Dipeptidyl peptidase-4 in the tumor microenvironment of the digestive system: Molecular mechanisms and therapeutic targets
Jingting Wang
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Conceptualization, Investigation, Writing - original draft
Search for more papers by this authorLei Zhao
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Writing - review & editing
Search for more papers by this authorDuo Yun
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Writing - review & editing, Investigation, Validation, Formal analysis, Supervision
Search for more papers by this authorHaishan Lin
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Funding acquisition, Resources
Search for more papers by this authorJing Wang
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Data curation
Search for more papers by this authorCorresponding Author
Bangwei Cao
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Correspondence
Bangwei Cao, Department of Oncology, Beijing Friendship Hospital, Capital Medical University, #95 Yong An Road, Xicheng District, Beijing, China.
Email: [email protected]
Contribution: Funding acquisition, Software
Search for more papers by this authorJingting Wang
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Conceptualization, Investigation, Writing - original draft
Search for more papers by this authorLei Zhao
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Writing - review & editing
Search for more papers by this authorDuo Yun
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Writing - review & editing, Investigation, Validation, Formal analysis, Supervision
Search for more papers by this authorHaishan Lin
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Funding acquisition, Resources
Search for more papers by this authorJing Wang
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Contribution: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Data curation
Search for more papers by this authorCorresponding Author
Bangwei Cao
Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Correspondence
Bangwei Cao, Department of Oncology, Beijing Friendship Hospital, Capital Medical University, #95 Yong An Road, Xicheng District, Beijing, China.
Email: [email protected]
Contribution: Funding acquisition, Software
Search for more papers by this authorAbstract
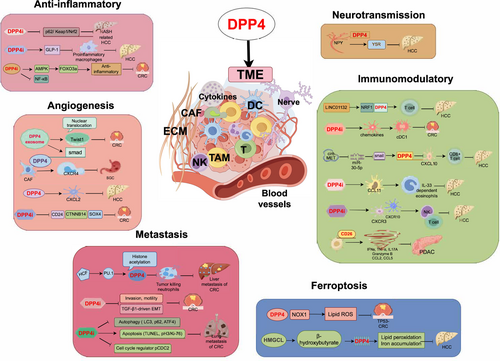
Digestive system cancers represent nearly half of all global cancer cases and are associated with high mortality rates. Although immunotherapy has ushered in a new era for the treatment of digestive system cancers, the complexity and diversity of the immunosuppressive tumor microenvironment (TME) pose significant challenges to effective immunotherapy for digestive tumors. Dipeptidyl peptidase 4 (DPP4), also known as CD26, has garnered considerable attention for its role within the TME, where it plays a crucial role in the initiation and progression of digestive system cancers and serves as a predictive biomarker for certain cancers. Additionally, it is involved in regulating immune responses, angiogenesis, ferroptosis activation, tumor-associated inflammation, neurotransmission, and metastasis. Thus, DPP4 inhibitors play a pivotal role in modulating the intricate tumor ecosystem, contributing to the control of digestive system cancer development. Therefore, a deeper understanding of DPP4's mechanisms in the TME holds promise for establishing essential theoretical frameworks for developing novel treatment strategies for digestive system cancers. This review explores the regulatory role of DPP4 in the TME of digestive system cancers and its clinical implications, aiming to provide insights into potential advancements in oncological therapies.
Graphical Abstract
CONFLICT OF INTEREST STATEMENT
All the authors declare no conflicts of interest.
REFERENCES
- 1O'Leary H, Ou X, Broxmeyer HE. The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation. Curr Opin Hematol. 2013; 20(4): 314-319.
- 2Elmansi AM, Eisa NH, Kondrikov D, et al. What doesn't kill you makes you stranger: dipeptidyl peptidase-4 (CD26) proteolysis differentially modulates the activity of many peptide hormones and cytokines generating novel cryptic bioactive ligands. Pharmacol Ther. 2019; 198: 90-108.
- 3Singh PHJ, Kamocka MM, Mohammad KS, et al. Neuropeptide Y regulates a vascular gateway for hematopoietic stem and progenitor cells. J Clin Invest. 2017; 127(12): 4527-4540.
- 4Elmansi AM, Eisa NH, Periyasamy-Thandavan S, et al. DPP4-truncated CXCL12 alters CXCR4/ACKR3 signaling, osteogenic cell differentiation, migration, and senescence. ACS Pharmacol Transl Sci. 2022; 6(1): 22-39.
