Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions
Abstract
Natural killer cells have been reported to be able to kill various transformed and virus-infected target cells. It was recently observed that NK cells also could kill syngeneic dorsal root ganglia (DRG) neurons by a perforin-dependent mechanism. We demonstrate here that this phenomenon does not reflect a general ability of NK cells to kill neurons in culture. While DRG neurons of the peripheral nervous system were readily killed, ventral spinal cord neurons and hippocampal neurons of the central nervous system (CNS) were resistant to lysis. The resistance to NK cell-mediated lysis of the latter neurons was not related to protection by MHC class I molecules, since similar β2-microglobulin–/– neurons were equally resistant to lysis. While exploring other possible molecular mechanisms for the selective triggering of lysis of DRG neurons, we observed that the retinoic acid early inducible gene-1 (RAE-1), the product of which is a ligand for the NK cell-activating receptor NKG2D, was expressed at high levels in the DRG neurons. In contrast, RAE-1 was expressed only at very low levels in the resistant CNS-derived neurons. Blocking NK cells withanti-NKG2D antibodies inhibited NK cell-mediated killing of the DRG neurons. Thus, we demonstrate that NK cell-mediated lysis of DRG neurons correlates with the expression of RAE-1 and that this lysis is dependent on activation of NK cells via NKG2D. This observation demonstrates that NK cells can kill non-pathogen-infected or non-transformed syngeneic cells through activation of the NKG2D receptor.
Abbreviations:
-
- B6:
-
C57BL/6
-
- β2m:
-
β2-microglobulin
-
- DRG:
-
Dorsal root ganglion
-
- RAE-1:
-
Retinoic acid early inducible gene-1
-
- RAG:
-
Recombinase-activating gene
1 Introduction
Natural killer (NK) cells are potent cytotoxic effector cells in the innate immune defense against cells infected by certain viruses, intracellular bacteria and parasites, as well as tumor cells 1–4. They can migrate to inflamed tissues in response to different chemoattractants. When activated, NK cells respond by direct cytotoxicity and by the releaseof cytokines and chemokines. By the latter process, they also contribute to inflammation and/or recruitment of other inflammatory cells.
NK cell function is tightly regulated by multiple signals transmitted via inhibitory and activating receptors 5–10. This fine specificity of NK cells has been worked out largely by relying on tumor-based model systems, in which tumor targets have been transfected with ligands for inhibitory or activating receptors, and receptor function addressed mainly by using blocking mAb. The killing of target cells by NK cells is often inhibited by MHC class I molecules 11–14. In mice, two C-type lectin-like receptor families have been described, the Ly49 and the CD94/NKG2 families, which recognize classical and non-classical MHC class I molecules, respectively 15. These families contain mainly inhibitory receptors that are expressed on overlapping subsets of NK cells. Although certain members of these receptor families can activate NK cells after binding to MHC class I molecules, NK cell activation generally appears to be elicited by a distinct set of molecules that have weak homology with MHC class I molecules 16–18. The activating receptor, NKG2D, which differs dramatically from the other NKG2 receptor proteins, is of particular interest since it, in contrast to other NK cell-activating receptors, is constitutively expressed on all NK cells 19–22.
Recently, two related families of previously cloned proteins were identified as ligands for the mouse NKG2D receptor, the retinoic acid early inducible gene-1 (RAE-1)-encoded proteins and the minor histocompatibility antigen H60 23–26. These molecules are distantly related to MHC class I molecules and are expressed in several tumor cell lines 24. Transfection of RAE-1 and H60 into non-expressing tumor target cells activates the killing capacity and IFN-γ production of NK cells in vitro 23, 24. RAE-1 transduced into the MHC class I-positive RMA cell line, which is not rejected upon injection into recipient mice, results in an NK cell-mediated rejection of the tumor cells 27, 28.
