Characterization of HLA DR3/DQ2 transgenic mice: a potential humanized animal model for autoimmune disease studies
Abstract
Linkage studies indicate close associations of certain HLA alleles with autoimmune diseases. To better understand how specific HLA alleles are related to disease pathogenesis, we have generated an HLA DR3/DQ2 transgenic mouse utilizing a 550-kb yeast artificial chromosome (YAC) construct containing the complete DRα, DRβ1, DRβ3, DQα, and DQβ regions. The transgenic mouse (4D1/C2D) in an I-Aβo background appears healthy with no signs of autoimmune diseases. Lymphoid tissues as well as CD4+ T cells develop normally. Characterization of the transgene expression demonstrates that ∼90% of B cells express high levels of DR3 and 50–70% of B cells express DQ2. CD11c+ dendritic cells express high levels of DR and DQ. Approximately12–18% of resting T cells are positive for DR expression, and further up-regulation to 40–50% expression is seen upon activation with anti-CD3/anti-CD28 mAb. These results suggest that the transgenic construct confers a high fidelity to the normal human temporal and spatial expression profile. Analysis of T cell receptor repertoire in transgenic mice confirms that DR3/DQ2 are able to mediate thymic selection. Furthermore, transgenic mice respond to a DR3-restricted antigen, demonstrating antigen processing and presentation by antigen-presenting cells (APC). Purified T cells from ovalbumin (OVA)-immunized 4D1 mice respond to human APC co-cultured with OVA, suggesting appropriate antigen/DR3 or DQ2 recognition by murine T cells. Immunoglobulin isotype switching is also observed, indicating functional T-B cognate interactions. Thus, the DR3/DQ2 transgenic mouse has normal lymphoid development and functionality that are mediated by HLA transgenes and can be used to investigate HLA-associated immunological questions.
Abbreviations:
-
- YAC:
-
Yeast artificial chromosome
-
- MFI:
-
Mean fluorescence intensity
1 Introduction
The etiopathology of autoimmunity is variably influenced by environmental factors, genetic background and immune regulation. The polygenic nature of autoimmune disease has led to a significanteffort to identify disease-associated loci and genes through genetic mapping. The best correlation thus far is to the host's human leukocyte antigen (HLA) loci. The most prevalent autoimmune diseases, including rheumatoid arthritis, Graves' disease, insulin-dependent diabetes, Celiac disease and multiple sclerosis, have close genetic linkages to a limited set of HLA class II DR or DQ (or both) alleles 1, 2. In comparison to the high degree of HLA allele polymorphism, the limited number of disease-associated alleles provides the basis for a more focused study on understanding the influence of genetic predisposition and pathogenesis at the molecular and structural level.
The well-defined function of class II molecules is to present antigens. Preferential binding and presentation of disease-inducing self peptide has been implicated as a potential mechanism to shape T cell repertoire, bias T effector function and influence autoimmunity. Recent structural studies pinpoint the amino acid residue and structural similarity in antigen-binding clefts between diabetes predisposing alleles of DQ8 in human and I-Ag7 in mouse, and suggest a correlation between better binding fit of autoantigen to these disease-prone MHC alleles as compared to non-disease associated alleles 3. A variety of HLA DR and DQ transgenic mice have been developed to evaluate the role of disease-associated alleles in: (1) processing and presentation of autoantigens, (2) shaping of the T cell repertoire, and (3) influencing T cell effector function leading to autoimmune pathology. The transgenes have been expressed using the murine I-Eα gene promoter 4–7, as human genomic clones 8–12 or as hybrids between human and murine domains 4, 5. Many of these lines have been crossed onto mice with a targeted mutation in mouse MHC class I or II genes. In correlation with the disease association, expression of HLA transgenes renders normally resistant mouse strains susceptible to disease onset and affects disease progression. Some transgenic mice develop early signs of disease in the absence of any immunological manipulation. For instance, spontaneous development of insulitis is found in DQ8/DR3 transgenic mice in a C57BL/6 background 13, and the development of spondyloarthropathies in HLA B27 transgenic rats 14. Progress has also been made utilizing these animals for some pathogenesis studies 15–18.
