ICOS+ Th cells produce distinct cytokines in different mucosal immune responses
Abstract
T cell activation, differentiation and effector functions depend on signals delivered through the antigen-specific TCR and non-clonal costimulatory receptors on the T cell. Activated T cells express the inducible costimulator (ICOS). We examined the co-expression of ICOS with Th cytokines in mucosal immune responses. ICOS+CD4+ Th cells expressed strikingly different cytokines depending on the type of infection encountered and the cells' anatomical localization. In the Th2-dominated response to Schistosoma mansoni, ICOS expression of CD4+ cells isolated from the liver was strongly associated with the expression of IL-5, IL-10, IL-13, and T1/ST2, but not with the chemokine receptor CXCR5, a pattern consistent with Th2 effector cells. In the secondary lymphatic organs of schistosome-infected mice, ICOS expression was randomly correlated with Th2 effector-cytokines, but positively correlated with CXCR5 expression; a pattern consistent with follicular Th cells. In Th cells isolated from gut or liver of mice infected with Toxoplasma gondii, ICOS expression was positively correlated with IFN-γ production. Finally, in the severe combined immunodeficiency transfer colitis model, ICOS expression was strongly positively associated with IFN-γ and IL-2. Thus, ICOS appears to costimulate distinct effector functions in different immune responses, depending on factors such as the nature of the antigen encountered and localization and chronicity of the immune response.
Abbreviations:
-
- DIG:
-
Digoxigenin
-
- ICOS:
-
Inducible costimulator
-
- SCID:
-
Severe combined immunodeficiency
1 Introduction
The activation, differentiation, and effector function of T lymphocytes require antigen recognition by the T cell receptor (TCR) together with signaling through costimulatory molecules. A particularly well-studied costimulatory pathway is the interaction of CD28, which is constitutively expressed on T cells, with CD80 (B7–1) and CD86 (B7–2), which are expressed on APC such as B cells, macrophages, and dendritic cells 1, 2. Naive Th cells usually depend on CD28-mediated signaling for IL-2 production and expansion. In contrast, the reactivation of effector or memory T cells is often CD28-independent, and effective T cell responses can be generated against several different pathogens in the absence of CD28 signaling 1, 2. CD152 (CTLA-4), a homologue of CD28, is induced during T cell activation and also binds both CD80 and CD86. In contrast to CD28, CD152 inhibits T cell proliferation, IL-2 production, and cell cycle progression 1, 3. Additional members of the CD28 family that participate in the costimulation of T cells and their B7-like ligands have been identified morerecently 2. One such CD28 family member is the inducible costimulator (ICOS), which is expressed essentially by activated but not by resting T cells 4, 5. The ICOS ligand is constitutively expressed on APC such as B cells, macrophages, and dendritic cells 2, 5–7.
The association of ICOS expression and costimulatory function with Th cytokine production has been a matter of debate. Initial studies demonstrated that ICOS was more effective than CD28 in costimulating IL-10 whereas it did not significantly enhance IL-2 production 4. Several lines of evidence support an association of ICOS with Th2 responses: in vitro studies demonstrated that Th2 cells express higher amounts of ICOS mRNA 8 and protein compared with Th1 cells 8–10, and ICOS was important for Th2 phenotype development in some 10 but not all 11 models. ICOS blockade inhibits Th2-mediated airway inflammation 8, 10, 11, and in one study Th1-mediated EAE was exacerbated in ICOS-deficient mice as compared to wild-type littermates 12. In addition, ICOS-deficient mice have defects in their ability to produce IL-4 and IL-13, but not IFN-γ, and generate reduced amounts of the Th2-dependent IgG1 and IgE antibodies 12–15. Finally, treatment with ICOS Ig drastically reduced IL-5, IL-4, and IL-10 production in mice infected with Nippostrongylus brasiliensis, but had no effect on the IFN-γ production in LCMV-infected mice 16. On the other hand there have been several reports suggesting that ICOS costimulation was also important for Th1 responses: the initial reports already showed that ICOS was not only effective in costimulating IL-10 and IL-4, but also IFN-γ and TNF-α 4. Moreover, it was demonstrated that ICOS is important for both chronic and Th1-dependent acute allograft rejection 17. Finally, ICOS costimulation appears to be important in the induction of IL-10-producing regulatory T cells which are capable of inhibiting Th2 responses and acute airway hyperreactivity in a transfer model 18.
