Incidence of late recurrence and second primary cancers 5–10 years after non-metastatic colorectal cancer
Abstract
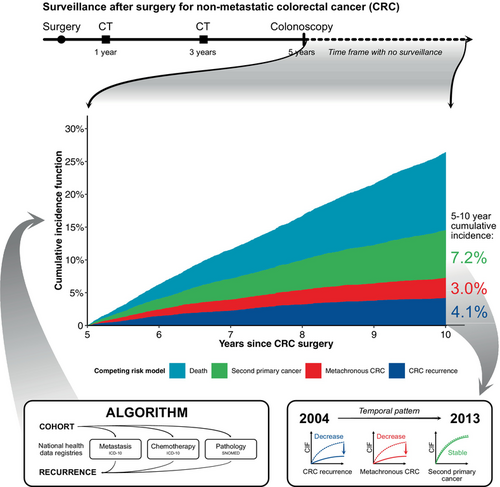
The fraction of patients who are cancer-free survivors 5 years after curative-intended surgery for colorectal cancer (CRC) is increasing, suggesting that extending surveillance beyond 5 years may be indicated. Here we estimate the incidence of late recurrence, metachronous CRC, and second primary cancers 5–10 years postoperative. All patients resected for UICC stage I–III CRC in Denmark through 2004–2013 were identified. Through individual-level linkage of nationwide health registry data, recurrence status was determined using a validated algorithm. Cancer-free survivors 5 years after surgery, were included. Cumulative incidence functions (CIF) of late recurrence, metachronous CRC, and second primary cancer 5–10 years postoperative were constructed. Subdistribution hazards ratios (sHR) were computed using Fine-Gray regression. Among 8883 patients, 370 developed late recurrence (5–10-year CIF = 4.1%, 95%CI: 3.7%–4.6%), 270 metachronous CRC (5–10-year CIF = 3.0%, 95%CI: 2.7%–3.4%), and 635 a second primary cancer (5–10-year CIF = 7.2%, 95%CI: 6.7%–7.7%). The risk of late recurrence was reduced for patients operated in 2009–2013 compared to 2004–2008 (2.9% vs. 5.6%, sHR = 0.52, 95% CI: 0.42–0.65). The risk of metachronous CRC was likewise reduced from 4.1% to 2.1% (sHR = 0.50, 95%CI: 0.39–0.65). While the risk of second primary cancer did not change between 2009–2013 and 2004–2008 (7.1% vs. 7.1%, sHR = 0.98, 95% CI: 0.84–1.15). Using nation-wide 10-year follow-up data, we document that the incidences of late recurrence and metachronous CRC are low and decreasing from 2004 to 2013. Thus, despite increasing numbers of long-term cancer survivors, the data do not advocate for extending CRC-specific surveillance beyond 5 years.
Graphical Abstract
What's new?
Recent advancements in colorectal cancer (CRC) management have reduced the risk of late recurrence and metachronous CRC 5–10 years after curative surgery for non-metastatic CRC—a timeframe with no surveillance according to current guidelines. Despite more patients are cancer-free survivors at the 5-year mark, late events remain uncommon. Our study does not support extending current surveillance protocols, as late recurrence or metachronous CRC don't impact more patients than late second non-CRC primary cancers.