The DR3 and DQ2 alleles are found in association with a number of autoimmune disorders 19, 20. In an attempt to address the immunological nature of these molecules in disease susceptibility and modulation, we generated a DR3/DRw52/DQ2 transgenic mouse in the MHC class II I-Aβ–/– background. A 550-kb yeast artificial chromosome (YAC) clone spanning the human TAP1 through DRα genes 21 was used to provide a significant portion of linked genes of the class II locus under the control of human regulatory sequences. This strategy has been demonstrated to be effective at expressing human transgenes in a copy number-dependent and position-independent manner 22, 23. Here, we report phenotypic characterization of the immune system and functional assessment of cellular and humoral immunity in the DR3/DQ2 transgenic mice.
2 Results
2.1 Transgenic mice develop normally and have normal numbers of CD4+ T cells
To ensure specific recognition of human HLA class II by murine CD4+ T cells, we crossed the transgenic mouse to the MHC I-Aβ–/– mouse 24. The resulting transgenic mice homozygous for I-Aβ–/– were named 4D1/C2D.
These animals showed no gross developmental and behavioral abnormalities and no gross pathological signs in various tissues. This is in contrast to other MHC transgenic mice with high copy numbers of the transgene. Those animals developed inflammatory disease with progressive B cell deficiency, abnormal extramedullary granulopoiesis and increased susceptibility to infection 25. In 4D1/C2D mice, the thymus, spleen and LN appeared normal in size and cell number. Hematoxylin and eosin-stained tissue sections (Fig. 2A), displayed a normal cellular architecture of the cortex and medulla in the thymus and normal red and white pulps in the spleen. Primary follicles and periarteriolar lymphoid sheaths were detected in the white pulp and were separated from the red pulp by the marginal zone. B cell follicles and the paracortical T cell region in the LN appeared indistinguishable from control mice. More detailed analyses of cell types revealed that the number of CD4+ cells and the ratio of CD4 to CD8 T cells in the thymus, spleen and LN were similar to control animals (Fig. 2B). Collectively, the data indicate that the DR/DQ transgene products act similarly to endogenous MHC class II and are able to instruct thymic development as well as maintain T cell homeostasis in the periphery.
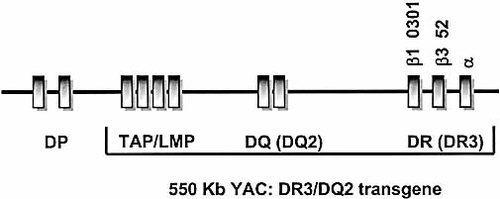
HLA DQ2/DR3 transgene. A 550-kb YAC clone (in the bracket) from a partial human chromosome that includes TAP and LMP genes as well as the HLA DQ2 and DR3 loci was used for making transgenic mouse.
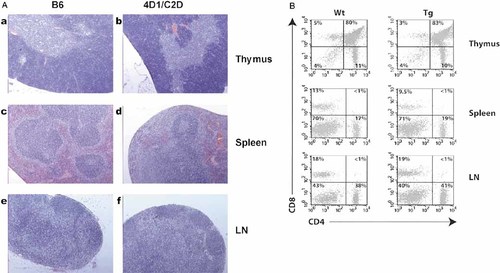
4D1/C2D mice have normal lymphoid organ and CD4 T cell development. (A) Hematoxylin and eosin staining of tissue sections of thymus, spleen and LN from B6 and 4D1/C2D mice. (B) The presence of CD4 and CD8 T cells were analyzed in these tissues and numbers in each quadrant represent the percentage of each population.
2.2 Expression profile of HLA DR3 and DQ2 molecules in 4D1/C2D mice
LN cells of 4D1/C2D and B6 mice were examined for the expression of DR and DQ by flow cytometry. Our results show that ∼98% of LN B cells expressed high levels of DR and 60–80% expressed DQ (Fig. 3A). In comparison, 100% of human B cells were positive for DR and DQ (Fig. 3A). Examination of T cells showed that the overall population shifted to the DR+ direction with an approximately threefold increase in the mean fluorescence intensity (MFI). Among them, 12–18% of CD3+ cells expressed relatively high levels of DR. In contrast, only 2–6% of T cells expressed DQ, the majority of the cells remained negative. In comparison, human T cells showed similar percentages of DR- and DQ-expressing cells with higher levels of DR and DQ expression in both T and B cells when quantified by the MFI (Fig. 3A). The DR- and DQ-positive T cells in transgenic mice included both CD4+ and CD8+ cells. Additionally, most DR-positive T cells were among the CD44+ memory population (Fig. 3B), suggesting that DR expression may be acquired during the transition from naïve to memory phenotype.