Taken together, the published data suggest that whereas ICOS preferentially stimulates the functions of Th2 cells, costimulation of T cells through ICOS can also costimulate the production of Th1 cytokines and may be important for the induction of regulatory T cells under some circumstances. To date, most of the published data stem either from in vitro experiments 9, 10, in vivo experiments that require immunization, cell transfer, or both 8, 10, 11, 18, or genetically manipulated ICOS deficient mice 12–15. To understand the association of ICOS with Th cytokine production in vivo, we studied the generation of parasite-induced Th2 and Th1 responses in normal mice. Here we show that ICOS+CD4+ Th cells produce a variety of different Th effector cytokines including IL-2, IL-4, IL-5, IL-10, IL-13, and IFN-γ. Importantly, the pattern of cytokines produced by ICOS+CD4+ Th cells depended on the infectious challenge. During an infection with the nematode Schistosoma mansoni, which induces a Th2-dominated response 19, 20, ICOS expression of CD4+ cells was most prominently associated with IL-10 production. In contrast, during an infection with the Th1-inducing protozoon Toxoplasma gondii 21, 22, ICOS+CD4+ Th cells produced preferentially IFN-γ. Finally, in the severe combined immunodeficiency (SCID) transfer colitis model, ICOS expression was positively correlated with IL-2 and IFN-γ production. Thus, ICOS appears to costimulate distinct effector functions in different immune responses, depending on factors such as the nature of the antigen encountered and localization and chronicity of the immune response.
2 Results
2.1 Expression of ICOS is associated with both Th2 or Th1 immune responses to gastrointestinal parasites
To determine the association of ICOS expression with Th cytokine production ex vivo, we investigated ICOS expression in CD4+ T cells obtained from the secondary lymphoid organs and effector sites of mice that were infected with either S. mansoni or T. gondii and of age-matched controls. Eight weeks after the infection with S. mansoni, ICOS expression was strongly up-regulated in infected CBA mice. Among the secondary lymphoid organs, ICOS expression was highest in the Peyer's patches. Of the CD4+ cells isolated from the Peyer's patches of schistosome-infected mice, 26.1% ± 4.1% (mean ± SD in three independent experiments) were ICOS+ (Fig. 1). This rate decreased to 11.6% ± 4.6% in the mesenteric LN and 11.3% ± 3.2% in the spleen. ICOS expression in peripheral LN, distant from the schistosome infection, was similar in infected and non-infected mice (approximately 2%). ICOS expression was also strongly up-regulated in T cells isolated from the liver and the gut of schistosome-infected mice. Twenty percent of the CD4+ cells isolated from liver granulomas of schistosome-infected mice and approximately 15% of the CD4+ intraepithelial cells from the small and large intestines were ICOS+ (Fig. 1).
Similar findings were obtained in a strongly Th1-polarized model. CD4+ T cells were obtained from the secondary lymphoid organs, liver, and gut from C57BL/6 mice 9 days after infection with T. gondii and compared with non-infected controls. As shown in Fig. 1B, ICOS was strongly expressed on CD4+ cells from the infected organs and the local secondary lymphatic organs. In both infection models, ICOS expression was up-regulated only in CD4+ cells. Less than 0.5% of the CD8+ T cells were ICOS+, and the percentage of ICOS+CD4+CD8+ intraepithelial T cells was significantly smaller than the percentage of ICOS+CD4+CD8– intraepithelial T cells (data not shown).
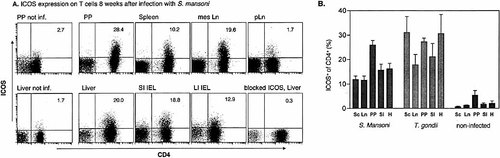
ICOS expression on CD4+ cells from mice infected with S. mansoni or T. gondii. (A) CBA mice were infected with S. mansoni 8 weeks before the analysis. Cells from Peyer's patches (PP), liver, spleen, mesenteric LN (mesLN), popliteal LN (pLN), small intestine intraepithelial lymphocytes (SI IEL), and large intestine intraepithelial lymphocytes (LI IEL) were stained with mAb against CD3, CD4 and ICOS. To control the specificity, liver cells from infected mice were incubated with unconjugated ICOS mAb before the staining was performed. Gates were set on viable CD3+ cells. Data are representative of three experiments. Upper right corner shows percentage of ICOS+ cells of the CD4+ T cells. (B) CBA mice were infected with S. mansoni 8 weeks before the analysis (left panel); C57BL/6 mice were infected with T. gondii 9 days before the analysis (middle) or not infected (right panel). Cells from spleen, mesenteric LN (LN), Peyer's patches (PP), liver (H), and small intestine intraepithelial lymphocytes (SI IEL) were isolated and FACS staining was performed as described in (A). Data are mean ± SD from three to seven independent experiments.