Under certain circumstances, NK cells can also kill non-pathogen-infected or non-transformed syngeneic cells in vitro. The molecular mechanisms for these reactions are poorly understood. We previously observed that mouse embryonic dorsal root ganglia (DRG) neurons in culture were rapidly killed upon exposure to syngeneic NK cells. These NK cells exerted a direct cytotoxic effect on the DRG neurons that was cell contact-dependent and mediated by perforin 29. Here, we provide a molecular mechanism for this effect. We demonstrate that DRG neurons, in contrast to other neurons, express high levels of RAE-1, a ligand for the NKG2D receptor. In addition, we show that the killing of these neurons by NK cells is critically dependent on NKG2D.
2 Results
2.1 Selective NK cell-mediated lysis of DRG neurons
Upon exposure to syngeneic IL-2-activated NK cells (hereafter denoted as NK cells), DRG neurons in 2-day primary cultures were totally destroyed as revealed by analyzing neuronal morphology and by detection of apoptotic bodies (Fig. 1A, B) 29. When lower concentrations of NK cells were used, nerve cell bodies were less affected but nerve fiber alterations, such as fragmentation and spheroid formations as described by Medana et al. 30 were conspicuous. In contrast, upon exposure of NK cells to 2-day primary cultures of CNS-derived hippocampal (Fig. 1C) and ventral horn neurons (data not shown), no significant effects on the neurons were observed. Thus, NK cells appeared to selectively kill the DRG-derived neurons, while sparing other populations of neurons cultured under the same conditions for the same length of time. This observation led us to explore the molecular mechanisms behind this selective difference in susceptibility to NK cell-mediated lysis between the different populations of neurons. When not otherwise noted, detailed comparisons between DRG and hippocampal neurons were performed.
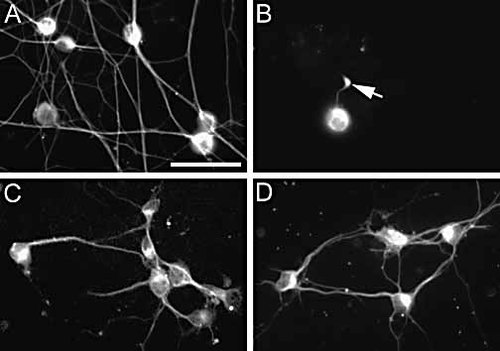
Selective NK cell-mediated lysis of DRG neurons. (A) Control DRG neurons and (B) DRG neurons exposed to nylon wool-purified NK cells (2×106 cells/ml) from B6 mice for 4 h. Note the severe loss of DRG neurons when exposed to NK cells and a spheroid formation in the axon of a remaining neuron (arrow). (C, D) Hippocampal neurons from (C) wild-type and (D) β2m-deficient mice. Both cultures of hippocampal neurons were exposed to nylon wool-purified NK cells (2×106 cells/ml) from B6 mice for 4 h. The neurons were immunolabeled with an Ab to the neuronal marker, neuron-specific class III β-tubulin. Bar 50 μm.
2.2 Two-day hippocampal neurons from β2-microglobulin–/– mice are resistant to NK cell-mediated cytotoxicity
One hypothesis that could explain the resistance of the hippocampal neurons to NK cell-mediated lysis was a general inhibition of NK cell activity by MHC class I molecules expressed by the neurons. To test this hypothesis, hippocampal neuronal cell cultures from MHC class I-deficient β2 -microglobulin (β 2m)–/– mice were established and exposed to NK cells. However, hippocampal neurons from β2m–/– mice were equally resistant to lysis as corresponding neurons derived from wild-type β2m+/+ mice (Fig. 1C, D). This shows that lack of inhibition of the NK cells via MHC class I-binding inhibitory receptors is not sufficient to elicit killing of these neurons.