- 5Zhong JRX, Deiuliis J, Braunstein Z, et al. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013; 62(1): 149-157.
- 6Jixin Zhong XR, Braunstein Z, Bromberg JS, Rajagopalan S. Dipeptidyl peptidase-4 promotes T cell migration. J Immunol. 2016; 196(1_Supplement): 54.1.
10.4049/jimmunol.196.Supp.54.1 Google Scholar
- 7Hui YXZ, Li J, Kuang L, et al. Nonenzymatic function of DPP4 promotes diabetes-associated cognitive dysfunction through IGF-2R/PKA/SP1/ERp29/IP3R2 pathway-mediated impairment of Treg function and M1 microglia polarization. Metabolism. 2023; 138:155340.
- 8Zeyer KA, BO N, Bao X, et al. Dipeptidyl peptidase-4-mediated fibronectin processing evokes a profibrotic extracellular matrix. J Invest Dermatol. 2024; 144(11): 2477-2487.e13.
- 9Yang Y, Chen B, Zheng C, et al. Association of glucose-lowering drug target and risk of gastrointestinal cancer: a mendelian randomization study. Cell Biosci. 2024; 14(1): 36.
- 10Busek P, Vanickova Z, Hrabal P, et al. Increased tissue and circulating levels of dipeptidyl peptidase-IV enzymatic activity in patients with pancreatic ductal adenocarcinoma. Pancreatology. 2016; 16(5): 829-838.
- 11de Chiara L, Barcia-Castro L, Gallardo-Gómez M, et al. Evaluation of blood soluble CD26 as a complementary biomarker for colorectal cancer screening programs. Cancers (Basel). 2022; 14(19): 4563.
- 12Boccardi VML, Rossetti RR, Rizzo MR, di Martino N, Paolisso G. Serum CD26 levels in patients with gastric cancer: a novel potential diagnostic marker. BMC Cancer. 2015; 15(703).
- 13Chen TILF, Hsu WL, Chen YC, Chen M. Association of dipeptidyl peptidase-4 inhibitors use with reduced risk of hepatocellular carcinoma in type 2 diabetes patients with chronic HBV infection. Cancers (Basel). 2023; 15(4): 1148.
- 14Chou CL, Juan SH, Li CH, et al. Association between DPP-4 inhibitors and events of colorectal and liver cancers in patients with diabetes receiving second-line agents: a nested case-control study. Front Oncol. 2022; 12:840142.
- 15Abrahami D, Douros A, Yin H, et al. Incretin based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: population-based cohort study. BMJ. 2018; 363:k4880.
- 16Laeeq T AM, Sattar H, Zeeshan MH, Ali MB. Role of SGLT2 inhibitors, DPP-4 inhibitors, and metformin in pancreatic cancer prevention. Cancers (Basel). 2024; 16(7): 1325.
- 17Bishnoi R, Hong YR, Shah C, et al. Dipeptidyl peptidase 4 inhibitors as novel agents in improving survival in diabetic patients with colorectal cancer and lung cancer: a surveillance epidemiology and endpoint research Medicare study. Cancer Med. 2019; 8(8): 3918-3927.
- 18Ng LFD, Wong CK, Man AT, Lo OS, Law WL. Repurposing DPP-4 inhibitors for colorectal cancer: a retrospective and single center study. Cancers (Basel). 2021; 13(14):3588.
- 19de Visser KEJJ. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023; 41(3): 374-403.
- 20Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144(5): 646-674.
- 21Cordero OJ, Rafael-Vidal C, Varela-Calviño R, et al. Distinctive CD26 expression on CD4 T-cell subsets. Biomolecules. 2021; 11(10): 1446.
- 22Hollande C, Boussier J, Ziai J, et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat Immunol. 2019; 20(3): 257-264.
- 23Zheng X, Liu J, Li X, et al. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021; 497: 190-201.
- 24Wang L, Wang E, Prado Balcazar J, et al. Chromatin remodeling of colorectal cancer liver metastasis is mediated by an HGF-PU.1-DPP4 Axis. Adv Sci (Weinh). 2021; 8(19):e2004673.