2.2 Kinetics of ICOS expression during anti-parasitic immune responses
We examined the ICOS expression on CD4+ cells of C57/BL6 mice infected with T. gondii and BALB/c mice infected with N. brasiliensis over 3 weeks following infection (Fig. 2). In non-infected C57/BL6 mice, ICOS was expressed on the surface of approximately 1.5% of the CD4+ T cells from mesenteric LN. Six days after oral infection with T. gondii, 8.5% of the CD4+ cells were ICOS+ (Fig. 2A). At the height of the Th1-dominated immune response against T. gondii, at day 9 after infection, more than 20% of the CD4+ cells were ICOS+. Following the clearance of the parasites from the gut, at day 20 after infection only 9.5% of the CD4+ cells were still ICOS+. Similar kinetics of ICOS expression were observed on CD4+ cells isolated from the liver of T. gondii-infected mice (Fig. 2A).
Following s.c. infection of mice with N. brasiliensis larvae, the helminths migrate to the lung where they induce an inflammatory Th2-dominated immune response 23. This pulmonary immune response is most intense 14 days after s.c. infection of BALB/c mice (data not shown). In non-infected BALB/c mice, ICOS was expressed on the surface of approximately 2% of the CD4+ T cells isolated from the lungs (Fig. 2). Eight days after infection, 12% of the CD4+ cells were ICOS+, and on day 14, 16% of the CD4+ cells were ICOS+. Twenty-four days after infection, less than 2% of the CD4+ cells were still ICOS+ (Fig. 2B). Throughout the course of the infection the percentage of ICOS+CD4+ cells was much lower in the draining mediastinal LN than in the lung (Fig. 2B).
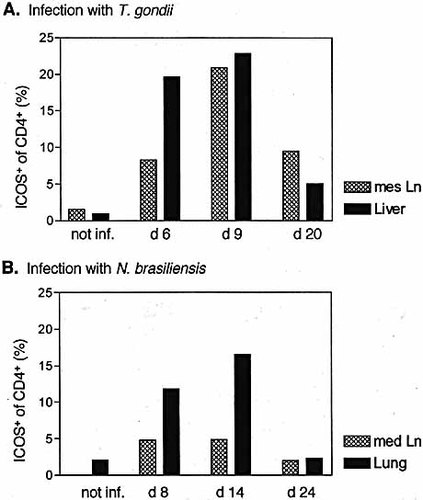
Kinetics of ICOS expression on CD4+ cells from effector sites and draining LN in parasitic infections. Expression of ICOS on CD4+ cells from mesenteric LN and liver of C57BL/6 mice 6, 9 and 20 days after infection with T. gondii (A) or from mediastinal LN and lung of BALB/c mice 8, 14 and 24 days after infection with N. brasiliensis (B). The left bars depict data from non-infected mice. In the mediastinal LN of uninfected BALB/c mice were too few cells for analysis. Data are representative of three or two (day 20) experiments.
2.3 Ex vivo association of ICOS expression with Th2 cytokine expression in schistosome-infected mice
To examine the association of ICOS expression with Th2 cytokines, we examined the co-expression of ICOS with IL-4, IL-5, IL-13 and IL-10 in CD4+ cells from effector sites and secondary lymphatic organs of mice infected with S. mansoni. Mononuclear cells were isolated from the liver and gut of CBA mice 8 weeks after the infection with S. mansoni, at the height of the Th2-dominated immune response against S. mansoni 20. These mononuclear cells where then stimulated with plate-bound anti-CD3/anti-CD28 mAb and stained for surface CD4 and intracellular ICOS and cytokines. The observed frequencies of ICOS/cytokine co-expressing cells were compared with those calculated for expected values (i.e. random coincidence) to determine whether co-expression is coordinated or independent. Phi correlation coefficients were calculated and are shown for each pair.