Next, the DR and DQ expression in dendritic cells (DC) was examined. Fig. 4A shows that, whereas nearly all the peritoneal CD11c+ cells from 4D1/C2D mice were positive for DR, the levels of DQ expression was much lower. Similar HLA-expressing DC were also found in the spleen and LN (data not shown). As a comparison, human mature DC generated by in vitro culture were also analyzed in parallel and robust expression of both DR and DQ was observed (Fig. 4A).
To address whether the expression of transgenic HLA on DC correlates with their maturity, we compared DR and DQ expression in CD11c+ cells from freshly isolated bone marrow cells, immature DC from GM-CSF-cultured bone marrow cells, and mature DC from GM-CSF + LPS-stimulated bone marrow cells (Fig. 4B). Unlike DC from the peritoneal cavity, BM CD11c+ cells were DR– and DQ–. Upon culture in the presence of GM-CSF, approximately 60% of CD11c+Mac-1lo cells became DR+ and a further increase in percentage (70–80%) and intensity (MFI 471–1,286) of DR expression was observed in mature DC upon LPS treatment. To our surprise, no induction of DQ expression was seen in these cells prior or post-cytokine/LPS treatment (data not shown), although its appearance on peritoneal CD11c+ cells was clearly identifiable. It is possible that the regulation of DQ expression is different from DR and the in vitro maturation process may not fully represent the in vivo differentiation milieu. Overall, we have demonstrated that mature DC in transgenic mice are capable of expressing DR and DQ and the expression is likely coupled with DC differentiation and maturation processes.
The identical expression pattern of HLA DR and DQ in 4D1/C2D mice as compared to human cells indicates that the transgene construct contains most, if not all, necessary control elements that confer cell-specific regulation of gene expression. It also suggests that the transcription regulation of the transgene is not influenced by the sequences flanking the integration site.
The expression of HLA DR can be further regulated by various stimuli in APC and T cells. To understand whether the same activation signals control DR gene expression in transgenic murine cells, DR expression was compared in splenocytes before and after LPS or anti-CD3/anti-CD28 mAb stimulation. Up-regulation of DR expression in B cells upon LPS treatment was shown by an eight- to tenfold increase in MFI, whereas that in T cells was demonstrated by both a threefold increase in DR+ cells (∼13% to ∼40%) as well as a two to fourfold increase in MFI (Fig. 5A). Further characterization of the T cells revealed that both CD4 and CD8 T cells up-regulated DR to a similar degree, and the results were comparable to that of human T cells (Fig. 5B). The shift of non-T cell fraction towards higher levels of DR expression upon anti-CD3/CD28 stimulation was most likely the effect of IFN-γ secreted by activated T cells on B cells. The data clearly show that the large fragment of human genomic DNA is not only able to confer cell-specific expression of DR and DQ genes it also contains cis-regulatory elements for inducible expression in the same fashion as seen in human cells.
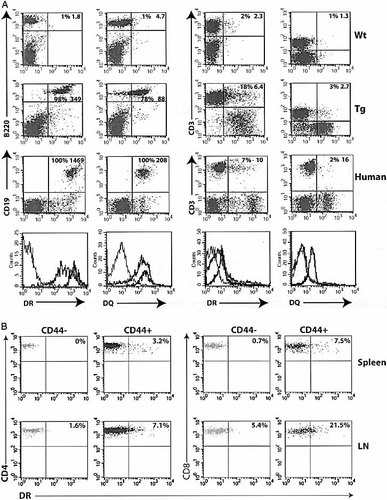
Expression of DR3 and DQ2 molecules on B and T lymphocytes. (A) Transgene expression was analyzed by co-staining LN cells with CD3, B220, and DR or DQ antibodies. Data shown are a representative of more than six experiments. As a comparison, expression of DR and DQ in human B (CD19+) and T (CD3+) cells was performed. Percentage of DR+ and DQ+ cells in total B or T cell population and the MFI of total B or T cell population are indicated. The results are summarized in histogram plots; where the thin lines indicate B6 control, gray lines 4D1/C2D, and dark lines human cells. (B) Correlation of memory phenotype and DR expression in T cells was examined by staining cells with CD4 or CD8, CD44 and DRα antibodies. Events shown in dot plots are gated on CD4+ or CD8+ T cells that are either CD44– or CD44+.