2.3 High expression of RAE-1 in primary cultures of DRG neurons
The data obtained this far could be explained by absence of ligands that activate NK cells on the hippocampal neurons. Of the two ligands recently described in the mouse system, RAE-1 and H60, only RAE-1 is expressed in C57BL/6 (B6) mice (used in this study) 23–25. Therefore, we analyzed the expression of RAE-1 in cultures of DRG, hippocampus and ventral horn. First we analyzed the expression of RAE-1 at the mRNA level. Total RNA was reverse-transcribed to cDNA and analyzed using primers designed to amplify cDNA-encoding RAE-1α, β, γ, δ and ϵ. Expression of RAE-1-encoding messages was high in DRG cultures but was substantially lower in hippocampal cultures (Fig. 2) and ventral horn cultures (data not shown). To determine whether the difference that we observed at the mRNA level also reflected a difference at the protein level, we used NKG2D tetramers to assess the surface expression of its ligand RAE-1 on neurons in the DRG and hippocampal cultures. The standard NK target cell line YAC-1, which expresses RAE-1, and the cell line EL4, which does not express RAE-1 24, were used as positive and negative controls, respectively. In agreement with the high expression of RAE-1 transcripts in the DRG cultures, the tetramers stained the neurons in these cultures brightly, but only weakly the neurons in the hippocampal cultures (Fig. 3). Thus, DRG, but not hippocampal, neurons express ligands for activating receptors on NK cells.
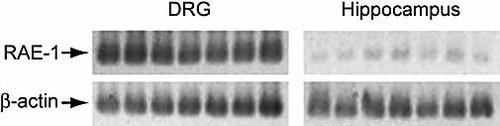
DRG neurons express mRNA encoding RAE-1. Total RNA was extracted and reverse-transcribed. The primary cultures were sampled from separate sets of experiments. The amplified cDNA corresponding RAE-1 and β-actin are indicated. The identity of the PCR products generated by the RAE-1-specific primers was verified by sequencing. One representative out of three individual PCR reactions is shown.
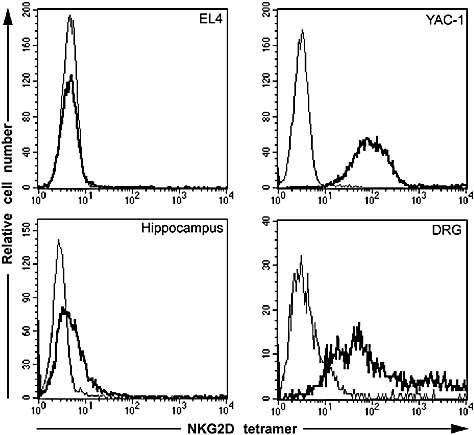
DRG neurons express ligands for NKG2D. Cell surface expression of ligands for mouse NKG2D on the EL4 cell line (negative control), the YAC-1 cell line (positive control), hippocampal and DRG neurons. Cells were stained with tetrameric NKG2D (bold line) or unstained (solid line) and analyzed by flow cytometry. Note that the DRG neurons stained brightly with NKG2D tetramers in contrast to hippocampal neurons that stained weakly.
2.4 Anti-NKG2D monoclonal antibodies block NK cell-mediated killing of DRG neurons
In order to test that the NK cell-mediated killing of DRG neurons is triggered via NKG2D, we first verified the presence of NKG2D on the NK cells. DX5-purified NK cells were derived from RAG–/– mice to yield a pure NK cell population, without DX5+ NKT or T cells. Using an anti-NKG2D mAb, we confirmed that all NK cells expressed NKG2D (Fig. 4), in agreement with previous results 23, 24. We then exposed DRG neurons to NK cells preincubated with the anti-NKG2D mAb. This procedure almost completely prevented the degeneration of DRG neurons, as assessed by direct counting of neurons and by analyzing the occurrence of spheroid formations and fragmented axons, which reflects a direct attack by lymphoid cells 30 (Fig. 5, 6). In contrast, incubation of NK cells with the control IgG did not prevent the degeneration of DRG neurons, but rather affected the neurons to the same extent as NK cells without pretreatment with Ab (Fig 6). The inhibitory effect of the anti-NKG2D mAb on the NK cell-mediated degeneration of DRG neurons was also assessed by an LDH release assay. Exposing DRG neurons (3×104 at the initiation of culture) to DX5-purified NK cells (5×105/ml) resulted in 6% specific cytotoxicity in the presence of anti-NKG2D mAb as compared to 42% specific cytotoxicity in the presence of control IgG. Taken together, these results suggest that the NK cell-mediated killing of DRG neurons occurs via activation of NKG2D.