Approximately 40% of the CD4+ cells from liver granulomas were ICOS+, and the co-expression of ICOS with IL-5 (ϕ correlation coefficient = 0.1), IL-13 (ϕ=0.21) and IL-10 (ϕ=0.29) occurred more frequently than expected for stochastic co-expression (Fig. 3). In the secondary lymphatic organs mesenteric LN and spleen, both the percentage of CD4+ICOS+ cells and the percentage of CD4+ cells producing IL-4, IL-5, IL-13, or IL-10 were reduced as compared with the liver. Moreover, in CD4+ cells from the secondary lymphatic organs, the association between ICOS expression and cytokine production was random for IL-4, IL-5, and IL-13. However, expression of ICOS and IL-10 were positively associated in the secondary lymphatic organs (ϕ=0.12 and 0.14 for mesenteric LN and spleen, respectively). Thus, ICOS expression was positively associated with the production of IL-5, IL-13, and IL-10 in the liver; and with IL-10, but not IL-4, IL-5, or IL-13 in the secondary lymphatic organs in schistosome-infected mice.
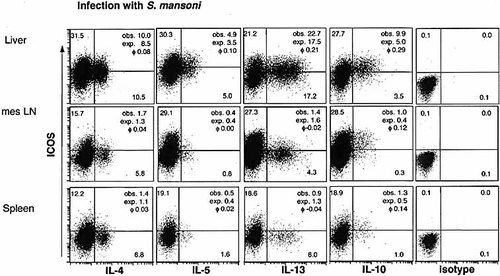
ICOS and Th2 cytokine production of CD4+ cells from schistosome-infected mice. Cells were isolated from spleen, mesenteric LN and liver of CBA mice 8 weeks after infection with S. mansoni. Cells were stimulated with anti-CD3 and anti-CD28 mAb and stained for surface CD4 and intracellular ICOS and IL-4, IL-5 IL-13 and IL-10 (as shown in Fig. 4 for IL-10 in the liver). The frequency of ICOS- and cytokine-producing CD4+ cells is indicated in the quadrants in percent. The observed (obs.) frequencies of ICOS/cytokine co-expression, the expected frequency (exp.) calculated for random coincidence of two independent variables, and the ϕ correlation coefficient (phi) of the respective ICOS-cytokine pair are indicated. Data are representative of three experiments.
2.4 Association of ICOS expression with IL-2, IL-10, and IFN-γ in gastrointestinal immune responses ex vivo
We used the infection models and the SCID transfer colitis model to study the association of ICOS expression with the expression of IL-10, IFN-γ, and IL-2 ex vivo. Mononuclear cells were isolated, stimulated with plate-bound anti-CD3/anti-CD28 mAb, and stained for surface CD4 and intracellular ICOS and cytokines. In CD4+ cells isolated from the liver of CBA mice 8 weeks after infection with S. mansoni, ICOS expression was strongly associated with IL-10 expression (ϕ=0.29; Fig. 4). In contrast, the association between ICOS expression and IFN-γ or IL-2 expression was random (ϕ=0.05 and –0.05, respectively). The ICOS+IL-2+ cells stained only weakly for ICOS and the cells that stained most intensively for IL-2 were ICOS–, further suggesting a negative association between ICOS and IL-2 in this model (Fig. 4).
CD4+ cells were isolated from the small intestine of C57BL/6 mice at the height of the Th1 immune response 9 days after infection with T. gondii. IFN-γ was the cytokine most frequently produced by these CD4+ cells, and the vast majority of the IFN-γ producers co-expressed ICOS (ϕ=0.20; Fig. 4). Few CD4+ cells produced IL-2, which was randomly associated with ICOS expression (ϕ=0.03). Only a small minority produced IL-10, and the association between ICOS expression and IL-10 expression was random (ϕ=0.09; Fig. 4). Similar associations were also observed in CD4+ cells isolated from the mesenteric LN, spleen, or liver of T. gondii-infected mice (data not shown).
A different picture emerged when we analyzed CD4+ cells that were isolated from the large intestine of SCID mice suffering from transfer colitis approximately 4 months after the transfer of in vitro activated syngeneic CD4+ cells. Here, ICOS expression was randomly associated with IL-10 production (ϕ=–0.03). In contrast, ICOS expression was positively associated with IFN-γ (ϕ=0.14) and even more with IL-2 (ϕ=0.24) expression in CD4+ cells from the large intestine of SCID mice suffering from transfer colitis (Fig. 4).
