Efficacy of trametinib in a metastatic urothelial carcinoma patient with a BRAF mutation
Abstract
Introduction
BRAF mutations in bladder cancer are rare. MEK inhibitors have excellent clinical benefits in the treatment of melanoma.
Case presentation
A 60-year-old male was diagnosed with muscle-invasive bladder cancer and underwent total cystectomy and ileal conduit diversion. Despite 4 cycles of gemcitabine and cisplatin chemotherapy and 3 courses of pembrolizumab, the left obturator lymph node enlarged. Cancer multi-gene panel testing confirmed the BRAF G469A mutation and trametinib was recommended. Three months after the initiation of trametinib (2 mg, qd), the left obturator lymph node shrank by more than 50%. The disease has remained stable for more than 18 months.
Conclusion
The present case indicates the potential of trametinib to treat mBUC patients with the BRAF G469A mutation in this setting.
Abbreviations & Acronyms
-
- CT
-
- computed tomography
-
- EV
-
- enfortumab vedotin
-
- GC
-
- gemcitabine and cisplatin
-
- mBUC
-
- metastatic bladder urothelial carcinoma
-
- NSCLC
-
- non-small cell lung cancer
-
- ORR
-
- objective response rate
-
- PD
-
- progressive disease
-
- PR
-
- partial response
-
- SD
-
- stable disease
-
- TURBT
-
- transurethral resection of bladder tumor
-
- UC
-
- urothelial carcinoma
Keynote message
This is the first case report that indicates the potential of trametinib to treat mBUC patients with the BRAF G469A mutation whose disease progresses after platinum-based chemotherapy and PD-1/PD-L1 blockade.
Background
Platinum-based chemotherapy has consistently been used as first-line therapy for metastatic UC since the 1980s. Immune checkpoint inhibitors for UC emerged as second-line therapy in the mid-2010s.1-3 However, since UC is aggressive and has a poor prognosis, the ORR has only been approximately 20%. Phase 2 and 3 trials on EV reported significant increase in ORR.4, 5 Therefore, EV has recently become available for treatment; however, its effectiveness and safety in real-world clinical practice have not yet been adequately tested.
Next-generation sequencing is used to identify mutations in cancer-related genes and target abnormal pathways. We herein encountered a case of UC with a BRAF mutation for which an MEK inhibitor was effective.
Case presentation
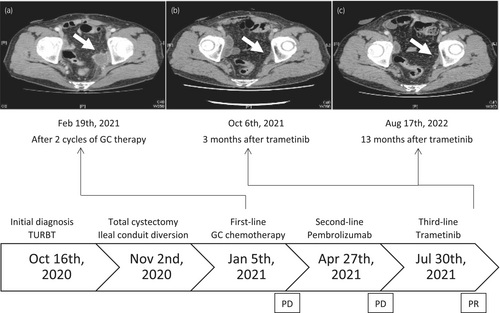
A 61-year-old male patient was diagnosed with a bladder tumor. TURBT was performed and the pathological result indicated UC with muscle invasion. One month after TURBT, the patient underwent total cystectomy and ileal conduit diversion. A pathological examination revealed UC with differentiation into the glandular epithelium, pT3b, without lymph node metastasis. After total cystectomy, the patient received adjuvant chemotherapy with GC. CT showed left obturator lymph node metastasis after 2 cycles of GC (Fig. 1a). Although the patient received 4 cycles of GC chemotherapy, no significant changes were noted in the metastatic lesion. Therefore, 3 courses of pembrolizumab were initiated as second-line therapy; however, CT suggested disease progression.
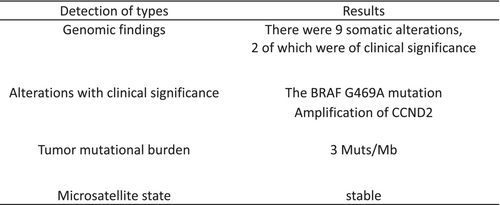
To provide further treatment for this patient, we conducted cancer multigene panel testing (FoundationOne®). We examined 324 cancer-related genes and identified the BRAF G469A mutation (Fig. 2). BRAF-activating mutations predict sensitivity to MEK inhibitors, such as trametinib. Significant clinical responses to trametinib have been achieved in patients with melanoma harboring the BRAF V600E mutation,6 and trials on MEK inhibitors reported objective responses in patients with solid tumors.7-9
Since accumulating evidence suggested the potential for a significant response, trametinib was recommended. The patient was fully informed and understood the use of Patient-Proposed Healthcare Services, which was started in Japan in 2016 to provide rapid access to new drugs to all patients. After providing his consent, the patient was administered trametinib (2 mg, qd). Three months after the initiation of treatment, CT showed shrinkage of the left obturator lymph node by more than 50% (Fig. 1b). The disease has remained stable for more than 18 months and the patient is still being followed up.