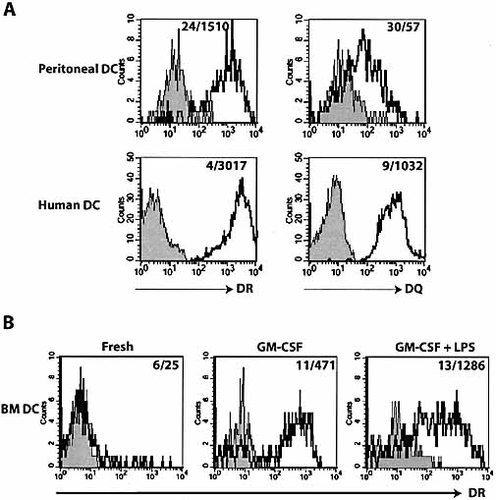
DC from transgenic mouse express DR3 and DQ2. (A) Peritoneal lavaged cells from B6 (gray) and 4D1/C2D (black) mice were stained with CD11c and DR or DQ antibodies. Human mature DC derived from in vitro cultured PBMC were included as a comparison. In these plots, gray curves are isotype controls and black curves are human DC. (B) BM cells freshly isolated, cultured in the presence of GM-CSF, or cultured in the GM-CSF and stimulated with LPS were stained with antibodies against CD11c, Mac-1, and DR or DQ. Cells shown in history plots were gated on CD11c+ and Mac-1lo. Numbers in the plots indicate MFI of DR expression in DC from B6 (gray curves) and 4D1/C2D mice (black curves).
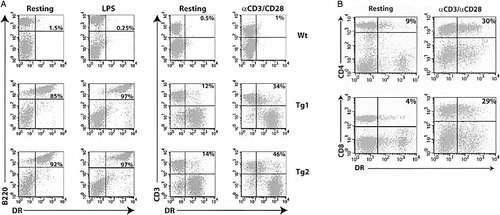
Up-regulation of DR expression in activated B and T cells. Splenocytes from one B6 and two 4D1/C2D transgenic mice were cultured in the presence of LPS or anti-CD3/anti-CD28 mAb for 24 h and expression of DR was analyzed and compared with freshly isolated cells. Numbers indicate the percentages of DR+ cells in total B or T cells (A) and in total CD4 or CD8 T cells (B). Data are representative of three experiments.
2.3 Thymic selection in transgenic mice
The formation of interspecies DRα/I-Eβ dimers has been reported in DRα-only transgenic mice 26. Our 4D1/C2D mice, although in the I-Aβ–/– background, express I-Eβ molecules intracellularly. To examine the presence of DRα/IEβ heterodimer formation in these mice, B lymphocytes were stained with antibodies recognizing DRα, DRβ and IEβ molecules. Among the DRα+ population, 80–93% were DRβ+ and >90% were IEβ+ (Fig. 6A). In agreement with the findings from a similar HLA transgenic line 27, these data indicate that DRα/DRβ and DRα/IEβ are co-expressed on the cell surface. We also addressed the possibility of I-Aα/DQβ dimer formation. Although C57BL/6 and 4D1 transgenic mice in a I-Aβ+ background showed high levels of I-Aα expression within the B cell population, 4D1/C2D mice were identical to C2D control mice and showed no detectable I-Aα expression (Fig. 6B).