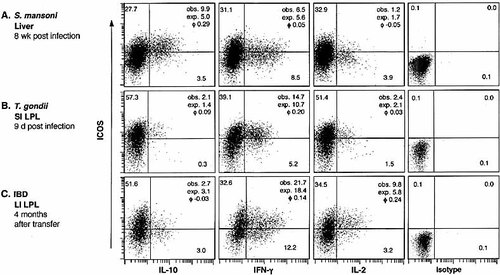
ICOS and cytokine production of CD4+ cells ex vivo. Liver cells were isolated 8 weeks after infection of CBA mice with S. mansoni (A), small intestine intraepithelial lymphocytes (SI IEL) were isolated 9 days after infection of C57BL/6 mice with T. gondii (B) and large intestine lamina propria lymphocyte (LI LPL) were isolated 3 months after transfer of in vitro activated CD4+ T cells into C.B-17 SCID mice (C). Cells were stimulated with plate-bound anti-CD3 and anti-CD28 mAb and stained for CD4. After fixation and permeabilization, cells were stained for intracellular ICOS and IL-10, IFN-γ, or IL-2. The frequency of ICOS- and cytokine-producing CD4+ cells is indicated in the quadrants in percent. The observed (obs.) frequencies of ICOS/cytokine co-expression, the expected frequency (exp.) calculated for random coincidence of two independent variables, and the ϕ correlation coefficient (phi) of the respective ICOS-cytokine pair are indicated. Data are representative of at least three experiments. The data shown for schistosome-infected mice are from the same experiment as shown in Fig. 3. Therefore, the dot plot showing ICOS/IL-10 co-expression in the liver of schistosome-infected mice (A, left panel) is the same as depicted in Fig. 3.
2.5 Association of ICOS expression with T1/ST2 and chemokine receptors ex vivo
To further evaluate the expression of ICOS in Th2 effector cells, we used mice infected with S. mansoni to examine the co-expression of ICOS with T1/ST2, a member of the IL-1R family that is expressed on Th2 but not on Th1 cells 24–27. The expression of ICOS was positively associated with T1/ST2 expression in CD4+ cells obtained from the liver, but much less in CD4+ cells obtained from the secondary lymphatic organs (Fig. 5B). Similar data were obtained from mice infected with N. brasiliensis where the positive correlation between ICOS and T1/ST2 expression was much stronger in the lung than in the secondary lymphatic organs (data not shown). Thus, the co-expression pattern of ICOS with T1/ST2 further affirms the finding that ICOS expression in these parasitic infections is associated with Th2 cytokine production (see Fig. 3) in effector sites more strongly than in secondary lymphatic organs.
The finding that ICOS expression was positively associated with Th2 cytokine production and T1/ST2 expression in effector sites but less in secondary lymphatic organs prompted us to investigate the co-expression of ICOS and the chemokine receptor CXCR5. CXCR5 expression enables Th cells to migrate into germinal centers, and CXCR5+ Th cells have been reported to be very effective in supporting antibody production 28–30 whereas they produce only small amounts of Th2 effector cytokines 31. Approximately 3% of the CD4+ cells isolated from the mesenteric LN, Peyer's patches or liver of non-infected CBA mice expressed CXCR5, and most of the CXCR5+ cells were ICOS– (Fig. 5A). Eight weeks after infection with S. mansoni, CXCR5 expression was strongly up-regulated on CD4+ cells from the secondary lymphatic organs but not from liver granulomas. Furthermore, CD4+ cells from the secondary lymphatic organs co-expressed ICOS and CXCR5 more frequently than expected for stochastic co-expression (Fig. 5B). Thus, in CD4+ cells from the secondary lymphatic organs, but not in CD4+ cells from the effector site (liver), ICOS expression is strongly positively associated with CXCR5 expression. In contrast, ICOS expression is strongly positively associated with T1/ST2 expression in CD4+ cells from the liver of schistosome-infected mice, but randomly or only weakly positively associated with T1/ST2 expression in CD4+ cells from the secondary lymphatic organs.
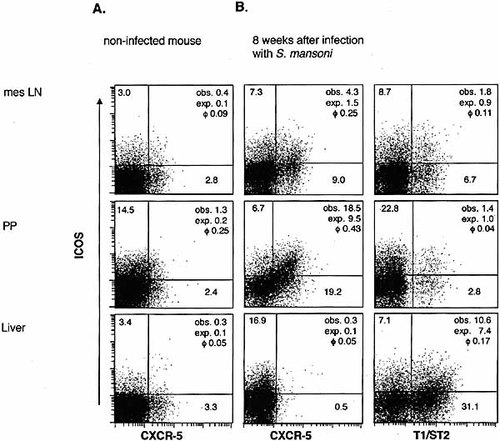
Co-expression of ICOS and T1/ST2 or CXCR5 on CD4+ cells isolated from the liver or from secondary lymphatic organs ex vivo. Cells were isolated from mesenteric LN, Peyer's patches and liver of non-infected CBA mice and of CBA mice 8 weeks after infection with S. mansoni. Data are representative of two experiments.