Abbreviations
-
- ASCO
-
- American Society of Clinical Oncology
-
- CIF
-
- cumulative incidence function
-
- CRC
-
- colorectal cancer
-
- DCCG
-
- Danish Colorectal Cancer Group
-
- DCR
-
- Danish Cancer Registry
-
- DNPR
-
- Danish National Patient Registry
-
- DPR
-
- Danish Pathology Registry
-
- ESMO
-
- European Society for Medical Oncology
-
- ICD-10
-
- International Classification of Diseases 10th Revision
-
- NMSC
-
- non-melanoma skin cancer
-
- OS
-
- overall survival
-
- sHR
-
- subdistribution hazard ratio
-
- SNOMED
-
- systematized nomenclature of medicine
-
- STROBE
-
- strengthening the reporting of observational studies in epidemiology
-
- UICC
-
- Union for International Cancer Control
-
- 95%CI
-
- 95% interval of confidence
1 INTRODUCTION
Colorectal cancer (CRC) is ranked third in global incidence and second in cancer-specific mortality worldwide.1 Death from CRC is primarily associated with disease recurrence. Despite curative-intended treatment, every fifth patient with stage I–III disease will develop recurrence with >80% of the recurrences diagnosed within 3 years of primary surgery.2, 3 Correspondingly, international guidelines recommend 5 years of postoperative surveillance to diagnose recurrence and metachronous CRC.4, 5
Over the last two decades, many countries have introduced different initiatives to reduce CRC recurrence and improve survival.6 Improvements in recurrence and survival within 5 years following primary treatment has been reported following the implementation of these initiatives.3 Furthermore, more patients are diagnosed with CRC at an earlier disease stage,7 and the life expectancy in the general population is increasing.8 Consequently, more patients are recurrence-free survivors at the end of standardized 5-year surveillance. Hence a higher proportion of CRC patients become at risk of late recurrence as well as metachronous CRC and other second primary cancers during a timeframe with no surveillance. Previous studies have reported late CRC recurrence (here defined as recurrence >5 years after primary resection) to occur in 1%–6% of patients treated from 1985 to 2006.9-11 However, new and preferably nationwide studies are needed to assess the risk of late recurrence following modern treatment of CRC. Also, the burden of late metachronous CRC and second primary cancers should be taken into account when considering prolonged surveillance specific for CRC late recurrence. If the incidence of late recurrence does not exceed that of second primary cancers, increased health costs to prolong surveillance specific for CRC recurrence may not be justified.
The aim of this study is to determine the incidence of late recurrence, metachronous CRC, and second primary cancer in a nationwide cohort of stage I-III CRC patients who underwent surgical resection with curative intent. Late recurrence, metachronous CRC and second primary cancer was defined as events occurring after completed postoperative surveillance 5 years after surgery. Furthermore, we aimed to describe the prognosis following diagnosis of late recurrence, metachronous CRC and second primary cancer with late recurrence as reference.
2 MATERIALS AND METHODS
2.1 Design and study population
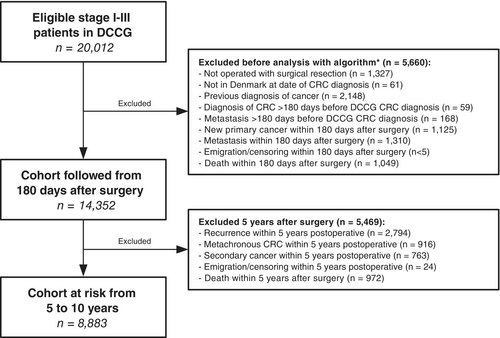
This study was conducted as a nationwide cohort study and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.12 All Danish patients younger than 80 years undergoing surgical resection of first-time Union for International Cancer Control (UICC) TNM stage I–III CRC between January 2004 and December 2013 were eligible for inclusion. Patients were identified from the Danish Colorectal Cancer Group (DCCG) database.13, 14
The cohort included all patients at risk of first time recurrence, metachronous CRC, and second primary cancers 5 years after surgery. The cohort was followed until 10 years postoperative or January 1, 2023, whichever came first.
2.2 Data sources and identification of recurrence
Individual-level data were obtained from Danish health care registries15 including the Danish Cancer Registry (DCR),16 the Danish National Patient Registry (DNPR),17 and the Danish Pathology Registry (DPR).18 Data records were linked using the unique 10-digit civil registration number assigned by the Danish Civil Registration System19 to all Danish citizens.
- International Classification of Diseases 10th Revision (ICD-10) codes of metastasis (ICD-10 DC76-DC80) in DNPR or DCR;
- ICD-10 codes of cytostatic treatment (BWHA1-2, BOHJ17 or BOHJ19B1) registered by an oncological department in DNPR 60 or more days from last cytostatic therapy code;
- Systematized Nomenclature of Medicine (SNOMED) combinations in DPR; or
- ICD-10 code specific to CRC local recurrence in DNPR (DC189X, DC209X or DC991).