The presence of mature CD4+ T cells in the periphery at levels equivalent to B6 mice suggests that the DR3 and DQ2 transgenes in the absence of mouse MHC class II molecules (I-Aα β and I-Eα β) are able to mediate thymic selection and peripheral homeostasis. To investigate how well HLA molecules could generate diverse TCR repertoire, T cells from thymus, spleen and LN were analyzed with a panel of TCR Vβ antibodies. LN T cells from 4D1/C2D mice contained clones that expressed most of the Vβ examined at levels comparable to B6 mice. Interestingly, Vβ5-, Vβ11- and Vβ12-expressing cells were under-represented or absent from the transgenic mice in both CD4 and CD8 populations. The same deletion was also observed in B10.A mice that express I-E molecules, as well as in the CD4 and CD8 single-positive thymocytes from both strains (Fig. 7 and data not shown). In addition, under-representation of Vβ4 and Vβ7 in CD4+ T cells and Vβ7, Vβ9 and Vβ14 in CD8+ T cells were almost identical between 4D1/C2D and B10.A mice. However, changes unique to 4D1/C2D mice were identified as well. These included the decrease in the percentages of Vβ3 and Vβ17 in both populations and Vβ14 in CD4+ T cells. In addition to under-utilization of certain TCR clones, Vβ6- and Vβ10-expressing clones were preferentially selected in both 4D1/C2D and B10.A mice, with slightly higher representation in the former. More importantly, higher percentages of Vβ8-expressing populations were found in 4D1/C2D but not B10.A animals. Consistent with the Vβ distribution of mature T cells, similar profiles of positive selection of Vβ6, Vβ8 and Vβ10 were also evident in the mature CD4 and CD8 single-positive thymocytes (data not shown). These results demonstrate that HLA DR3 and DQ2 molecules are able to shape TCR repertoire by mediating positive and negative selection processes during thymocyte development and by maintaining T cell peripheral homeostasis.
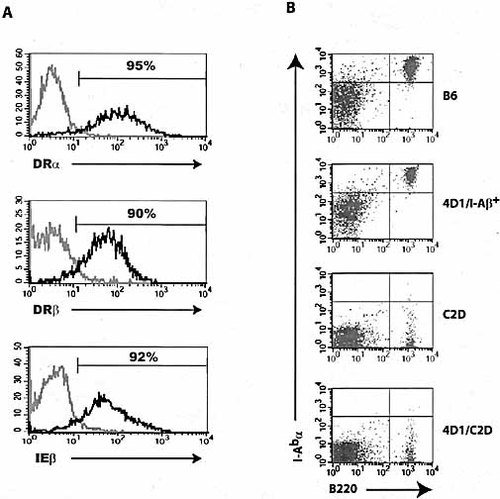
DRα forms dimer with both DRβ and IEβ, and I-Aα does not dimerize with DQβ and DRβ. (A) Total white blood cells from peripheral blood were analyzed for DRα, DRβ and IEβ expression with specific antibodies. Cells were gated on the B220+ population. Light lines represent B6 control and dark lines 4D1/C2D mice. (B) Dimerization and surface expression of I-Aα and DQβ or DRβ were examined in transgenic mice. LN cells from B6, 4D1/I-Aβ+, C2D and 4D1/C2D mice were stained with B220 and I-Abα. Data are representative of at least four experiments.
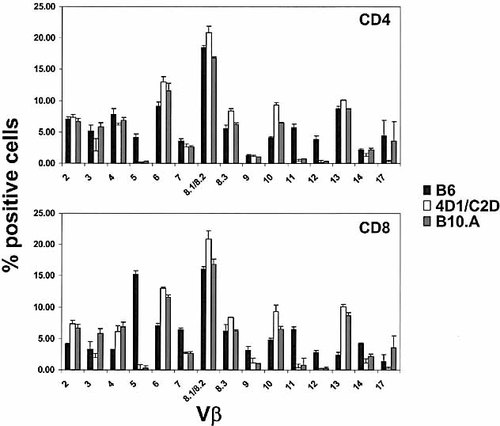
TCR repertoire selection in 4D1/C2D mice. Cells from LN of 4D1/C2D, B6 and B10.A animals were examined for Vβ usage by flow cytometry. Percentage of Vβ-specific cells was quantified in CD4+CD3+ (upper panel) or CD8+CD3+ (lower panel) gated populations. Results are graphed from a mean of three animals in each group and error bars indicate standard deviations. Data shown are results from one of two experiments.
2.4 Functional analyses of cellular and humoral immunity
To understand whether DRα/DRβ dimers are functionally competent in triggering immune response, 4D1/C2D mice were examined for their ability to process and present a known HLA DR3-restricted peptide epitope from an intact protein. Mice were immunized with CFA in which the Mycobacterium tuberculosis heat shock protein (hsp) 65 contains an HLA DR3-restricted CD4+ T cell epitope at amino acid position 3–13 28. As a comparison, mice were immunized with the hsp 1–20 peptide in either CFA or IFA. As shown in Fig. 8A, LN cells from 4D1/C2D mice immunized with CFA are capable of mounting a proliferative response to the DR3-restricted peptide derived from the protein present in the immunogen. In contrast, proliferative responses to the peptide in IFA and in CFA were twofold higher than with CFA alone as a result of an increased overall concentration of immunizing peptide. The slightly reduced response with CFA + hsp 1–20 was not statistically significant and likely due to the saturating amount of hsp 1–20 (100 μg/ml) used in both reactions that may plateau the responses and mask the potential quantitative difference by using different adjuvant. We conclude that the HLA-DR3 molecules present in the 4D1/C2D mouse enter into the class II presentation pathway and bind peptides of the appropriate specificity in conjunction with mouse processing intermediates.