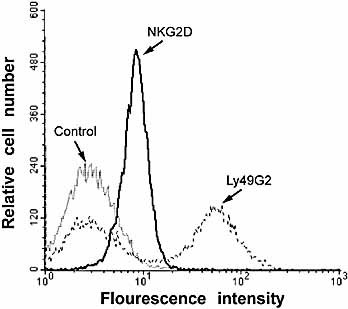
Expression of the NKG2D-activating receptor on the surface of NK cells. DX5-sorted cells from RAG1–/– mice were cultured for 4 days in rIL-2. NK1.1-positive cells from these cultures were stained with a control Ly49G2 mAb, the NKG2D mAb or an isotype-matched control Ig. Cells were analyzed by flow cytometry. Note that the anti-Ly49G2 mAb stains approximately 50% of the NK cells. In contrast, the anti-NKG2D mAb stains all NK cells, albeit at lower intensity.
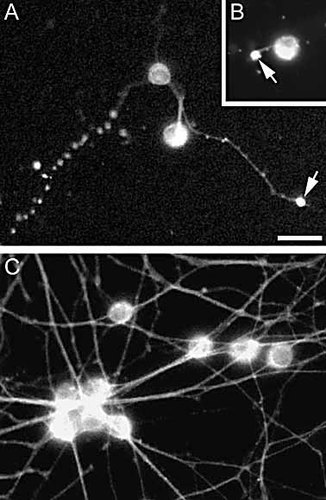
Inhibition of NK cell-mediated degeneration of DRG neurons upon NKG2D blockade. (A, B) DRG neurons exposed to DX5-sorted NK cells from RAG1–/– mice in the presence of isotype control Ig. Note the fragmented immunolabeling of the axons (A) and spheroid formations (A and B; arrows). (C) DRG neurons exposed to DX5-sorted NK cells from RAG1–/– mice in the presence of anti-NKG2D mAb. All cultures were exposed to 1×105 NK cells/ml for 4 h and subsequently immunolabeled with an Ab to the neuronal marker, neuron-specific class III β-tubulin. Bar 50 μm.
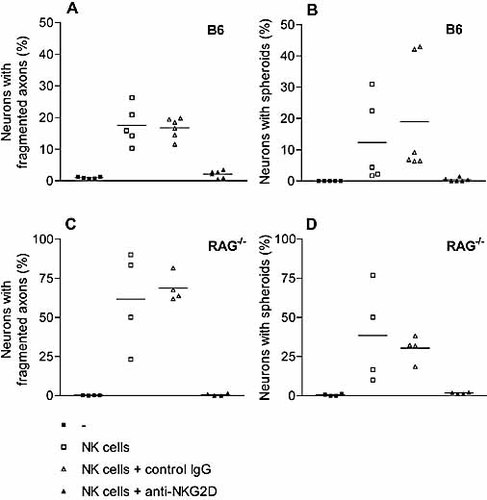
Quantification of NK cell-mediated effects on DRG neurons upon NKG2D blockade. DRG neurons exposed to DX5-sorted NK cells (1×105 cells/ml) derived from B6 (A, B) and RAG1–/– mice (C, D). Evaluation of the NK cell-mediated neurodegeneration was performed by analyzing neurons with fragmented immunolabeling of axons (A, C) and neurons with spheroid formations (B, D). Data represent affected neurons in percent of the total number of neurons. Each value represents results from one cell culture; mean values are indicated by horizontal bars. Differences between DRG neurons exposed to NK cells in the presence of control IgG and NKG2D were statistically significant (p<0.05).
3 Discussion
In the present study, we demonstrate the selective expression of RAE-1 in cultures of embryonic DRG neurons, and that the susceptibility of these neurons to NK cell-mediated degeneration is critically dependent on activation of NK cells via NKG2D.