- 25Kawaguchi T, Koga H, Torimura T. Effects of a DPP4 inhibitor on progression of NASH-related HCC and the p62/Keap1/Nrf2-pentose phosphate pathway in a mouse model. Liver Cancer. 2019; 8(5): 359-372.
- 26Cassetta L, Pollard JW. A timeline of tumor-associated macrophage biology. Nat Rev Cancer. 2023; 23(4): 238-257.
- 27Kim TK, Herbst RS, Chen L. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov. 2022; 21(7): 529-540.
- 28Zhang J, Pan T, Zhou W, et al. Long noncoding RNA LINC01132 enhances immunosuppression and therapy resistance via NRF1/DPP4 axis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022; 41(1): 270.
- 29Huang XY, Zhang PF, Wei CY, et al. Circular RNA circMET drives immunosuppression and anti-PD1 therapy resistance in hepatocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol Cancer. 2020; 19(1): 92.
- 30Nishina S, Yamauchi A, Kawaguchi T, et al. Dipeptidyl peptidase 4 inhibitors reduce hepatocellular carcinoma by activating lymphocyte chemotaxis in mice. Cell Mol Gastroenterol Hepatol. 2018; 7(1): 115-134.
- 31Ruterbusch MPK, Shehata L, Pepper M. In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. 2020; 38: 705-725.
- 32Bailey SR, Nelson MH, Majchrzak K, et al. Human CD26high T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat Commun. 2017; 8(1): 1961.
- 33Zhang C, Huang J, Xu M, et al. Eosinophil-activating semiconducting polymer nanoparticles for cancer photo-immunotherapy. Angew Chem Int Ed Engl. 2024; 63(30):e202405358.
- 34Peng SXF, Chen M, Gao H. Tumor-microenvironment-responsive Nanomedicine for enhanced cancer immunotherapy. Adv Sci (Weinh). 2022; 9(1):e2103836.
- 35del Prete A, Salvi V, Soriani A, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023; 20(5): 432-447.
- 36Ng II, Zhang J, Tian T, et al. Network-based screening identifies sitagliptin as an antitumor drug targeting dendritic cells. J Immunother Cancer. 2024; 12(3):e008254.
- 37Chen DS. MIEociatc-ispN-Elements of cancer immunity and the cancer-immune set point. Nature. 2017; 541(7637): 321-330.
- 38Enz N, Vliegen G, De Meester I, Jungraithmayr W. CD26/DPP4: a potential biomarker and target for cancer therapy. Pharmacol Ther. 2019; 198: 135-159.
- 39Nigam M, Mishra AP, Deb VK, et al. Evaluation of the association of chronic inflammation and cancer: insights and implications. Biomed Pharmacother. 2023; 164:115015.
- 40Anstee QMRH, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019; 16(7): 411-428.
- 41Song Y, Yang H, Kim J, et al. Gemigliptin, a DPP4 inhibitor, ameliorates nonalcoholic steatohepatitis through AMP-activated protein kinase-independent and ULK1-mediated autophagy. Mol Metab. 2023; 78:101806.
- 42Jung YA, Choi YK, Jung GS, et al. Sitagliptin attenuates methionine/choline-deficient diet-induced steatohepatitis. Diabetes Res Clin Pract. 2014; 105(1): 47-57.
- 43Kawakubo M, Tanaka M, Ochi K, et al. Dipeptidyl peptidase-4 inhibition prevents nonalcoholic steatohepatitis-associated liver fibrosis and tumor development in mice independently of its anti-diabetic effects. Sci Rep. 2020; 10(1): 983.
- 44Abdelhady R, Mohammed OA, Doghish AS, et al. Linagliptin, a DPP-4 inhibitor, activates AMPK/FOXO3a and suppresses NFκB to mitigate the debilitating effects of diethylnitrosamine exposure in rat liver: novel mechanistic insights. FASEB J. 2024; 38(4):e23480.
- 45Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 2022; 8(11): 901-914.
- 46Hou JKM, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021; 18(5): 261-279.
- 47Yuan SAJ, Fuchs E. Beyond genetics: driving cancer with the tumour microenvironment behind the wheel. Nat Rev Cancer. 2024; 24(4): 274-286.
- 48Longo V, Brunetti O, Gnoni A, et al. Angiogenesis in pancreatic ductal adenocarcinoma: a controversial issue. Oncotarget. 2016; 7(36): 58649-58658.