To determine if 4D1/C2D mice are capable of responding to protein antigen, we investigated antibody responses to OVA. Groups of mice were immunized with OVA in a protocol designed to induce high levels of antigen-specific antibody, most notably IgE. As shown in Table 1, 4D1/C2D mice produce robust levels of IgG1, IgG2b and IgE on the order of the levels expressed by BALB/c mice. Neither BALB/c nor 4D1/C2D mice produced significant levels of OVA-specific IgG2a. As expected, antigen-specific antibody production from control C2D animals remained at background levels in separate experiments performed in an identical manner as well as in studies by others (data not shown and 24). This suggests that antigen-primed CD4+ T cells from 4D1/C2D mice are capable of cognate interactions with antigen-specific B cells that result in isotype switching.
|
|
Polyclonal IgG |
IgG1 |
IgG2a |
IgG2b |
IgE |
|---|---|---|---|---|---|
|
BALB/c |
> 12 |
>10 |
1.12 ± 0.97 |
15.63 ± 1.23 |
11.45 ± 1.09 |
|
4D1/C2D |
>12 |
>10 |
0.90 ± 1.57 |
>10 |
11.07 ± 1.80 |
- a) Log2 titers of OVA-specific antibody are reported as averages for three mice. If a linear regression could not be performed the data represented as a value >the maximum dilution tested.
2.5 T cells from 4D1/C2D mice respond to antigen presented by human APC
To further evaluate whether cells from 4D1/C2D mice indeed recognized antigen complexed to human DR3 and DQ2, an antigen-presentation assay was performed with human APC. CD4+ T cells from OVA-immunized mice were cultured with OVA and human B-lymphoblastoid cells that are DR3+DQ2+ (8.1.6 cells) or DR null and DQ2+ (9.22.3 cells). A typical dose-dependent proliferative response to increasing amount of OVA was observed (Fig. 8B). Thus, T cells from immunized 4D1/C2D mice are proficient at recognizing and responding to antigen presented by human HLA-DR3+DQ2+ APC, suggesting that they have been selected against the right HLA haplotype in vivo.

4D1/C2D mice process and present HLA transgene-restricted epitopes and respond to antigen in the context of DR3 and DQ2. (A) Draining LN were removed from mice immunized with CFA, IFA + 100 μg hsp 1–20 peptide, or CFA + 100 μg hsp 1–20 peptide and tested in vitro with 10 μg/well hsp 1–20 peptide. ΔCPM was determined for each animal, and the results were averaged. (B) CD4+ T cells from OVA-immunized mice were co-cultured with human B-LCL and OVA. T cell stimulation was measured by [3H]thymidine uptake. Data were plotted as an average of triplicates. This experiment was performed three times with similar results.
3 Discussion
Here we describe phenotypic characterization of HLA DR3/DQ2 transgenic mice generated utilizing a 550-kb YAC construct containing the complete DRα, DRβ1, DRβ3, DQα, and DQβ loci. A large genomic DNA fragment (4D1) used in this study has a greater potential to mimic human physiological conditions and provide the cell-specific distribution and activation-dependent up-regulation of class II molecules seen in humans. Indeed, our data show the restricted expression of DR and DQ in B cells, DC and a low percentage of T cells, and the up-regulation of DR in B and T cells. One aspect of differential regulation of class II genes in mouse and human is the absence of class II in murine T cells 29. Unlike most HLA class II transgenic mice, our transgenic mouse with cognate cis-control elements showed low levels of DR expression in ∼12–18% of resting T cells and ∼40% of activated T cells. This suggests the presence of unique cis-acting regulatory sequences in the human class II locus that confer cell type- as well as activation-dependent gene expression. Other transgenic mice created with a large genomic construct encompassing both HLA-DR3/DQ2 did not show DR expression in resting or activated T lineage cells 27. This is likely due to the smaller size of the construct (320 kb). Alternatively, the other strain of mice may carry their transgene in a location that is subject to flanking-region effects.