The date of recurrence was defined as the earliest date on which at least one of the four indicators of the algorithm were met, and all other codes meeting an indication within the following 60 days were classified as belonging to the same recurrence event, acknowledging that there is some delay in getting the diagnostic workup of the recurrence registered in the national registries.
2.3 Definition of metachronous CRC
- An ICD-10 code of CRC (DC18 or DC20) registered in the DCR;
- Recurrence as defined by the algorithm followed by an ICD-10 code of CRC (DC18 or DC20) registered in DCR within 60 days from date of recurrence (meaning the recurrence was reclassified from CRC recurrence to metachronous CRC); or
-
A mapping of the remaining bowel at time of recurrence for each patient was constructed from surgical procedure codes in the DNPR (available from 1996 and onwards, Table S1). Recurrence as defined by the algorithm was reclassified from CRC recurrence to metachronous CRC in the following scenarios based on SNOMED:
- If specimens registered in the DPR contained adenocarcinoma with SNOMED topography code corresponding to colon or rectum (without P30755; “supplementary analysis on previously assessed material”) in a remaining bowel segment at time of recurrence, or
- If specimens registered in the DPR contained adenocarcinoma with SNOMED topography code corresponding to colon or rectum (without P30755) within 2 weeks after a procedure involving polypectomy, biopsy or mucosal resection.
2.4 Definition of second primary cancer
Second primary cancer was defined as ICD-10 code other than CRC (DC18 or DC20) or non-melanoma skin cancer (NMSC; DC44) registered in the DCR. Furthermore, recurrence as defined by the algorithm (other than DC189X, DC209X or DC991) was reclassified from CRC recurrence to secondary primary cancer if a secondary primary cancer was registered in DCR within 60 days after the date of recurrence.
2.5 Studied covariates
Eligible patients were categorized by sex, age-group at surgery or event (<50, 50–59, 60–69, 70–79 or ≥80 years old), UICC stage of the primary CRC (I, II or III according to UICC TNM classification 5th edition22), Charlson comorbidity score (0, 1, 2 or ≥3),23 location of primary CRC tumor (colon and rectum), and surgical priority (elective or emergency). Time of primary treatment was grouped in two 5-year calendar periods (2004–2008 and 2009–2013).
2.6 Statistical method
Patients were followed from 5 years after primary surgery until date of recurrence (event), metachronous CRC (event), second primary cancer other than NMSC (event), death (competing event), emigration, or end of follow-up (January 1st 2023) whichever came first.
Descriptive characteristics were presented for all patients at risk 5 years after primary surgery stratified by calendar period of CRC surgery.
Risk of late recurrence, metachronous CRC, and second primary cancer were presented as a 5–10-year cumulative incidence function (CIF), treating death as a competing event. Cumulative incidence curves were constructed using the Aalen-Johansen estimator for visualization. The association between calendar period of primary treatment and the risk of late recurrence, metachronous CRC and second primary cancer was estimated using the Fine-Gray competing risk method treating death as a competing event, and was reported as subdistribution hazard ratios (sHR) with 95% interval of confidence (95%CI).24 Both a univariate analysis and a multivariable analysis adjusted for all covariates were calculated using sHR.
To evaluate the overall survival (OS) following late events (recurrence, metachronous CRC, and second primary cancer), the patients who developed late recurrence, metachronous CRC or second primary cancer between 5 and 10 years postoperative were followed from the time of event and until death, 5 years, or end of follow-up (January 1, 2023) whichever came first. The OS was plotted for visualization using the Kaplan–Meier method. Cox proportional hazards regression was used to compare mortality following metachronous CRC and second primary cancer with late recurrence as reference. These analyses were adjusted for age at event, sex and comorbidity at event.
Analyses were performed using R version 4.3.1 (https://www.r-project.org/) between January and October 2023. No hypothesis testing was executed in this observational study.
