminescence (PL) and time-resolved photoluminescence (TRPL) tests are performed to investigate the dynamics of photogenerated carrier separation and transfer.11, 50 With the introduction of Ce-S bonds, the PL peak intensity of the samples gradually decreases and the carrier lifetime correspondingly extends, and Mo-ZIS/OV-CeO2 exhibits the lowest PL peak intensity and the longest carrier lifetime of 1.76 ns (Figure 4D,E, and Supporting Information S1: Table S4). These results suggest that interfacial Ce-S bonds can effectively enhance the separation efficiency of photogenerated carriers, effectively suppressing recombination.51, 52 Benefiting from the enhanced carrier separation and transport capability, Mo-ZIS/OV-CeO2 exhibits the highest photocurrent density of 2.45 μA cm−2, being 10.6, 5.1, 24.5, and 1.8 times greater than ZIS, Mo-ZIS, OV-CeO2, and ZIS/OV-CeO2, respectively (Figure 4F).53, 54 Additionally, the doping of Mo atoms and the introduction of OV-CeO2 result in a more loose structure for Mo-ZIS/OV-CeO2, exposing the largest specific surface area and electrochemically active surface area compared to other samples, thereby providing more active sites for photocatalytic OWS (Supporting Information S1: Figures S16 and S17, and Table S5).55 Meanwhile, EPR radical trapping tests and linear sweep voltammetry (LSV) are crucial methods for assessing the photocatalytic activity of the H2 evolution reaction (HER) and O2 evolution reaction (OER).56, 57 Compared to ZIS/OV-CeO2, Mo-ZIS/OV-CeO2 exhibits stronger EPR signals and lower overpotentials, indicating superior photocatalytic HER and OER activity in Mo-ZIS/OV-CeO2 (Supporting Information S1: Figures S18 and S19).
1.4 Mechanism of Mo-ZIS/OV-CeO2 photocatalytic OWS
The energy band structure of ZIS, Mo-ZIS, and OV-CeO2 are determined using the Kubelka-Munk equation and ultraviolet photoelectron spectroscopy (UPS) (Supporting Information S1: Figure S20).29 The UPS results reveal that the Fermi levels of ZIS, Mo-ZIS, and OV-CeO2 are −4.17, −3.94, and −4.39 V (vs. vacuum), respectively. The detailed energy band structures of ZIS, Mo-ZIS, and OV-CeO2 are constructed by combining the bandgap and band edge potentials, as shown in Supporting Information S1: Figure S21. In situ XPS is an efficient technique for identifying the direction of carrier migration and explaining the mechanism of photocatalysis.11, 58 Under illumination, the XPS peaks of S 2p, In 3d, Zn 2p, and Mo 3d in Mo-ZIS/OV-CeO2 and ZIS/OV-CeO2 shift toward lower binding energies, while those of Ce 3d and O 1s shift to higher binding energies, demonstrating that photogenerated electrons transfer from OV-CeO2 to Mo-ZIS or ZIS during illumination (Figure 5A, Supporting Information S1: Figures S22, and S23). Based on the energy band structure and the direction of the photogenerated electron migration, it can be concluded that both Mo-ZIS/OV-CeO2 and ZIS/OV-CeO2 follow the S-scheme carrier transfer pathway.59
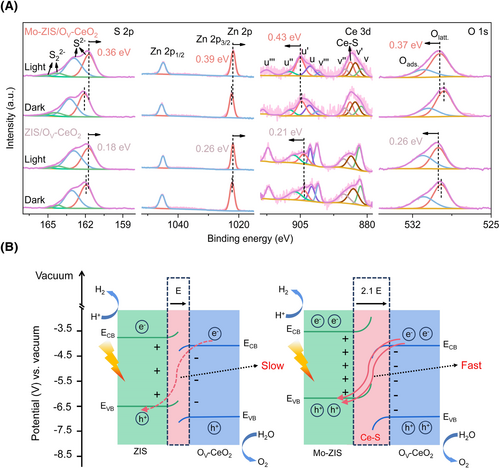
The mechanism of Mo-ZIS/OV-CeO2 photocatalytic OWS. (A) In situ XPS spectra of S 2p, Zn 2p, Ce 3d, and O 1s for ZIS/OV-CeO2 and Mo-ZIS/OV-CeO2 under dark and light conditions and (B) schematic diagram of photoinduced carrier transfer of Mo-ZIS/OV-CeO2 and ZIS/OV-CeO2, the red dashed arrow denotes the slow charge transfer between ZIS and OV-CeO2, the two red solid arrows denote the fast charge transfer between Mo-ZIS and OV-CeO2, E represents the build-in electric field intensity at ZIS/OV-CeO2 interface, Mo-ZIS/OV-CeO2 exhibits 2.1 E, compared to the ZIS/OV-CeO2, Mo-ZIS/OV-CeO2 exhibits interfacial Ce–S bonds and an enhanced E, promoting the migration of photogenerated carriers on the interface, energy of conduction band (ECB) and energy of valence band (EVB) are depicted in diagram. OV-CeO2, CeO2 with O vacancies; OWS, overall water splitting; XPS, X-ray photoelectron spectroscopy; ZIS, ZnIn2S4.