The inclusion of DR and DQ present in their natural linkage positions in the transgenic construct allows comparisons to single DR and DQ transgenic mice. Studies along this line have shown that, whereas DR3 mediates the onset and susceptibility of the disease, DQ8 modifies its severity 30. Separate studies have shown that the DR2 molecule modulates collagen-induced arthritis in association with the expression of DQ8, and DR4 reduces the incidence of spontaneous diabetes in DR4/ DQ8/RIP-B7 transgenic mice 2, 31. Events that may attribute to disease modification include: (1) epitope masking and epitope spreading in autoantigens, either allowing certain autoreactive T cells to remain ignorant or resulting in recruitment and expansion of T cells with additional autoantigen specificities, respectively 13, 30; (2) switching of the CD4 T cell response from Th1 to Th2; and (3) reshaping the TCR repertoire 2, 18, 31. These studies elegantly demonstrate the interplay between different DR and DQ alleles in mediating autoimmunity and demonstrate the usefulness of HLA transgenic mice in understanding disease pathogenesis and immune regulation. However, all of these double transgenic models carry two discrete transgenes that are not linked as in their naturally occurring configuration, with control elements and potential loci-associated genes in attendance. It will be interesting to evaluate these studies in genomic HLA loci transgenic mice such as 4D1/C2D.
The role of murine I-E molecules in modulating disease processes has been demonstrated in several different autoimmune disease models. For instance, expression of I-E renders resistant strains susceptible for autoimmune thyroiditis 32 and confers resistance to diabetes in NOD mice 33, 34. DRα/I-Eβ dimers have been reported to be functionally indistinguishable from I-Eα β 26. It has also been demonstrated that DRα/DRβ, DRα/ I-Eβ and I-Eα/I-Eβ dimers select TCR repertoire with similar Vβ usage 26, 35, 36. Although our 4D1/C2D mice did show near complete deletion of Vβ5, Vβ11 and Vβ12 cells and partial deletion or under-representation of a few other Vβ that are consistent with I-E-expressing mice, reduced utilization of Vβ3, Vβ14 and Vβ17 and over-representation of Vβ clones that are unique to 4D1/C2D mice suggest the participation of DR3, DRβ52 and DQ2 molecules in the selection process. The combination of multiple diverse class II molecules confers a unique pattern of TCR repertoire. Interestingly, others have reported that trans-species dimerization of DRα/I-Eβ is less efficient than DRα/DRβ in triggering antigen-specific T cell activation and that less than ∼5% of antigen-specific T cell hybridomas raised in DR4 transgenic mice are restricted to DRα/I-Eβ 15. This observation corroborates findings made by us and others that despite the presence of DRα/I-Eβ dimer, APC in the transgenic mice are able to process and present a DR3-restricted antigen and induce an antigen/DR3-dependent T cell response 37. These observations suggest that the 4D1/C2D transgenic mice in their current genetic background can be used to investigate HLA-related immunological questions. To unequivocally resolve the I-Eβ interference, breeding DQ2/DR3 transgenic mice to I-A/E double knockout background is required 38.
In conclusion, we demonstrate the importance of using a large fragment of human genomic DNA to confer cell-specific expression of DR and DQ genes and its ability to provide cis-regulatory elements for inducible expression in the same fashion as seen in human cells. We also show that the HLA-DR3 and -DQ present in the 4D1/C2D mouse enter into the class II presentation pathway and bind peptides of the appropriate specificity in conjunction with mouse processing intermediates and that antigen-primed CD4+ T cells from 4D1/C2D mice are capable of cognate interactions with antigen-specific B cells that result in isotype switching. We anticipate that the 4D1/C2D mouse will serve as a relevant disease model and allow for more reliable target identification and validation forimmunotherapies.