3 RESULTS
We identified 20,012 patients undergoing surgery for first-time UICC TNM stage I–III CRC between 2004 and 2013 in the DCCG database. Of these, 8883 were alive and event-free 5 years after surgery, and therefore at risk of a late event, Figure 1. Characteristics of the cohort are provided in Table 1.
| Variable | Overall N = 8883a | Calendar period of CRC surgery | |
|---|---|---|---|
| 2004–2008 N = 4136 | 2009–2013 N = 4747 | ||
| Gender, n (%) | |||
| Female | 4122 (46%) | 1934 (47%) | 2188 (46%) |
| Male | 4761 (54%) | 2202 (53%) | 2559 (54%) |
| Age group at CRC surgery, n (%) | |||
| <50 | 648 (7%) | 304 (7%) | 344 (7%) |
| 50–59 | 1482 (17%) | 737 (18%) | 745 (16%) |
| 60–69 | 3442 (39%) | 1574 (38%) | 1868 (39%) |
| 70–79 | 3311 (37%) | 1521 (37%) | 1790 (38%) |
| Charlson comorbidity index score at CRC surgery, n (%) | |||
| CCI 0 | 6406 (72%) | 3049 (74%) | 3357 (71%) |
| CCI 1 | 1717 (19%) | 756 (18%) | 961 (20%) |
| CCI 2 | 415 (4.7%) | 177 (4%) | 238 (5%) |
| CCI ≥ 3 | 345 (3.9%) | 154 (4%) | 191 (4%) |
| CRC tumor location, n (%) | |||
| Colon | 5691 (64%) | 2600 (63%) | 3091 (65%) |
| Rectum | 3192 (36%) | 1536 (37%) | 1656 (35%) |
| UICC stage, n (%) | |||
| I | 1961 (22%) | 890 (22%) | 1071 (23%) |
| II | 4078 (46%) | 1927 (47%) | 2151 (45%) |
| III | 2844 (32%) | 1319 (32%) | 1525 (32%) |
| Surgical priority, n (%) | |||
| Elective | 8447 (95%) | 3913 (95%) | 4534 (96%) |
| Emergency | 434 (5%) | 222 (5%) | 212 (4%) |
| Adjuvant chemotherapy, n (%) | 2414 (27%) | 829 (20%) | 1585 (33%) |
| Neoadjuvant therapy,b n (%) | 1022 (12%) | 541 (13%) | 481 (10%) |
- Abbreviation: CRC, colorectal cancer.
- a Including both chemo- and/or radiotherapy.
- b n (%).
3.1 Risk of late recurrence and metachronous CRC
Among the 8883 included patients, late recurrence occurred in 370 patients (305 [82.4%] histologically verified in the DPR) and metachronous CRC in 270 patients (265 [98.1%] histologically verified in the DPR). The recurrences were categorized as local recurrence in 18 patients (4.9% of recurrences).
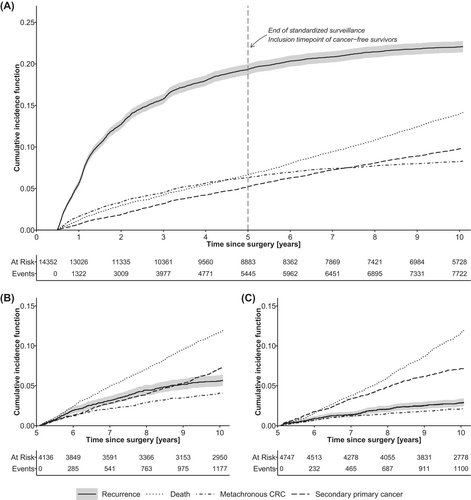
Between years 5 and 10, the CIF of late recurrence was 4.1% (95%CI: 3.7%–4.6%), Figure 2A. The corresponding 5–10 year CIF of metachronous CRC was 3.0% (95%CI: 2.7%–3.4%).