The adverse events of trametinib in the present case included grade 1–2 skin rash, diarrhea, and hyperuricemia. Skin rash appeared 2 weeks after the initiation of trametinib and was treated with a macrolide and steroid ointment. The timeline of treatment is shown in Fig. 1.
Discussion
After total cystectomy, cisplatin-based adjuvant chemotherapy is considered for patients who have not received neoadjuvant chemotherapy, including cisplatin. Platinum-based chemotherapy may prolong overall survival in patients with mBUC; however, cancer progression is inevitable. PD-1/PD-L1 inhibitors were recently shown to be clinically beneficial for patients with mBUC. Avelumab is used in cases with no evidence of disease progression after primary chemotherapy, while pembrolizumab is administered if disease progression or relapse occurs. In addition, EV, a nectin-4-targeting antibody-drug conjugate, has been approved for cases with progression after the administration of these immune checkpoint inhibitors following primary chemotherapy. However, UC is aggressive, has a poor prognosis, and therapeutic strategies remain unsatisfactory.
Histological variants are well recognized in UC. In this case, the pathological examination showed UC with differentiation into the glandular epithelium. Glandular differentiation is the second most common variant and occurs in up to 18% of invasive UC.10 Although response to systemic therapies in patients with variant histological subtypes remains controversial, survival outcomes are similar to pure UC. Therefore, patients should be managed as would be appropriate for UC of the same stage.11 Despite this indication, this case developed the left obturator lymph node metastasis while adjuvant GC chemotherapy and disease progression was observed after the administration of pembrolizumab. EV was not available in Japan at that time, and cancer multigene panel testing revealed the BRAF G469A mutation.
BRAF mutations in bladder cancer are rare. BRAF induces intracellular signaling through the RAS–RAF–MEK–ERK pathway, which regulates cell differentiation and growth. BRAF mutations have been detected in various carcinomas, including malignant melanoma, colorectal cancer, and non-small cell lung cancer.12 An amino acid substitution at codon 600 of the BRAF protein activates BRAF kinase and induces transformation to malignant cells via the constant phosphorylation of downstream signals.
BRAF mutations are classified into three classes. Class 1 is the BRAF V600E mutation, the kinase activity of which is the highest of the three subtypes at approximately 500-fold that of wild-type BRAF. Non-V600E mutations are divided into class 2, the kinase activity of which is 50-fold higher than that of wild-type BRAF, and class 3, the kinase activity of which is reduced.13 The BRAF G469A mutation in the present case was classified as class 2.
BRAF mutations have been detected in 4% of bladder UC cases,14 and only one case of bladder UC registered in the Cancer Genome Atlas had the BRAF G469A mutation. MEK inhibitors, such as trametinib, exert their antitumor effects by binding to the allosteric site of MEK, the downstream gene of BRAF, thereby inhibiting its activity. They are generally used in combination with BRAF inhibitors and have excellent clinical benefits in the treatment of melanoma. There are a limited number of case reports of solid tumors with the BRAF G469A mutation (Table 1). Three out of 4 cases treated with trametinib maintained SD or better. A previous study characterized BRAF non-V600E mutations through cell viability and pharmacological assays in NSCLC cell lines.15 Cell viability assays indicated that trametinib alone or in combination with the BRAF inhibitor, dabrafenib, effectively inhibited the growth of cell lines with the BRAF G469A mutation.
| Author | Year | Cancer type | Age | Sex | Treatment | Tumor response |
|---|---|---|---|---|---|---|
| Dagogo-Jack16 | 2020 | NSCLC | 62 | Female | Dabrafenib | PR |
| Trametinib | ||||||
| Mu17 | 2020 | NSCLC | Unknown | Unknown | Chemotherapy | SD |
| Kronish18 | 2021 | Glioma | 15 | Male | Dabrafenib | PD |
| Trametinib | ||||||
| Julius19 | 2022 | Melanoma | 53 | Male | Dabrafenib | PR |
| Trametinib | ||||||
| Toutain20 | 2022 | Neuroblastoma | 7 | Male | Trametinib | SD |
To the best of our knowledge, this is the first case report that used trametinib to treat mBUC with the BRAF G469A mutation. A partial response was achieved and the disease has remained stable for more than 18 months. The present case indicates the potential of trametinib to treat mBUC patients with the BRAF G469A mutation whose disease progresses after platinum-based chemotherapy and PD-1/PD-L1 blockade. However, since we herein described only one case, further study is needed to evaluate the efficacy and safety of this treatment.
Author contributions
Hiroyuki Karasawa: Data curation; investigation; resources; visualization; writing – original draft; writing – review and editing. Yota Yasumizu: Conceptualization; data curation; investigation; project administration; resources; supervision; writing – review and editing. Takeo Kosaka: Supervision. Tatsunori Shimoi: Data curation; supervision. Mototsugu Oya: Supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
All informed consent was obtained from the patient.
Registry and the Registration No. of the study/trial
Not applicable.




