Favorable response to pembrolizumab in granulocyte colony-stimulating factor-producing upper urinary tract urothelial carcinoma
Abstract
Introduction
Granulocyte colony-stimulating factor-producing upper urinary tract urothelial carcinoma is rare, with a poor prognosis. Advanced urothelial carcinoma is currently treated with immune checkpoint inhibitors, whose efficacy for granulocyte colony-stimulating factor-producing upper urinary tract urothelial carcinoma remains unclear.
Case presentation
A 66-year-old male diagnosed with clinical stage T3N1M0 urothelial carcinoma of the right ureter with giant hydronephrosis underwent right radical nephroureterectomy. Local recurrence, leukocytosis, and elevated serum granulocyte colony-stimulating factor levels were observed approximately 3 months after surgery. Chemotherapy was started but failed to control the disease. Therefore, pembrolizumab was chosen as the second-line treatment. After this treatment, the blood leukocyte count rapidly normalized, and a clinically favorable response was achieved. There was no recurrence 10 months after the beginning of pembrolizumab treatment, which is still ongoing.
Conclusion
Pembrolizumab may be a treatment option for advanced granulocyte colony-stimulating factor-producing upper urinary tract urothelial carcinoma.
Abbreviations & Acronyms
-
- CT
-
- computed tomography
-
- G-CSF
-
- granulocyte colony-stimulating factor
-
- irAE
-
- immune-related adverse event
-
- PD-L1
-
- programmed death-ligand 1
-
- PET
-
- positron emission tomography
-
- UC
-
- urothelial carcinoma
-
- UTUC
-
- urinary tract urothelial carcinoma
-
- WBC
-
- white blood cell
Keynote message
G-CSF-producing UTUC is rare and is known to be a highly malignant disease. Herein, we presented a patient with G-CSF-producing UTUC successfully treated with pembrolizumab. Immune checkpoint inhibitors might be one of the treatment options in patients with this rare disease that progresses after chemotherapy.
Introduction
The G-CSF is known as a hormone that stimulates the bone marrow to produce stem cells and granulocytes. Furthermore, G-CSF is functionally a cytokine and produced by different tissues including the endothelium, as well as by macrophages and malignant cells.1 G-CSF-producing tumor is a highly malignant disease with a very poor prognosis. In the field of urological oncology, several G-CSF-producing bladder cancers and upper UTUC have been reported to date, with the effects of chemotherapy on these diseases being limited.2, 3 Although immune checkpoint inhibitors such as pembrolizumab are an effective treatment for patients with chemotherapy-refractory UC, their effectiveness against G-CSF-producing UTUC remains unclear. In this report, we present a case of G-CSF-producing UTUC with local recurrence after nephroureterectomy that responded and was controlled with pembrolizumab.
Case presentation
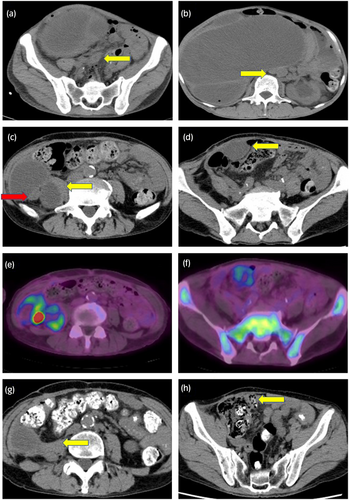
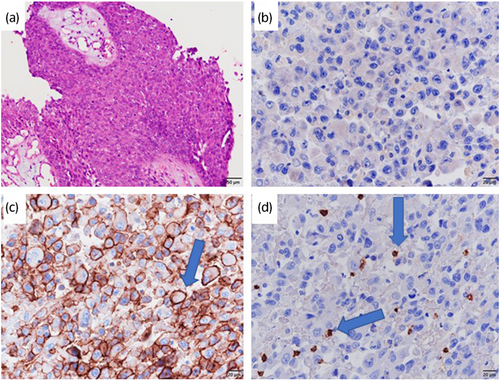
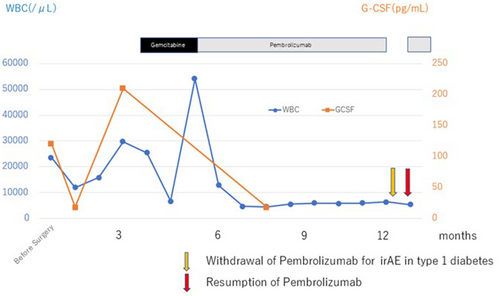
A 66-year-old male whose chief complaint was abdominal distension was referred to our hospital with the diagnosis of locally advanced right ureteral carcinoma with giant hydronephrosis (Fig. 1a). Blood test revealed a WBC count of 14 400/μL, neutrophil count of 12 936/μL, and serum creatinine of 3.49 mg/dL. We suspected G-CSF-producing ureteral carcinoma because his G-CSF serum concentration was 121 pg/mL (Human G-CSF Immunoassay; SRL, Tokyo, Japan: reference values <39 pg/mL). Although CT revealed retroperitoneal lymph node metastasis (Fig. 1b), preoperative chemotherapy was not performed due to renal dysfunction. The patient underwent right radical nephroureterectomy and retroperitoneal lymph node dissection. Histopathological examination showed invasive high-grade UC without lymph node metastasis (pT2N0M0) (Fig. 2a). After surgery, leukocytosis and high G-CSF serum concentration promptly improved to 10 100 cells/μL and 19.5 pg/mL, respectively. However, 3 months later, the WBC count and serum G-CSF increased to 29 800/μL and 210 pg/mL, respectively, and CT findings led to suspicion of local and intra-abdominal recurrence (Fig. 1c,d). Furthermore, PET-CT showed fluorodeoxyglucose uptake in a suspected recurrence site (Fig. 1e), with increased diffuse bone marrow fluorodeoxyglucose uptake likewise indicated (Fig. 1f). Based on these clinical findings, the patient was diagnosed with recurrence of G-CSF-producing UTUC. We decided to treat the patient with gemcitabine monotherapy due to his decreased renal function. However, before the second cycle of gemcitabine, WBC count increased to 54 100 cells/μL. Moreover, CT-scan showed progression of local recurrence and suspected peritoneal dissemination, indicating progressive disease. As a second-line treatment, we treated the patient with the PD-1 antibody pembrolizumab. Three months after this therapy, the WBC count and G-CSF concentration became normalized (Fig. 3), and all recurrent lesions shrank remarkably on CT-scan (Fig. 1g,h). After 12 cycles of pembrolizumab, the patient developed type 1 diabetes, which was presumed to be an irAE. Following control of the patient’s diabetes, pembrolizumab was restarted, which is still ongoing. Despite interruption of therapy, he was progression-free for 10 months, maintaining a normal range of WBC count.