Specific CIF for late recurrence, metachronous CRC, and second primary cancers according to clinicopathological characteristics are presented in Table 2.
| Characteristic | Late recurrence | Metachronous CRC | Second primary cancers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative incidencea | Univariate analysisa | Multivariable analysisa | Cumulative incidenceb | Univariate analysisb | Multivariable analysisb | Cumulative incidencec | Univariate analysisc | Multivariable analysisc | |||||||
| 5–10 year CIF | sHR | CI | sHR | CI | 5–10 year CIF | sHR | CI | sHR | CI | 5–10 year CIF | sHR | CI | sHR | CI | |
| Exposure variable | |||||||||||||||
| Calendar period of CRC surgery | |||||||||||||||
| 2004–2008 | 5.6% (4.9%, 6.3%) | 1 | Reference | 1 | Reference | 4.1% (3.5%, 4.7%) | 1 | Reference | 1 | Reference | 7.1% (6.4%, 8.0%) | 1 | Reference | 1 | Reference |
| 2009–2013 | 2.9% (2.4%, 3.4%) | 0.50 | 0.40, 0.62 | 0.52 | 0.42, 0.64 | 2.1% (1.7%, 2.5%) | 0.52 | 0.41, 0.67 | 0.50 | 0.39, 0.65 | 7.1% (6.4%, 7.9%) | 0.99 | 0.85, 1.16 | 0.98 | 0.84, 1.15 |
| Confounder variables | |||||||||||||||
| Gender | |||||||||||||||
| Female | 4.0% (3.5%, 4.7%) | 1 | Reference | 1 | Reference | 2.9% (2.5%, 3.5%) | 1 | Reference | 1 | Reference | 6.4% (5.7%, 7.2%) | 1 | Reference | 1 | Reference |
| Male | 4.2% (3.7%, 4.8%) | 1.08 | 0.88, 1.32 | 1.07 | 0.87, 1.32 | 3.1% (2.7%, 3.6%) | 1.04 | 0.82, 1.32 | 1.05 | 0.83, 1.34 | 7.8% (7.1%, 8.6%) | 1.20 | 1.03, 1.41 | 1.21 | 1.03, 1.42 |
| Age group at CRC surgery | |||||||||||||||
| <50 | 5.1% (3.6%, 7.0%) | 1 | Reference | 1 | Reference | 3.9% (2.6%, 5.6%) | 1 | Reference | 1 | Reference | 3.5% (2.2%, 5.1%) | 1 | Reference | 1 | Reference |
| 50–59 | 4.8% (3.8%, 5.9%) | 0.94 | 0.62, 1.42 | 0.92 | 0.61, 1.40 | 2.7% (2.0%, 3.7%) | 0.70 | 0.42, 1.15 | 0.70 | 0.42, 1.16 | 5.4% (4.3%, 6.7%) | 1.58 | 0.99, 2.53 | 1.58 | 0.99, 2.53 |
| 60–69 | 4.0% (3.4%, 4.7%) | 0.79 | 0.54, 1.16 | 0.79 | 0.54, 1.16 | 2.9% (2.4%, 3.5%) | 0.76 | 0.49, 1.17 | 0.78 | 0.50, 1.20 | 8.0% (7.1%, 8.9%) | 2.40 | 1.55, 3.70 | 2.34 | 1.52, 3.61 |
| 70–79 | 3.8% (3.2%, 4.5%) | 0.74 | 0.50, 1.09 | 0.72 | 0.48, 1.08 | 3.1% (2.6%, 3.8%) | 0.81 | 0.52, 1.25 | 0.84 | 0.53, 1.31 | 7.8% (6.9%, 8.8%) | 2.37 | 1.53, 3.66 | 2.27 | 1.47, 3.52 |
| Charlson comorbidity index score at CRC surgery | |||||||||||||||
| CCI 0 | 4.1% (3.6%, 4.6%) | 1 | Reference | 1 | Reference | 3.1% (2.7%, 3.6%) | 1 | Reference | 1 | Reference | 6.8% (6.2%, 7.5%) | 1 | Reference | 1 | Reference |
| CCI 1 | 4.3% (3.4%, 5.4%) | 1.04 | 0.81, 1.35 | 1.11 | 0.86, 1.45 | 2.5% (1.9%, 3.4%) | 0.84 | 0.61, 1.17 | 0.87 | 0.62, 1.20 | 7.9% (6.7%, 9.2%) | 1.16 | 0.96, 1.41 | 1.07 | 0.88, 1.30 |