- 49Rizzolio S, Giordano S, Corso S. The importance of being CAFs (in cancer resistance to targeted therapies). J Exp Clin Cancer Res. 2022; 41(1): 319. doi:10.1186/s13046-022-02524-w
- 50Joshi RS, Kanugula SS, Sudhir S, Pereira MP, Jain S, Aghi MK. The role of cancer-associated fibroblasts in tumor progression. Cancers (Basel). 2021; 13(6): 1399.
- 51Hurwitz HFL, Novotny W, Cartwright T, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004; 350: 2335-2342.
- 52van Cutsem ETJ, Lakomy R, Prenen H, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012; 30(28): 3499-3506.
- 53Grothey A, van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381(9863): 303-312. doi:10.1016/S0140-6736(12)61900-X
- 54Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013; 31(26): 3219-3225.
- 55Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383(9911): 31-39.
- 56Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 389(10064): 56-66.
- 57Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020; 382(20): 1894-1905.
- 58Kushiyama SYM, Yamamoto Y, Sera T, et al. Dipeptidyl Peptidase-4 from cancer-associated fibroblasts stimulates the proliferation of Scirrhous-type gastric cancer cells. Anticancer Res. 2022; 42(1): 501-509.
- 59Qin CJ, Zhao LH, Zhou X, et al. Inhibition of dipeptidyl peptidase IV prevents high fat diet-induced liver cancer angiogenesis by downregulating chemokine ligand 2. Cancer Lett. 2018; 420: 26-37.
- 60Dorafshan S, Razmi M, Safaei S, Gentilin E, Madjd Z, Ghods R. Periostin: biology and function in cancer. Cancer Cell Int. 2022; 22(1): 315.
- 61Winkler JA-OA, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020; 11(1): 5120.
- 62Shih JW, Wu ATH, Mokgautsi N, Wei PL, Huang YJ. Preclinical repurposing of Sitagliptin as a drug candidate for colorectal cancer by targeting CD24/CTNNB1/SOX4-centered signaling hub. Int J Mol Sci. 2024; 25(1): 609.
- 63Huang QHB, Zhang P, Yuan Y, et al. Neuroscience of cancer: unraveling the complex interplay between the nervous system, the tumor and the tumor immune microenvironment. Mol Cancer. 2025; 24(1): 24.
- 64Sánchez MLRF, Coveñas R. Neuropeptide Y peptide family and cancer: antitumor therapeutic strategies. Int J Mol Sci. 2023; 24(12): 9962.
- 65Dietrich P, Wormser L, Fritz V, et al. Molecular crosstalk between Y5 receptor and neuropeptide Y drives liver cancer. J Clin Invest. 2020; 130(5): 2509-2526.
- 66Mahmoud AMA, Mantawy EM, Wahdan SA, Ammar RM, El-Demerdash E. Vildagliptin restores cognitive function and mitigates hippocampal neuronal apoptosis in cisplatin-induced chemo-brain: imperative roles of AMPK/Akt/CREB/ BDNF signaling cascades. Biomed Pharmacother. 2023; 159:114238.
- 67Chen XKR, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021; 18(5): 280-296.
- 68Lei GZL, Gan B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell. 2024; 42(4): 513-534.
- 69Yang K, Wang X, Song C, et al. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics. 2023; 13(6): 1774-1808.
- 70Jiang Z, Lim SO, Yan M, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021; 131(8):e139434.
- 71Cui X, Yun X, Sun M, et al. HMGCL-induced β-hydroxybutyrate production attenuates hepatocellular carcinoma via DPP4-mediated ferroptosis susceptibility. Hepatol Int. 2023; 17(2): 377-392.
- 72Xie Y. The tumor suppressor p53 limits Ferroptosis by blocking DPP4 activity. Cell Rep. 2017; 20(7): 1692-1704.
- 73Zhang S, Fang W, Zhou S, et al. Single cell transcriptomic analyses implicate an immunosuppressive tumor microenvironment in pancreatic cancer liver metastasis. Nat Commun. 2023; 14(1): 5123.
- 74Wang Y, Zhong X, He X, et al. Liver metastasis from colorectal cancer: pathogenetic development, immune landscape of the tumor microenvironment and therapeutic approaches. J Exp Clin Cancer Res. 2023; 42(1): 177.
- 75Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nat Rev Dis Primers. 2021; 7(1): 27.