Discussion
G-CSF-producing tumors are malignant tumors that exhibit marked leukocytosis with no cause, such as infection, blood disease, or myelocarcinomatosis. Since Asano et al. proved that human lung carcinoma cells produce G-CSF in 1977, there have been many similar reports in Japan.4 They reported the following diagnostic criteria for a G-CSF-producing tumor: (i) increased WBC count without infection or other diseases; (ii) increase in serum G-CSF concentration; (iii) reduction in WBC count after treatment; and (iv) G-CSF-positive staining from immunohistochemical tumor analysis. In the current case, leukocytosis and increased serum G-CSF without infection were observed before the surgery and tumor recurrence and decreased WBCs were observed after treatment. Furthermore, the present case had a diffuse fluorodeoxyglucose uptake in the bone marrow before pembrolizumab treatment, which is suggested to have occurred due to bone marrow hyperplasia induced by G-CSF produced by recurrent UTUC. G-CSF-producing tumors typically show diffuse accumulation of 18F- fluorodeoxyglucose in the bone marrow on PET scans, indicating enhanced glucose metabolism by promoting hematopoietic capacity.5, 6 In the present case, only weak positives for G-CSF immunostaining of cancer cells in nephroureterectomy specimens were found (Fig. 2b). However, we diagnosed G-CSF-producing UTUC based on criteria (1–3), as well as the findings on diffuse bone marrow fluorodeoxyglucose uptake. Notably, positive immunostaining with G-CSF antibody is reportedly not a definitive finding for the diagnosis of G-CSF-producing tumors.7, 8 A possible reason is the quick turnover of the G-CSF protein and its short intracellular retention time because of its rapid release to the cell’s external environment.9
Based on a previous case report and literature review on G-CSF-producing UTUC, a poor prognosis and high mortality rate are possible. Matsumoto et al. reviewed 46 patients with G-CSF-producing UTUC, and in their report, the median survival time of their cases was only 4.5 months.2 Therefore, novel systemic therapies for G-CSF-producing UTUC need to be identified to improve clinical outcomes. Pembrolizumab monotherapy as second-line treatment significantly prolonged the overall survival of advanced UC patients compared to the standard second-line chemotherapy in the KEY-NOTE 045 clinical study.10 However, there are few reports on the antitumor effect of pembrolizumab in G-CSF-producing advanced UC. Only one case report demonstrated the effective cancer control of pembrolizumab as a second-line therapy.3 This study reported a case of G-CSF-producing bladder cancer, in which complete response was achieved with pembrolizumab therapy. Only two cases, including ours, have shown the efficacy of pembrolizumab in patients with advanced G-CSF producing UC. However, it might have been better to switch to pembrolizumab as soon as possible when the first-line chemotherapy was not effective because most cases were associated with aggressive progression and a poor prognosis.
Several studies examining the pathological characteristics of responders to immunotherapy have suggested that the predictive biomarkers of immune checkpoint inhibitor therapy are PD-L1 expression, tumor infiltration by CD8+ T lymphocytes, and tumor mutation burden.10-12 In the present case, immunohistochemical testing of PD-L1 was performed using IHC 22C3 antibody, and positive PD-L1 expression in the tumor cell membrane was observed (Fig. 2c). Furthermore, tumor infiltration with CD8+ T lymphocytes was observed (Fig. 2d). Although there were limitations in which tumor mutation burden was not evaluated and recurrent lesions had not been assessed, these findings might explain our patient’s response to pembrolizumab.
Conclusion
This is the first case report on the successful treatment of G-CSF-producing UTUC with pembrolizumab as far as we searched. Immune checkpoint inhibitors can be a treatment option for patients with this rare disease that progresses after chemotherapy.
Author Contributions
Hiroki Takeda: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft. Ryuji Matsumoto: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing. Emi Takakuwa: Data curation; Methodology; Visualization. Kanta Hori: Data curation; Supervision. Takuya Moriguchi: Data curation; Supervision. Shuhei Yamada: Data curation; Supervision. Hiroshi Kikuchi: Conceptualization; Supervision. Takahiro Osawa: Conceptualization; Supervision. Takashige Abe: Conceptualization; Supervision. Nobuo Shinohara: Conceptualization; Supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Informed consent was obtained from the patient.
Registry and the Registration No. of the study/trial
Not applicable.