| CCI 2 | 4.6% (2.9%, 6.9%) | 1.12 | 0.70, 1.78 | 1.24 | 0.77, 2.00 | 3.0% (1.6%, 5.1%) | 0.94 | 0.52, 1.67 | 0.97 | 0.54, 1.76 | 10% (7.4%, 13%) | 1.49 | 1.08, 2.05 | 1.32 | 0.95, 1.83 |
| CCI ≥ 3 | 3.5% (1.9%, 5.9%) | 0.84 | 0.47, 1.50 | 0.89 | 0.49, 1.62 | 4.4% (2.6%, 6.9%) | 1.43 | 0.84, 2.41 | 1.47 | 0.86, 2.51 | 6.8% (4.4%, 9.8%) | 0.99 | 0.65, 1.50 | 0.87 | 0.57, 1.33 |
| CRC tumor location | |||||||||||||||
| Colon | 4.0% (3.5%, 4.5%) | 1 | Reference | 1 | Reference | 3.2% (2.8%, 3.7%) | 1 | Reference | 1 | Reference | 7.5% (6.9%, 8.3%) | 1 | Reference | 1 | Reference |
| Rectum | 4.4% (3.8%, 5.2%) | 1.11 | 0.90, 1.37 | 1.02 | 0.82, 1.28 | 2.7% (2.2%, 3.4%) | 0.84 | 0.65, 1.09 | 0.88 | 0.68, 1.16 | 6.5% (5.7%, 7.4%) | 0.88 | 0.74, 1.03 | 0.86 | 0.73, 1.03 |
| UICC stage | |||||||||||||||
| I | 3.0% (2.3%, 3.8%) | 1 | Reference | 1 | Reference | 2.6% (1.9%, 3.3%) | 1 | Reference | 1 | Reference | 7.5% (6.3%, 8.7%) | 1 | Reference | 1 | Reference |
| II | 4.1% (3.5%, 4.7%) | 1.34 | 1.00, 1.80 | 1.38 | 1.02, 1.87 | 3.2% (2.7%, 3.7%) | 1.25 | 0.90, 1.73 | 1.17 | 0.85, 1.63 | 7.2% (6.4%, 8.0%) | 0.95 | 0.78, 1.16 | 0.92 | 0.75, 1.13 |
| III | 5.0% (4.2%, 5.8%) | 1.66 | 1.23, 2.24 | 1.93 | 1.38, 2.71 | 3.2% (2.6%, 3.9%) | 1.25 | 0.88, 1.76 | 1.05 | 0.70, 1.56 | 7.0% (6.1%, 8.0%) | 0.93 | 0.75, 1.15 | 0.96 | 0.74, 1.25 |
| Adjuvant chemotherapy | |||||||||||||||
| No | 4.3% (3.8%, 4.8%) | 1 | Reference | 1 | Reference | 2.9% (2.5%, 3.4%) | 1 | Reference | 1 | Reference | 7.3% (6.7%, 7.9%) | 1 | Reference | 1 | Reference |
| Yes | 3.8% (3.1%, 4.6%) | 0.90 | 0.71, 1.14 | 0.75 | 0.56, 1.01 | 3.3% (2.6%, 4.1%) | 1.14 | 0.88, 1.48 | 1.29 | 0.92, 1.81 | 6.9% (5.9%, 7.9%) | 0.93 | 0.78, 1.11 | 0.95 | 0.75, 1.20 |
- Abbreviations: CI, confidence interval; CIF, cumulative incidence function; sHR, subdistribution hazard ratio.
- a 370 events.
- b 270 events.
- c 635 events.
The 5–10 year CIF of late recurrence decreased over time from 5.6% (95%CI: 4.9%–6.3%) in patients operated in 2004–2008 to 2.9% (95%CI: 2.4–3.4%) in 2009–2013, Table 2. Thus, estimates of the Fine-Grey regression adjusted for confounding covariates showed that the risk of late recurrence was reduced for patients operated in 2009–2013 (Figure 2B) compared to 2004–2008 (Figure 2C, adjusted sHR = 0.52, 95% CI: 0.42–0.64). For metachronous CRC the corresponding 5–10 year hazard in 2009–2013 compared to 2004–2008 patients also displayed a decrease in the adjusted analysis (adjusted sHR = 0.50, 95%CI: 0.39–0.65) as the 5–10 year CIF decreased from 4.1% (95%CI: 3.5%–4.7%) in 2004–2008 to 2.1% (95%CI: 1.7–2.5%) in 2009–2013.