More strikingly, after doping with Mo atoms, the Fermi level of Mo-ZIS shifts upward, increasing the Fermi level difference between the different structures of ZIS and OV-CeO2 (Supporting Information S1: Figure S21). This enhanced Fermi level difference and the formation of interfacial Ce-S bonds in Mo-ZIS/OV-CeO2 lead to an increased built-in electric field intensity compared to ZIS/OV-CeO2. These results reduce the interfacial resistance and strengthen the driving force, thereby facilitating the swifter migration of photogenerated carriers at the interface in Mo-ZIS/OV-CeO2 (Figure 5B). Impressively, Mo-ZIS/OV-CeO2 exhibits a greater shift in binding energy (S 2p (0.36 eV shift) and Ce 3d (0.43 eV shift)) compared to ZIS/OV-CeO2 (S 2p (0.18 eV shift) and Ce 3d (0.21 eV shift)) under illumination, indicating a higher quantity of photogenerated carriers are separated, accumulated, and participate in the photocatalytic OWS in Mo-ZIS/OV-CeO2 (Figure 5A).
Based on the previous analysis, the OWS mechanism for the Mo-ZIS/OV-CeO2 heterostructure photocatalyst was proposed (Figure 5B and Supporting Information S1: Figure S24). Upon illumination, Mo-ZIS/OV-CeO2 is excited, generating photoexcited electrons and holes in the conduction band (CB) and valence band (VB), respectively, of Mo-ZIS and OV-CeO2.60 The photogenerated electrons in the CB of OV-CeO2 combine with photogenerated holes in the VB of Mo-ZIS through the low interfacial barrier Ce-S bonds, while the photoinduced electrons in the CB of Mo-ZIS and photoinduced holes in the VB of OV-CeO2 are retained. Under the combined effects of the interfacial Ce-S bonds and enhanced internal electric field, efficient charge separation is achieved and the S-scheme carrier transfer pathway is accelerated. Consequently, more photogenerated electrons in the CB of Mo-ZIS are transferred to the Pt sites to participate in HER, while more photogenerated holes in the VB of OV-CeO2 are separated to the CoOx sites for OER.
2 CONCLUSION
We have designed a low-interfacial barrier Ce-S bond-enhanced Mo-ZIS/OV-CeO2 S-scheme heterojunction photocatalyst using a doping-defect coupling strategy, achieving H2 and O2 evolution rates with a value of 512.7 and 256.3 μmol g−1 h−1, respectively, along with a STH efficiency of 0.14%. Introducing Mo atoms into ZIS and O vacancies into OV-CeO2 facilitates the formation of Ce-S bonds at the heterogeneous interface, leading to a 43% decrease in carrier transport activation energy and a 2.1-fold increase in build-in electric field intensity. Driven by robust interfacial Ce-S bonds, the S-scheme carrier transfer pathway in Mo-ZIS/OV-CeO2 is accelerated, effectively promoting the photocatalytic OWS process. This study obtains an efficient ZIS-based photocatalyst for OWS through the introduction of doping and defect engineering, providing innovative insights into developing and constructing heterojunction photocatalysts with efficient charge migration interfaces.
ACKNOWLEDGMENTS
This research is supported by National Natural Science Foundation of China (52372225, 22261142666, 52072228, 52302311, 52402308), the Science, Technology, and Innovation Commission of Shenzhen Municipality (JCYJ20220818103417036), Guangdong Basic and Applied Basic Research Foundation (2023B1515120098), the Fundamental Research Funds for the Central Universities, the China National Postdoctoral Program for Innovative Talents (BX20230496, BX20240490), the Youth Talent Support Program of Shaanxi province (20240463), and Project supported by State Key Laboratory of Solidification Processing (2024-BJ-04).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




