Testicular seminoma in transverse testicular ectopia
Abstract
Introduction
Transverse testicular ectopia is a rare anomaly in which both testes descend toward the same side of the hemiscrotum.
Case presentation
A 35-year-old man presented with right inguinal enlargement. Computed tomography showed a normal testis in the right hemiscrotum and a 58 mm heterogeneous mass in the right inguinal area. No testis was observed in the left hemiscrotum. The vascular structures extended from the right inguinal mass to the left renal vein. Consequently, the left testicular tumor was diagnosed as transverse testicular ectopia, and a left orchiectomy was performed. The histological diagnosis was seminoma stage pT2. Furthermore, left para-aortic lymph node metastasis developed 10 months postoperatively. A complete response was obtained after systemic chemotherapy.
Conclusion
Awareness of seminomas in transverse testicular ectopia could facilitate appropriate diagnosis and treatment. Furthermore, the location of the lymph node metastasis indicated that the ectopic testis could have originated from the left side.
Abbreviations & Acronyms
-
- BEP
-
- bleomycin, etoposide phosphate, and cisplatin
-
- CT
-
- computed tomography
-
- LDH
-
- lactate dehydrogenase
-
- MRI
-
- magnetic resonance imaging
-
- PMDS
-
- persistent Müllerian duct syndrome
-
- TTE
-
- transverse testicular ectopia
Keynote message
In the present report, we describe a rare case of seminoma with transverse testicular ectopia. Awareness of this condition may lead to accurate diagnosis and appropriate treatment. Furthermore, the location of lymph node metastasis could indicate the side of origin of the ectopic testis.
Introduction
TTE is a rare anomaly in which both testis descend toward the same side of the hemiscrotum.1 Although more than 260 cases of TTE have been reported, testicular tumors associated with TTE are rare. Preoperative imaging studies can help diagnose TTE.2, 3 However, it is occasionally diagnosed intraoperatively during inguinal exploration or laparoscopy. Here, we report a case of testicular seminoma with TTE in a 35-year-old male, for whom preoperative diagnosis was possible based on clinical and radiological findings.
Case report
A 35-year-old man presented with right inguinal enlargement for 1 month. The patient was married and had two children. The patient's medical history included asthma and ureteral calculus. He had undergone surgery for a left undescended testicle during childhood at another hospital; however, no testicles were found intraoperatively. On clinical examination, the patient was of average built and well-nourished, and his secondary sexual characteristics were well-developed. An abdominal examination revealed a lump in the right inguinal area. Upon genitourinary examination, the penis was well-developed, the right testis was palpable and normal in size in the right hemiscrotum, whereas the left testis was absent in the left hemiscrotum. Hypospadias and epispadias were not observed.
Following the clinical examinations, a right inguinal tumor was suspected; therefore, a CT was performed and revealed a large mass in the right inguinal area, with the right testis presented in the right scrotum. The vascular structure of the right inguinal mass extended into the left renal vein through the ventral side of the bladder (Fig. 1a). MRI showed a heterogeneous mass measuring 58 mm in the right inguinal area (Fig. 1b). Blood tests revealed a LDH level of 483 IU/L, whereas other tumor markers were negative. Based on these findings, our preoperative diagnosis was a left testicular tumor with TTE without metastasis.
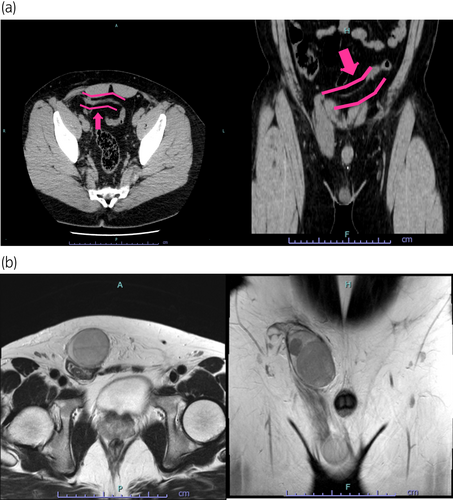
A left orchiectomy was performed a right inguinal incision. During surgery, the inguinal mass was found along the right spermatic cord with no apparent communication with the right spermatic cord. The left spermatic cord was parallel to the right and reached the peritoneum. No other findings, such as PMDS, were observed.
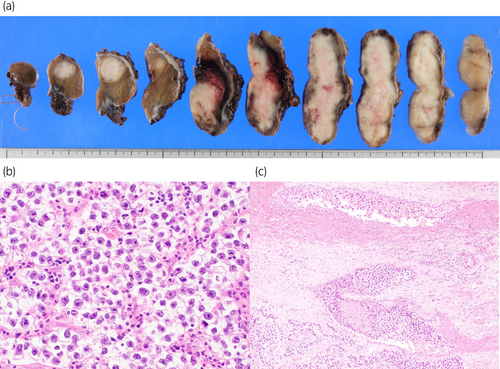
Macroscopically, two grayish-white, solid tumors of sizes 7.3 × 6.8 × 3.2 cm and 4.6 × 4.0 × 4.0 cm were found (Fig. 2a). Histologically, the tumor cells contained pale-to-clear cytoplasm with abundant nuclei (Fig. 2b). The tumors presented seminoma features. Vascular invasion of the tumor cells was observed (pT2) (Fig. 2c).