3 Discussion
The CD28 family member ICOS is an inducible costimulatory receptor expressed on activated, but not resting, T cells 2, 4. Here, we report on the association of ICOS expression with Th effector function at the single-cell level in mucosal immune responses directly ex vivo. To study the association of ICOS expression with the production of particular Th cytokines in mucosal and systemic immune responses to different parasitic infections in mice, we investigated both short-lived (T. gondii, N. brasiliensis) and chronic infections (S. mansoni) as well as both Th1-(T. gondii) and Th2-(S. mansoni, N. brasiliensis) dominated responses. In addition we studied the co-expression of ICOS with Th cytokines in a well-established model of pathogenic mucosal Th1 immune responses, the SCID transfer colitis 32.
The initial studies on ICOS had already shown that in comparison with CD28, ICOS was particularly effective in costimulating IL-10 production, but ineffective in costimulating IL-2 production 4. Other groups have confirmed this finding 9, 33, 34. In addition, several lines of evidence have pointed towards a preferential, albeit not exclusive, expression of ICOS by Th2 cells 2. Our ex vivo analyses of Th cells from mucosal immune responses revealed some striking differences compared with these previous findings. Depending on the type, the localization and the chronicity of the immune response, ICOS expression was associated with different cytokines.
Our analysis of Th cells from liver granulomas of schistosome-infected mice affirmed the initial observations; there was a strong positive correlation between ICOS expression and the expression of IL-5, IL-13, and IL-10 (Fig. 3, 4). In striking contrast, in the effector Th cells isolated from the large intestine's lamina propria of mice suffering from SCID transfer colitis, ICOS expression was randomly associated with IL-10, but strongly positively correlated with IFN-γ and IL-2 expression (Fig. 4). The lack of an association between ICOS expression and IL-10 in the SCID transfer colitis model (Fig. 4) is particularly striking given the chronicity of this immune response. The CD4+ effector cells were examined several months after the transfer of in vitro activated syngeneic Th1 cells into SCID mice, and there were both plenty ICOS+CD4+ cells (∼55%) and IL-10 producers (∼6% of the CD4+ cells). Such lack of correlation between ICOS and IL-10 expression is, however, not unprecedented: ICOS-deficient mice are fully capable of secreting IL-10 in response to stimulation with anti-CD3 antibody 12. Clearly then, ICOS is not necessary for the differentiation of IL-10-producing Th cells.
Finally, in the Th1-dominated mucosal immune response against T. gondii, ICOS expression was strongly associated with the expression of IFN-γ. These latter findings are in agreement with and extend recent data, which indicate a functional role for ICOS in mediating resistance to T. gondii in CD28-deficient mice 35. Thus, ICOS expression was associated with the expression of distinct cytokine patterns at the effector sites (liver, small or large intestine) in the different immune responses. Together with previous findings that show expressionof the ligand for ICOS not only in lymphatic organs but also in several non-lymphoid tissues 6, 36, these findings would argue that ICOS costimulates the cytokine production of previously activated Th cells at the effector sites of immune responses.
The association of ICOS with Th cytokine expression varied not only between the different mucosal immune responses. Within the context of one particular immune response there were also striking differences depending on the anatomical site from which the Th cells were isolated. In Th cells isolated from liver granulomas of schistosome-infected mice 8 weeks after infection, ICOS expressionwas strongly associated with the expression of IL-10 and the Th2 cytokines IL-5 and IL-13. In contrast, expression of IL-5 and IL-13 was only randomly associated with ICOS expression in Th cells isolated from the secondary lymphatic organs (mesenteric LN and spleens) of the same mice at the same time point during the infection (Fig. 3, 4). These findings were complemented and confirmed by the finding that expression of ICOS and the Th2-specific molecule T1/ST2 20, 24–27 are strongly positively associated in CD4+ cells from the hepatic granulomas, but randomly or only weakly positively associated in CD4+ cells from the secondary lymphatic organs (mesenteric LN and spleens) of the same mice at the same time point during the infection (Fig. 5).