MHC class I molecules expressed by target cells can influence the outcome of an NK cell/target cell interaction. Of several MHC class I-specific receptors on NK cells that have been cloned, most inhibit NK cell activity. The CNS poses an intriguing problem in this respect, because the adult CNS expresses no or only low levels of MHC class I molecules 31. This would, according to a strict interpretation of the "missing self" hypothesis 13, be devastating in that it would render neurons sensitive to an NK cell-mediated attack. Three strategies may prevent neurons from such an attack by NK cells: 1) absolute seclusion from NK cells, 2) induction of MHC class I molecules during inflammation, or 3) absence of molecules that activate NK cells. In the present study, we show that lack of self MHC class I molecules is not enough to elicit NK cell-mediated killing of neurons, as assessed in hippocampal neuronal cultures from β2m-deficient mice. This observation led us to hypothesize that the expression of a ligand for an activating receptor may determine whether a particular neuron is or is not susceptible to NK cell lysis.
Overlapping subsets of NK cells express different sets of both inhibitory and activating receptors 5–10. Unlike most other NK cell receptors, which are expressed on minor subsets of NK cells, the activating receptor NKG2D is expressed on all NK cells including freshly isolated NK cells as well as IL-2-activated ones 23, 24. NKG2D forms a homodimer that associates with the signaling adapter molecule DAP10 21, which upon binding recruits the p85 subunit of phosphatidylinositol-3 kinase. This pathway has recently been shown to be important in regulating NK cell cytotoxicity by promoting perforin and granzyme B movement towards the target cell 32, 33.
By using reverse transcription (RT)-PCR, RAE-1 transcripts were detected at high levels in DRG cultures. Surface expression of RAE-1 protein was also shown using NKG2D tetramers. The expression of RAE-1 correlated with the susceptibility of DRG neurons to NK cell-mediated lysis. In contrast, only low levels of RAE-1 were detected, either at the mRNA or at the protein level, in the hippocampal cultures, which resisted NK cell lysis. The expression of RAE-1 in the neuronal primary cultures therefore paralleled their susceptibility to an NK cell-mediated attack. This is in agreement with a study in which the G-5 tumor cell line was shown to express high levels of RAE-1 and succumb to NK cell cytotoxicity, whereas the G-1 tumor cell line expressed low levels of RAE-1 and was resistant 34. Furthermore, NKG2D-dependent killing of the DRG neurons in our study was verified by blocking NKG2D with an anti-NKG2D mAb. It should be pointed out, however, that despite blocking NKG2D, our experiments do not provide formal evidence that RAE-1 protein interacts with NKG2D. The theoretical possibility remains that NKG2D might recognize a still unknown ligand whose expression correlates with the presence of RAE-1. However, it should be noted that NKG2D ligands were identified using expression cloning, and the RAE-1 and H60 ligands were the only ones detected. Inaddition, H60 transcripts have not been found in B6 mice 23–25. Thus, this strongly suggests that the killing of DRG neurons is dependent on the NKG2D/RAE-1 interaction.
