Spontaneous remission of rectal ulcer associated with SpaceOAR® hydrogel insertion in radiotherapy for prostate cancer
Abstract
Introduction
The SpaceOAR® hydrogel system separates the prostate and rectum to reduce rectal irradiation during prostate radiotherapy. However, it could induce rectal toxicity.
Case presentation
A 75-year-old man with localized prostate cancer underwent external beam radiotherapy with the use of SpaceOAR® System. However, postimplant magnetic resonance imaging showed hydrogel infiltration to the rectum. Three months after implantation, he complained of bowel symptoms, including bloody stool. Colonofiberscopy and computed tomography revealed a rectal ulcer associated with SpaceOAR® hydrogel insertion. He was treated with fasting, fluid replacement, and blood transfusion. One year after implantation, complete healing was confirmed during outpatient follow-up.
Conclusion
To our knowledge, this is the first report of a rectal ulcer associated with SpaceOAR® hydrogel insertion assessed by magnetic resonance imaging beforehand. Postimplant magnetic resonance imaging evaluation might be a useful follow-up tool in such cases.
Abbreviations & Acronyms
-
- AE
-
- adverse event
-
- CF
-
- colonofiberscopy
-
- CT
-
- computed tomography
-
- EBRT
-
- external beam radiotherapy
-
- MRI
-
- magnetic resonance imaging
-
- RT
-
- radiotherapy
Keynote message
We report a case of rectal ulcer associated with the use of SpaceOAR® hydrogel system in RT for prostate cancer. It was assessed by MRI before its onset and treated conservatively.
Introduction
RT is a well-established treatment for localized prostate cancer. Irradiation dose escalation is related to its efficacy, but also to the risk of rectal toxicity. RT must be carefully planned because of the proximity of the prostate and the rectum. The SpaceOAR® System (Boston Scientific, Marlborough, MA, USA) is a synthetic polyethylene glycol hydrogel, designed to separate the prostate and rectum, to reduce rectal RT exposure.1 Although the injection procedure for the SpaceOAR® System is not very complicated, some AEs associated with SpaceOAR® hydrogel insertion have been reported, including hematuria, perianal pain, prostatic abscess, and rectal ulcer.1-4 Few of these reported cases with AEs had been evaluated by MRI before their onset. Therefore, MRI findings that could predict the risk for AEs, especially of the rectum, are unclear. However, at our hospital, we routinely perform postimplant MRIs within a week after the procedure to confirm proper placement.
Here, we present a case of a conservatively treated rectal ulcer associated with SpaceOAR® hydrogel insertion before EBRT for prostate cancer, in a patient who had been assessed by MRI before onset of the ulcer.
Case presentation
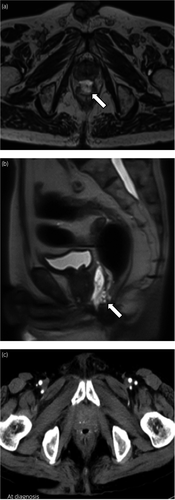
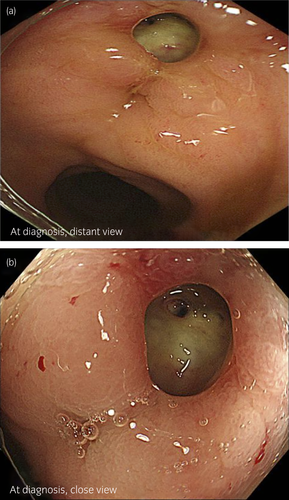
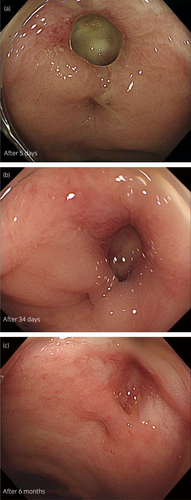
A 75-year-old man with a prostate-specific antigen level of 9.1 ng/ml underwent a prostate biopsy and was diagnosed with low-risk prostate cancer (cT2aN0M0; Gleason score: 3 + 3). Definitive EBRT using the SpaceOAR® System was planned. He had a history of brain infarction, atrial fibrillation, chronic heart failure, and glaucoma. MRI revealed the suspicion of cancer in the transitional zone of the left prostate lobe. One week before RT, he underwent SpaceOAR® hydrogel insertion through the ultrasonography-guided transperineal technique under spinal anesthesia.5 The space between the prostate and rectum seemed small, and the injection needle was inserted several times before reaching a perirectal space. Next day, postimplant MRI images showed that some SpaceOAR® hydrogel had infiltrated his anterior rectal wall (Fig. 1a,b). Despite this finding, he complained of no symptoms, and we considered that the rectum could be protected from irradiation. He underwent EBRT; a total dose of 74 Gy in 37 fractions was delivered to his prostate gland as planned. On his visit at 3 months after implantation, he complained of increased bowel frequency, urgency, and intermittent pain around his anus. Six months after implantation, he complained of bloody stools. Although he did not have systemic symptoms, such as abdominal pain or fever, his blood hemoglobin level had decreased from 14.0 to 10.4 g/dL. While the postimplant MRI images showed no air around the hydrogel, an enhanced abdominal CT scan revealed air in the space between the prostate and anterior rectal wall that was inconsistent with