The opposite pattern was observed for the co-expression of ICOS and CXCR5 in schistosome-infected mice. In Th cells isolated from the secondary lymphatic organs, expression of ICOS was strongly associated with expression of the chemokine receptor CXCR5. In contrast, Th cells isolated from liver granulomas of schistosome-infected mice do not express CXCR5. CXCR5, the receptor for CXCL13, mediates follicular homing of a subset of Th cells, named TFH cells. TFH cells are especially equipped to support antibody production 28–30, although they produce very little effector cytokines 31. Since ICOS is important for antibody production 8, 12, 14, 37, our finding that ICOS expression in the secondary lymphatic organs is strongly associated with CXCR5 expression but not with cytokine production is compatible with the hypothesis that ICOS costimulates the B cell help mediated by CXCR5+ TFH cells.
Taken together, our data show that ICOS is co-expressed with strikingly different cytokines and chemokine receptors depending on the type and localization of the immune response. This is consistent with the idea that ICOS costimulates different effector functions, including Th1, Th2, or TFH, depending on the inciting antigen, the anatomical localization and the chronicity of theimmune response. There appears to be an ordered sequence of interactions between B7 family members and their ligands during immune responses. The interaction between CD28, which is constitutively expressed on T cells, and CD80 or CD86, both of which are expressed on professional APC, is crucial for the induction of most immune responses. Upon activation and differentiation, the Th cells up-regulate ICOS. The interaction between ICOS and its ligand costimulates very different effector T cell responses both in the secondary lymphatic organs and in target tissue infiltrated by T cells. Finally, the interaction between CD152 and CD80/86 contributes to the down-regulation of the ongoing immune response. Of course, there is considerable cross-talk and overlap between these different phases (see e.g. the possible association of ICOS with both the "early" cytokine IL-2 and the "late" IL-10). Since ICOS costimulates very different Th effector functions including B cell help it seems to be a natural therapeutic target for the modulation of pathogenic immune responses regardless of the inciting antigen or the cytokine pattern produced by the pathogenic T cells.
4 Materials and methods
4.1 Mice
Female C57BL/6, BALB/c, CBA, and C.B-17 scid/scid mice were bred and maintained under specific pathogen-free conditions in the animal facilities of the Bundesinstitut für Risikobewertung (Berlin, Germany). CBA mice were purchased from Jackson (Bar Harbor, ME). Mice were used at 8–10 weeks of age. All animal experiments involved groups of 2–5 mice and were performed according to institutional and state guidelines.
4.2 Infection with parasites
Female CBA mice were infected intraperitoneally with 70 S. mansoni cercariae (Puerto Rico strain) obtained from infected Biomphalaria glabrata snails, provided by the Biomedical Research Institute (Rockville, MD) as described 38. Cysts of the ME49 strain of T. gondii were obtained from brains of NMRI mice that had been infected intraperitoneally with ten cysts for 2–3 month as previously described 21. For peroral infection, C57BL/6 mice were infected with ten cysts by gavage. C57BL/6 and BALB/c mice were infected s.c.with 750 N. brasiliensis L3 larvae.
4.3 Induction of inflammatory bowel disease
Inflammatory bowel disease was induced as described 39. In brief, single-cell suspensions of BALB/c spleen and LN cells were depleted of CD8+ T cells on LD columns using the MidiMACS system (Miltenyi Biotec) and cultured in the presence of soluble anti-CD3 mAb (3 μg/ml), anti-CD28 mAb (2.5 μg/ml), anti-IL-4 mAb (5 μg/ml) and rmIL-12 (5 ng/ml). After 3 days, cell medium supplemented with 100 U/ml rhIL-2 (provided by Dr. C. Reynolds, NCI-FCRDC, Frederick, MD) was added. At day 6, dead cells were removed using a Nycodenz gradient (Nycomed PharmaAS), and 5–105 cells were transferred intraperitoneally into C.B-17 scid/scid mice.
4.4 Isolation of cells from spleen, lymph nodes, Peyer's patches, gut, liver, and lungs
Mice were sacrificed by cervical dislocation, and single-cell suspensions were prepared from spleen, LN, and Peyer's patches in RPMI 1640 (Gibco) supplemented with antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin), 2 mM glutamine, 25 μM 2-ME (Gibco), 10 mM Hepes (PAA) and 10% FCS (Sigma) (complete medium). Intraepithelial lymphocytes and lamina propria lymphocytes were isolated as described 40. Mononuclear cells from livers or lungs were prepared as described 20. Cells from three to five mice were pooled for analysis.