NKG2D ligands have been detected on numerous tumor cell lines. In normal tissues, RAE-1 transcripts appear only at low levels in adult liver and spleen, and freshly isolated splenocytes, lymphnode cells and thymocytes from B6 mice did not show any surface expression of the NKG2D ligands 23, 24. However, during development, mRNA encoding RAE-1 has beendetected in embryos from developmental day 7 to 14, specifically in the nervous system from day 9 to 11, by RT-PCR, Northern blot analysis and in situ hybridization on whole-mount embryos 35, 36. Substantial NKG2D expression has been detected in fetal NK cells as early as day 15 of gestation 37. In addition, fetal NK cells have been shown to kill cells of the NK-sensitive cell line YAC-1, which have also been shown to express RAE-1 24, 38, 39. However, NK cells develop their fulllytic capacity after birth, with an increasing cytolytic competence 39. Thus, a temporal separation between the expression of RAE-1 and the appearance of NK cells in vivo couldprevent nerve cell killing during embryonic development. In mice, retinoic acid is the only factor presently known to induce RAE-1 expression. Receptors for retinoic acid are expressed in populations of neurons not only during development, but also in the adult brain 40, 41. Therefore, it would be of interest to analyze whether enhanced receptor stimulationof these cell populations induces RAE-1 postnatally, after functionally competent NK cells have appeared. In addition, the human NKG2D ligands, MICA and MICB, have been shown to be up-regulated by heat shock stress in intestinal epithelium 42, 43. Furthermore, cytomegalovirus infection was recently shown to potently induce the MICA and MICB proteins in primary skin fibroblast cultures, while MHC class I molecules were down-regulated 44. Even though expression of NKG2D ligands is low or absent in normal adult tissues, there are several other ligands for activating receptors that are widely distributed in normal non-neuronal tissues. It appears that, for NK cell-activating receptors specific for these constitutively expressed ligands, inhibitory receptors recognizing self MHC class I molecules are essential in that they may prevent NK cell-mediated attack on a cell. An NK cell may only be activated when several of these receptors are simultaneously engaged by a target cell or when target cell MHC class I molecules are down-regulated as discussed e.g. by Cerwenka et al. 5.
A precisely regulated balance between inhibitory and activating signals for NK cells is particularly important in the nervous system with its limited regenerative capacity. Important tasks with respect to the understanding of neurodegeneration are to define factors that can regulate the expression of RAE-1 molecules in neurons and in the postnatal or adult nervous system during microbialinfections, autoimmune diseases or other stressful events. The potential of NK cells to cause neurodegeneration in vivo is illustrated by the effects of guanethidine on superior cervical ganglia in rats. In this classical example of NK cell-mediated nerve cell killing, NK cells infiltrate the ganglia and cause an extensive nerve cell death 45, 46. In the light of our present results, analyzing the expression of NK cell-activating ligands in these ganglia after guanethidine treatment could clarify whether these molecules also play a role in vivo. Additionally, since expression of NKG2D ligands is likely regulated by viral infections or transformation, and MHC class I expression is low or absent in the nervous system, a viral infection could possibly be deleterious with respect to an NK cell-mediated attack.
4 Materials and methods
4.1 Mice
For preparation of nerve cell cultures, embryos from pregnant B6 or β2m–/– 47 mice were used. Effector cells were prepared from male B6 and RAG1–/– 48 mice, 6–10 weeks of age. All mice were bred and maintained at the Microbiology and Tumor Biology Center, Karolinska Institutet, Stockholm, Sweden. The experiments were performed with ethical permission from Stockholm's Norra Djurförsöksetiska Nämnd, and animal care was in accordance with institutional guidelines.
4.2 Establishment of neuronal cell cultures
Hippocampal cultures were established as described previously 49. Briefly, hippocampi from E16 embryos (day of vaginal plug = E0) were dissected and collected in ice-cold calcium- and magnesium-free Hank's balanced salt solution (HBSS; pH 7.3; Life Technologies, Paisley, Scotland). The tissues were incubated in 0.1% trypsin (Life Technologies) for 20 min at 37°C, rinsed and subsequently dissociated. The cells were plated on poly-L-lysine-(Sigma, St. Louis, MO) coated 35-mm tissue culture plates (Corning Costar, Cambridge, MA) at a density of 4 105 cells/dish. Cultures of ventral horns of spinal cords were prepared similarly to those described of dorsal horns 50. In principal, the spinal cords from E13 embryos were collected in ice-cold HBSS, the meninges and dorsal horns removed and the ventral thirds dissected and incubated in 0.1% trypsin for 20 min at 37°C. The cells were then dissociated in culture medium and seeded on poly-L-lysine-coated 35-mm tissue culture plates at a density of 4×105 cells/dish. DRG cultures were established as previously described 29. In short, DRG were dissected from E15 embryos and collected in Neurobasal medium (Life Technologies). The DRG were then dissociated and seeded onto collagen-(Sigma) and Matrigel-coated (Becton DickinsonLabware, Bedford, MA) 35-mm tissue culture plates at a density of 4×105 cells/dish or in 96-well plates (Corning Costar) at a density of 3×104 cells/well. All neurons were grown in Neurobasal medium with the addition of B27 supplement (1×), 2 mM L—glutamine and gentamicin (all from Life Technologies). Nerve growth factor (10 ng/ml; Sigma) was added tothe media of DRG cultures.