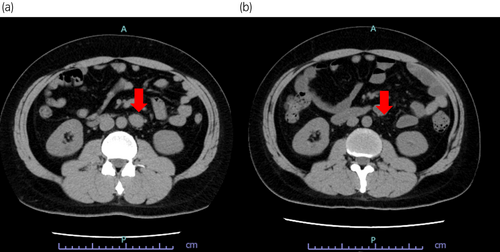
The patient underwent a postoperative follow-up. Left para-aortic lymph node metastasis developed 10 months postoperatively (Fig. 3a), and systemic chemotherapy with bleomycin, etoposide phosphate, and cisplatin (BEP) was initiated. During the first cycle of chemotherapy, the patient developed Grade 3 pneumonitis. Therefore, bleomycin was omitted from the subsequent three cycles. The overall efficacy evaluation showed a complete response after chemotherapy (Fig. 3b). Treatment efficacy was assessed according to the new guidelines to evaluate the response to treatment in solid tumors v1.1.
Discussion
We report a case of a testicular tumor associated with TTE. Although this disease is rare, appropriate preoperative diagnosis is possible. Furthermore, a durable and complete response against lymph node metastasis can be achieved with systemic chemotherapy (Fig. 4).

The prevalence of TTE is unknown. To date, over 260 cases have been reported in the literature, and more 80 such cases have been reported in Japan. Literature that specifically mentions the timing of the occurrence could not be confirmed. Generally, testicular decent begins approximately during the third month of fetal development and passes through the inguinal canal by 7–8 months, suggesting the possibility of TTE occurring during this period. Although literature addressing TTE etiology is scarce, it is discussed based on Gauderer's classification.4, 5 Type 1 (50%) is the most common subtype associated with inguinal hernia. Although no clear cause has been identified, testosterone and the HOXA10, HOXA11, and RXFP2/LGR8/GREAT, which are involved in testicular descent, may be affected. Type 2 (30%) is associated with PMDS. Homozygous G > A (p.W8X) mutations in the AMHR2 gene causing AMH receptor resistance may cause Type 2 TTE.6 Type 3 (20%) involves anomalies other than PMDS (low-lying, scrotal anomalies, fused vasa deferentia, seminal vesicle cysts, and testicular microlithiasis); however, the mechanism remains unclear.
Undescended testis in TTE have a high risk of developing malignant tumors at a rate of 18%, similar to cryptorchidism.7 The histological subtypes of malignant TTE include seminoma and mixed germ cell tumors. In most cases, the exact diagnosis is made during surgical intervention. However, MRI could reveal the location of the non-palpable testis. Laparoscopy is useful in the diagnosis and management of TTE. Considering the risk of malignancy in undescended testis, extensive examinations using MRI or laparoscopy are required for impalpable testis. In this case, the preoperative diagnosis was based on the presence of the right testis and radiological evidence of tumor vascularity in the left renal vein.
Different hypotheses have been proposed to explain the causes of TTE. A unilateral origin of both testes from the semen genital ridge has been proposed.8 This is further supported when both the spermatic ducts arise from one side. In the present case, the MRI revealed that the left and right spermatic ducts were parallel to the seminal vesicles. Moreover, the development of lymph node metastasis in the left para-aortic region suggests that the affected testis developed from the left genital ridge, with a descending anomaly into the right inguinal area. Overall, a review of the incidence of TTE due to lymph node metastasis would be valuable.
Conclusions
Herein, we report a rare case of testicular seminoma with TTE. Based on clinical characteristics and radiological findings, an exact preoperative diagnosis can be made. Increased awareness of these rare conditions can facilitate accurate diagnosis. Furthermore, the location of the lymph node metastasis indicated that the ectopic testis may have originated from the left side.
Author contributions
Minoru Inoue: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; writing – original draft; writing – review and editing. Akiyoshi Osaka: Conceptualization; investigation; writing – review and editing. Erika Ikezoe: Investigation. Hiroki Tsujioka: Investigation. Asumi Nirazuka: Investigation. Kintaro Hasegawa: Investigation. Toshiyuki Iwahata: Investigation. Akinori Nakayama: Investigation. Kiyoshi Setoguchi: Investigation. Kazutaka Saito: Conceptualization; writing – review and editing.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
The study protocol was reviewed and approved by the Dokkyo Medical University Saitama Medical Center Ethics Committee (approval number: 22017).
Informed consent
Written informed consent was obtained from the patient.
Registry and the Registration No. of the study/trial
Not applicable.