4 Materials and methods
4.1 Generation of transgenic mouse
A 550-kb human genomic fragment was isolated as a YAC (clone 4D1) and used to generate transgenic mice. It encompasses a region of human chromosome 6 that contains the TAP1/2, LMP2/7 and HLA DQw2 (DQA*0501/DQB1*00201) and DR3 (DRA*0101/DRB1*0301/DRB3*52) genes (Fig. 1) 21. The YAC was co-transfected into AB-1 embryonic stem cells 39 with a plasmid carrying the phosphoglycerate kinase promoter-driven neor gene 40 using transfectam (Promega, Madison, WI). G418-resistant clones were screened for transgene integration by PCR and Southern blotting analyses. Embryonic stem cells identified as containing at least one intact copy of the 4D1 YAC were injected into blastocysts derived from C57BL/6 mice. Offspring derived from transgenic ES cells were screened by tail DNA analysis and transgene positive founders were crossed to C57BL/6 mice and to MHC class II I-Aβ–/– mice (Taconic, Germantown, NY) 24.
4.2 Cells
Lymphocytes were obtained from thymus, spleen and LN of 4D1/C2D and B6 mice. Mature DC were obtained by peritoneal lavage. BM-derived immature and mature DC were generated as described 41. Briefly, bone marrow cells from B6 and 4D1/C2D mice were cultured with 20 ng/ml GM-CSF (Endogen, Inc. Woburn, MA) for 10 days followed by 1 μg/ml LPS (Sigma) for 1 day. CD11c+Mac-1lo cells were analyzed for DR and DQ expression. Human PBMC were prepared from buffy coat material (Stanford University Blood Center). Mature human DC were derived as previously described 42. Human B lymphoblastoid cell line (B-LCL) 8.1.6 cells expressing DRB1*0301, DRw52, DQ2 and DP4, and its derivative 9.22.3 cells lacking DRα and expressing DQ2 andDP4 were a kind gift from Dr. E. D. Mellins (Stanford University) 43.
4.3 Phenotypic analysis
Cells were stained with phycoerythrin (PE), fluorescein isothiocyanate (FITC), or allophycocyanin-conjugated antibodies that recognize B220, CD3, CD4, CD8, CD19, CD44, CD11c, I-Eβ (clone 17-3-3), I-Abα (clone AF6-120.1), HLA DR (clones L243 and TÜ36), and DQ (clone 1a3). TCR repertoire was analyzed using a panel of Vβ antibodies. All antibodies used in this study were purchased from BD PharMingen (San Jose, CA), except anti-DQ antibody (Biodesign International, Saco, ME). Flow cytometry was performed using FACSCalibur and CellQuest software (Becton Dickinson, San Jose, CA).
4.4 Functional analysis
Mycobacterium tuberculosis hsp65 peptide 1-20 (MAKTIAYDEEARRGLERGLN) was constructed in house using FastMoc chemistry with the ABI peptide synthesizer. Mice were immunized in their hind footpads, and popliteal LN were removed 7 days later. LN cells were cultured with 10 μg/ well of the peptide for 24 h. Proliferation was assessed 18 h later 28.
OVA-specific IgG1 and IgE responses were induced as described 44. OVA-specific antibodies were measured by ELISA using biotinylated anti-IgG1 (A85-1), anti-IgE (R35-118), anti-IgG2b (R12-3), horseradish peroxidase-conjugated anti-IgG2a (R19-15) (all from PharMingen), and anti-IgG Fc-specific antibodies (Jackson Immunoresearch).
The mouse T cell response to antigen presented by human APC was performed as follows. Mice were immunized as described above; with schedule modifications of intraperitoneal immunization (day 1) followed by intranasal immunization on days 7, 8 and 9. Mice were killed on day 10. CD4+ T cells purified from spleen and LN using Miltenyi beads (Miltenyi Biotec, CA) were cultured with B-LCL 8.1.6 or 9.22.3 cells pretreated with mitomycin C (Sigma-Aldrich, MO) in the presence of increasing concentrations of OVA (Worthington Biochemical, NJ) for 72 h at 37°C. Cells were pulsed with[3H]thymidine and proliferation was assessed.
Acknowledgements
The authors thank Jiannis Ragoussis for the 4D1 YAC construct, Dr. E. D. Mellins for human B lymphoblastoid cell lines, Doug Hodges for mouse genotypic analysis, Regina Chin and Nargol Faravashi for assistance in proliferation assays, and Jim McCabe and Daniel Brigham for animal facility management and assistance in surgical procedures. We also thank Dr. Mark Goldsmith and members of the Immunology group for helpful discussion and comments, and Roopa Ghirnikar for her editorial assistance. This work was partially supported by the National Institute of Standards and Technology, Advanced Technology Program, Cooperate Agreement no. 70NAB4H151.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