4.5 Antibodies and flow cytometry
The ICOS-specific mAb MIC-280 41 was conjugated to digoxigenin (DIG) or biotin. This mAb was used together with phycoerythrin (PE)- and FITC-conjugated mAb against CD4 (GK1.5), CD62L (MEL-14), CD25 (2E4) and CD154 (MR1, PharMingen), or biotinylated CD45RB (23G2, PharMingen), T1/ST2 (3E10, 25), CD69 (H1.2F3, PharMingen) and CD134 (OX-86, PharMingen). To prevent unspecific binding, the samples were incubated with blocking anti-FcγRII/III mAb 2.4G2/75 (100 μg/ml) and purified rat IgG (200 μg/ml, Jackson) 10 min before and during staining. Digoxigenized MIC-280-labeled cells were detected by anti-DIG Fab fragments (Boehringer Mannheim) conjugated to Cy5; biotinylated primary mAb were detected with streptavidin coupled to allophycocyanin or PE (PharMingen). To control the specificity, staining of MIC-280 and CD154 was blocked by preincubating cells with a 100-fold excess of unconjugated mAb.
For CXCR5 staining, cells were incubated in 10% mouse serum and 10% goat serum (Sigma) to prevent unspecific binding. Anti-CXCR5 mAb (2G8, 42) was added for 15 min. After washing, cells were incubated with 10 μg/ml mouse adsorbed anti-rat IgG-FITC (Jackson). Cells were washed and incubated with 200 μg/ml rat IgG before staining was performed as described above.Samples were analyzed on a FACScalibur using CellQuest software (Becton Dickinson). Gates were set on viable cells according to forward and side scatter and exclusion of propidium iodide-binding particles.
4.6 Analysis of cytokine production by flow cytometry
For intracellular detection of cytokines, cells (2.5×106/ml) were stimulated with plate-bound anti-CD3 mAb (145–2c11, 3 μg/ml) and anti-CD28 mAb (37.51, 2.5 μg/ml) for 4 h. Brefeldin A (Sigma) was added at 5 μg/ml for the last 2 h of stimulation. In these experiments, ICOS was stained intracellularly, and control stainings were performed to make sure that the stainings were comparable to ICOS levels on the cell surface. Short-term stimulation with anti-CD3 and anti-CD28 did not enhance the ICOS expression significantly. Cells were incubated with anti-FcγR mAb and rat IgG and stained with biotinylated anti-CD4 mAb before fixation with 2% formaldehyde for 20 min. CD4 was detected on the fixed cells using streptavidin coupled to peridinin chlorophyll protein (PerCP, 1 μg/ml, PharMingen). Cells were washed and then permeabilized with saponin (0.5%, Sigma) for intracellular staining of ICOS with digoxigenized 12A7 mAb 43 together with the following FITC- and PE-labeled mAb: anti-IFN-γ-FITC (AN 18.17.24, 1.5 μg/ ml), anti-IL-4-FITC (BVD4–11D11, 3 μg/ ml), anti-IL10-PE (JES5–16E3, 3 μg/ml), anti IL-13-PE (38213.11, R&D) anti-IL-2-PE (JES6–5H4, 3 μg/ml) and anti-TNF-α-PE (MP6-XT22, 0.4 μg/ml). ICOS staining was detected with anti-DIG conjugated to Cy5. To control the specificity, staining of 12A7 was blocked by preincubating cells with a 100-fold excess of unconjugated mAb. FITC- or PE-labeled isotype-control mAb (PharMingen) were used at the same concentrations as the respective anti-cytokine mAb. Samples were analyzed by four-color cytometry on a FACScalibur.
4.7 Statistical analysis of co-expression
The observed value for cells co-expressing ICOS with other surface molecules or with cytokines was compared with the expected value calculated for random coincidence of two independent variables. Correlations were calculated using the test for ϕ correlation coefficients as described 20. Coefficients of ϕ <–0.1 or ϕ ≥0.1 were considered significantin this analysis 44.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft Ka 755/4 (to T.K.), SFB 506 and Kr 827/13–2 (to R.A.K.) and by the Charité Forschungskommission (to T.K.)
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