4.3 Effector cells and cell lines
Effector cells were obtained from mouse spleens. Erythrocytes were depleted and spleen cells were resuspended in RPMI 1640 medium supplemented with 5% FBS, 1 mM sodium pyruvate, 1× nonessential amino acids, penicillin/streptomycin (all from Life Technologies), 1 mM L-glutamine and 2×10–7 M 2-ME (Merck, Darmstadt, Germany). The cell suspensions were loaded ontoequilibrated nylon wool (Polyscience, Eppelheim, Germany) columns and incubated at 37°C for 1 h. Non-adherent cells were eluted from the column using α-MEM, 10 mM Hepes (both from Life Technologies), 10% FBS, 2×10–7 M 2-ME, 1 mM L-glutamine and penicillin/streptomycin. These cells were seeded in 25-cm2 culture flasks (Corning Costar), 25×106–30×106 cells/flask. Finally, rIL-2 (1,000 U/ml; PeproTech EC, London, GB) was added, and the cells were incubated at 37°C at 10% CO2 for 4 days. The cells were then harvested and used as effector cells. In some experiments, freshly isolated splenocytes were purified using anti-NK cell (DX5) MicroBeads (Miltenyi Biotech, Auburn, CA) before incubation in rIL-2 (1,000 U/ml) for 4 days. The YAC-1 and EL4 cell lines were grown in RPMI 1640 medium supplemented with 5% FBS, 1 mM sodium pyruvate, 1× nonessential amino acids and penicillin/streptomycin.
4.4 Immunocytochemistry
The cultured cells were fixed in 4% formalin and 4% sucrose in PBS for 10 min at room temperature. After pretreatment with 5% BSA and 0.3% Triton X-100 (Sigma) in PBS for 30 min at room temperature, the cultures were incubated overnight at 4°C with the primary Ab mouse anti-neuron-specific class III β–tubulin (1:250; clone TUJ1; Babco, Richmond, CA). Immunoreactivity was detected by using goat anti-mouse conjugated to Alexa Fluor 568 Ab (1:200; Molecular Probes, Leiden, The Netherlands), incubated for 1 h at room temperature and mounted in glycerol containing 2.5% 1,4-diazabicyclo[2,2,2]octane (Sigma). All Ab were diluted in 2% BSA, 0.3% Triton X-100 in PBS. Images of the immunolabeled cell cultures were obtained with a CCD camera (AxioCam, Carl Zeiss Vision, Hallbergmoos, Germany) connected to a fluorescence microscope (Microphot-FX, Nikon Corp. Tokyo, Japan) using AxioVision software (Carl Zeiss Vision) and prepared for illustration with Adobe software (Adobe Systems, San Jose, CA).
4.5 Cytotoxicity assays
Effector cells, at the indicated concentrations, or medium only (for controls) were added to target cells and incubated for 4 h. After immunostaining, evaluation of the NK cell-mediated cytotoxic effects was performed under coded numbers. For Ab blocking experiments, the NK cells were preincubated with the anti-NKG2D mAb (clone 3C7.4.1/10B; 30 μg/ml; 51) or controlhamster IgG (30 μg/ml; Jackson ImmunoResearch Laboratories, West Grove, PA) for 15 min at room temperature before addition of effector cells to the target cells. Cytotoxicity was also determined using an LDH-release assay kit (Roche Diagnostics Scandinavia, Stockholm, Sweden). The effector cells were added to the target cells and incubated for 4 h. The released LDH activity was determinedby incubating the supernatant with the reaction mixture and measuring the absorbance at 490 nm. Lysis was calculated by using the formula: [(LDH experimental – LDH effector cells – LDH spontaneous)/(LDH total – LDH spontaneous)]×100, according to manufacturer's recommendations.