- 76Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021; 21(3): 162-180.
- 77Gao YBI, Wang H, Zhang W, Rosen JM, Zhang XH. Metastasis organotropism: redefining the congenial soil. Dev Cell. 2019; 49(3): 375-391.
- 78Gerstberger SJQ, Ganesh K. Metastasis. Cell. 2023; 186(8): 1564-1579.
- 79Karras PBJ, McGranahan N, Marine JC. Decoding the interplay between genetic and non-genetic drivers of metastasis. Nature. 2024; 629(8012): 543-554.
- 80Varela-Calviño R, Rodríguez-Quiroga M, Dias Carvalho P, et al. The mechanism of sitagliptin inhibition of colorectal cancer cell lines' metastatic functionalities. IUBMB Life. 2021; 73(5): 761-773.
- 81Xia HGD, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021; 21(5): 281-297.
- 82Jang JH, Baerts L, Waumans Y, et al. Suppression of lung metastases by the CD26/DPP4 inhibitor Vildagliptin in mice. Clin Exp Metastasis. 2015; 32(7): 677-687.
- 83Das M. Monoclonal antibody YS110 for refractory solid tumours. Lancet Oncol. 2017; 18(5):e247.
- 84Angevin E, Isambert N, Trillet-Lenoir V, et al. First-in-human phase 1 of YS110, a monoclonal antibody directed against CD26 in advanced CD26-expressing cancers. Br J Cancer. 2017; 116(9): 1126-1134.
- 85Fitzgerald AA, Wang S, Agarwal V, et al. DPP inhibition alters the CXCR3 axis and enhances NK and CD8+ T cell infiltration to improve anti-PD1 efficacy in murine models of pancreatic ductal adenocarcinoma. J Immunother Cancer. 2021; 9(11):e002837.
- 86Hu Y, Sarkar A, Song K, et al. Selective refueling of CAR T cells using ADA1 and CD26 boosts antitumor immunity. Cell Rep Med. 2024; 5(5):101530.
- 87Li CQ, Shi JH, Mu J, Wang AQ, Zou LW, Ge GB. Licochalcone a derivatives as selective dipeptidyl peptidase 4 inhibitors with anti-inflammatory effects. J Nat Prod. 2023; 86(7): 1824-1831.
- 88Nakagawa K, Kijima T, Okada M, et al. Phase 2 study of YS110, a recombinant humanized anti-CD26 monoclonal antibody, in Japanese patients with advanced malignant pleural mesothelioma. JTO Clin Res Rep. 2021; 2(6):100178.
- 89Wagner L, Klemann C, Stephan M, von Hörsten S. Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins. Clin Exp Immunol. 2016; 184(3): 265-283.
- 90Puré E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene. 2018; 37(32): 4343-4357.
- 91Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009; 30(11): 600-607.
- 92Wang S, Fitzgerald A, Ajina R, et al. Therapy with BXCL701 (B) a. therapy with BXCL701 (B), a DPP8, DPP9, DPPIV and FAP inhibitor, in combination with anti-PD1 antibody (PD1) in a syngeneic murine pancreatic ductal adenocarcinoma (PDAC) model improves treatment outcomes and induces intratumoral NK cell infiltrates and a marked reduction in tumor stromal fibrosis. Cancer Res. 2020; 80: 6636.
10.1158/1538-7445.AM2020-6636 Google Scholar
- 93Plas S, Pircher A, Heidegger I. Pembrolizumab in mCRPC: combination therapies as breakthrough to success? Curr Opin Urol. 2023; 33(6): 458-471.
- 94Weinberg BA, Lekan A, Fitzgerald A, et al. Phase II trial of BXCL701 and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (EXPEL-PANC): preliminary findings. J Clin Oncol. 2024; 42:LBA4132.
- 95Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023; 20(7): 429-452.
- 96Liu L, Shah K. The potential of the gut microbiome to reshape the cancer therapy paradigm: a review. JAMA Oncol. 2022; 8(7): 1059-1067.
- 97Cao C, Yue S, Lu A, Liang C. Host-gut microbiota metabolic interactions and their role in precision diagnosis and treatment of gastrointestinal cancers. Pharmacol Res. 2024; 207:107321.
- 98Wang K, Zhang Z, Hang J, et al. Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target. Science. 2023; 381(6657):eadd5787.
Online Version of Record before inclusion in an issue