contamination during the insertion procedure (Fig. 1c). He underwent a CF, which revealed an ulcer with an exposed vessel at the anterior rectal wall (Fig. 2). No findings indicated proctitis. He was diagnosed with a rectal ulcer and hospitalized for careful monitoring. Treatment was conservative: fasting, fluid infusion, and blood transfusion. Five days after diagnosis, his hemoglobin level stabilized, and he underwent a follow-up CF. The previously exposed vessel was not observed, and the rectal ulcer did not get worse (Fig. 3a). Oral food intake was resumed with a low-fiber diet; no bloody stool or bowel symptoms were observed since the second CF. On a subsequent follow-up CF, 34 days after diagnosis of his ulcer, mucosal restoration was observed (Fig. 3b). Six months after diagnosis (1 year after implantation), epithelization was observed, which was consistent with healing (Fig. 3c). One and a half years after implantation, the bowel symptoms and bloody stool have not recurred.
Discussion
The rectum is the main dose-limiting organ for prostate cancer RT because of its proximity to the prostate. The SpaceOAR® System can reduce the risk of rectal irradiation. Using the Common Terminology Criteria for Adverse Events v.4, the frequency of grade 2 AEs associated with SpaceOAR® hydrogel insertion is about 3.3–4.1%.1, 6 The few rectal ulcers that have been reported were treated conservatively without hospitalization.2, 3 Although the mechanism leading to rectal ulcer associated with SpaceOAR® hydrogel is unclear, several other causes of rectal injuries have been reported, including infection, inflammation, radiation, mechanical injury, and ischemic change. Bacterial infection and inflammatory bowel disease could lead to a local inflammation.7, 8 Excessive RT doses to the rectum can result in radiation proctitis, which causes bowel frequency, urgency, fecal incontinence, bleeding, and ulcer.9 Mechanical injury or ischemic change could cause tissue necrosis.10 Rectal ulcer associated with SpaceOAR® hydrogel may also occur through these mechanisms. The patient in this case did not develop systemic symptoms (e.g. abdominal pain, fever, and elevated inflammatory markers), which suggests that he did not have local infection or inflammation. The CF findings and the healing process were inconsistent with those of radiation proctitis. We inserted the needle several times and postimplant MRI showed hydrogel infiltration to the rectum. Taken together, the hydrogel could have infiltrated through the piercing site or previous needle tracks that may lead to mechanical injury or ischemic change. The SpaceOAR® hydrogel can be delineated on CT image, but its density is similar to that of the prostate.11 MRI detects hydrogel more clearly than CT.12 Routine MRI evaluation of SpaceOAR® hydrogel is reportedly unnecessary in terms of treatment management,2 and the insertion procedure will become refined with gained experience, which could decrease the need of postimplant MRI. SpaceOAR® hydrogel rectal wall infiltration has reportedly occurred in 9/149 cases (6%).13 In these cases, no correlations were observed between rectal wall infiltration and procedure-related AEs, and RTs were performed as planned. Meanwhile, in a report on proton therapy, two cases of hydrogel infiltration into the rectum led to consecutive occurrence of rectal fistulas.14 In our case, postimplant MRI images suggested infiltration of hydrogel into the anterior rectal wall, which led us to follow-up the patient carefully. Because the patient was asymptomatic immediately after hydrogel insertion and the prostate was sufficiently separated from the rectum, we performed RT as planned despite the hydrogel infiltration. However, if the infiltration caused any symptoms, or the SpaceOAR® hydrogel did not create space to prevent rectal irradiation, we should reconsider the treatment policy. To our knowledge, this is the first report of rectal ulcer associated with SpaceOAR® hydrogel insertion which was assessed by MRI before its onset. We should recognize that SpaceOAR® hydrogel insertion is an invasive procedure and could cause AEs. Postimplant MRI evaluation could be a useful part of careful follow-up, especially in patients for whom hydrogel insertion was difficult.
Acknowledgments
We thank Alison Sherwin, PhD, and Marla Brunker from Edanz Group (www.en-authorservices.edanzediting.com) for editing a draft of this manuscript.
Conflict of interest
The authors declare no conflict of interest.