4.6 Extraction of RNA and reverse transcription
Total RNA was extracted from 2–3-day primary cultures of DRG, hippocampal and ventral horn neurons using the RNeasy Kit (Qiagen, Hilden, Germany). The amount and purity of the RNA was assessedby spectrophotometry (Ultrospec Plus, Pharmacia LKB Biotechnology). Total RNA (0.4 μg) was subsequently treated with 1 U of amplification grade DNase I (Life Technologies) for 15 min at room temperature and inactivated by the addition of 2.5 mM EDTA followed by incubation at 65°C for 10 min according to the manufacturer's instructions. The DNase-treated RNA was reverse-transcribed ina 20 μl reaction containing the following reagents from Life Technologies: 500 ng of oligo(dT)12–18 primers, 1× RT buffer, 10 mM DTT and 500 μM of each dNTP. The reaction was heated to 65°C and chilled on ice before the addition of 200 U murine Moloney leukemia virus reverse transcriptase (Superscript II, Life Technologies). cDNA synthesis was allowed to proceed for 1 h at 42°C before inactivation at 70°C for 15 min.
4.7 Semiquantitative PCR
For detection of gene-specific messages encoding RAE-1 and β–actin, 1 μl of cDNA template was amplified in a 25-μl reaction containing 1× Titanium Taq DNA Polymerase and 1 Polymerase buffer (Clontech Laboratories Inc., Palo Alto, CA), 200 μM of each dNTP and 1 μM of the following primers: for RAE-1 cDNA sense 5′—GCTGTTGCCACAGTCACATC-3′; antisense 5′—CCTGGGTCACCTGAAGTCAT-3′; and for β-actin cDNA sense 5′—AAAAACTGGAACGGTGAAGG-3′, anti-sense 5′—CAGAAGCAATGCTGTCACCT-3′ (all from Life Technologies). A GeneAmp PCR system 9700 (Applied Biosystems) with the following cycling conditions was used: for RAE-1 cDNA 94°C for 30 s and 68°C for 1 min, 31 cycles; and for β-actin cDNA 94°C for 30 s, 68°C for1 min, 22 cycles (both within the linear range of amplification). PCR products were electrophoresed in 2% agarose gels in 40 mM Tris, 20 mM acetic acid and 1 mM EDTA (TAE) buffer, visualized by staining in 1× SYBR gold (Molecular Probes, Leiden, The Netherlands) in TAE buffer and documented on a Gel Doc 2000 (Bio-Rad, Hercules, CA). The primers were designed to amplify all the RAE-1 isoforms, α, β, γ, δ and ϵ (GenBank accession numbers: NM1_009016, NM_009017, NM_009018, NM_020030 and AY056835, respectively).
4.8 Flow cytometry
All Ab were used according to the manufacturer's recommendations. PE-conjugated anti-NK1.1 (PK136) and FITC-conjugated anti-Ly49G2 (4D11) were obtained from PharMingen (San Diego, CA). The anti-NKG2D mAb was detected using a FITC-conjugated goat anti-hamster IgG secondary Ab (Southern Biotechnology Associates, Birmingham, AL). Soluble NKG2D was synthesized and biotinylated as previously described 52, multimerized with streptavidin-PE at a molar ration of 4:1 and used at a concentration of 7.5 μg/ml. Incubations were performed on ice for 30 min, and cells were analyzed on a FACScan cytometer (Becton Dickinson, Mountain View, CA).
4.9 Statistical analysis
Statistical analysis was performed using Kruskal-Wallis nonparametric test (Graph Pad Prism 3.02 for Windows; GraphPad Software Inc., San Diego, CA).
Acknowledgements
We would like to thank J. Brask for establishment of hippocampal cultures. We also thank Dr. H. Karlsson for technical support and advise. This work has been supported by grants from the Swedish Research Council (04480), the Swedish Cancer Society, the Swedish Society for Medical Research and the Stanley Medical Research Institute.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