3.2 Second primary cancers 5–10 years after surgical resection of CRC
Among the 8883 included patients, 635 patients were diagnosed with a second primary cancer (CIF = 7.2%, 95%CI: 6.7%–7.7%). Histological verification was achieved in 611 (96.2%) of the cases. The most frequently occurring second primary cancers in women (n = 267) were breast cancer (34%) and lung cancer (20%), Figure S1. Correspondingly, the most frequent second primary cancers in men (n = 368) were prostate cancer (31%) and lung cancer (17%).
In adjusted Fine-Gray regression, the risk of second primary cancer was similar in 2009–2013 compared to 2004–2008 with 5–10 year CIFs of 7.1% (6.4%–7.9%) vs. 7.1% (6.4%–8.0%) resulting in an adjusted sHR of 0.98 (95% CI: 0.84–1.15).
3.3 Overall survival following late events
Characteristics of the patients who developed late recurrence, metachronous CRC or second primary cancer between 5 and 10 years postoperative are provided in Table S2.
Of the 370 patients with late recurrence, 192 died within 5 years of diagnosis, Table 3. Correspondingly, among the 270 metachronous CRC patients, 72 died within 5 years, and among the 635 patients with secondary primary cancers, 316 died within 5 years.
| Characteristic | No. of patients | PY | No. of deaths | 5-years OS | Crude HRs | Adjusted HRs | ||
|---|---|---|---|---|---|---|---|---|
| HR | CI | aHRa | CI | |||||
| Event | ||||||||
| Late recurrence | 370 | 1050 | 192 | 46% (41%, 52%) | 1 | Reference | 1 | Reference |
| Metachronous CRC | 270 | 1040 | 72 | 72% (66%, 78%) | 0.41 | 0.31, 0.53 | 0.37 | 0.28, 0.49 |
| Second primary cancer | 635 | 1864 | 316 | 48% (44%, 52%) | 0.92 | 0.77, 1.10 | 0.78 | 0.65, 0.93 |
- Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; OS, overall survival; PY, person years at risk from event.
- a Adjusted for gender, age at event, and Charlson Comorbidity Index Score at diagnosis of late event.
The 5-year OS was higher following metachronous CRC (5-year OS = 72%, 95% CI: 66%–78%) compared to late recurrence (5-year OS = 46%, 95% CI: 41%–52%), with an adjusted HR of 0.37 (95% CI: 0.28–0.49), Table 3 and Figure S2. In comparison, the 5-year OS was 48% (95% CI: 44%–52%) following second primary cancer, with an adjusted HR of 0.78 (95% CI: 0.65–0.93) compared to patients diagnosed with late recurrence.
4 DISCUSSION
International guidelines recommend 5 years of recurrence surveillance after curative intent treatment for CRC.4, 5 However, over the last decades the fraction of CRC patients who are cancer-free survivors at 5 years has been continuously increasing,3 which made us raise the question if extended recurrence surveillance beyond 5 years is indicated. To address this, we have investigated the risk of late events including late recurrence, metachronous CRC and second primary cancer after 5-years of surveillance in a nationwide, contemporary cohort of CRC patients with 10 years of follow-up. In patients operated from 2004 to 2013 the risk of late recurrence from CRC was low, affecting approximately one in 25 patients. Furthermore, the risk of recurrence was nearly halved for patients operated during 2009–2013 compared to 2004–2008. An older French cohort (1985–2000) reported higher rates of late recurrence, affecting one of 12 colon cancer patients and one of 13 rectal cancer patients.10, 11 Similar to our findings, they report decreasing rates of late recurrence over time. Thus, the lower and decreasing rates of late recurrence observed in our contemporary cohort indicate a continued decrease during recent decades.
Second primary cancers are common following primary CRC and have been found to affect up to 8.6% of the patients, with 20% of the cancers being metachronous CRC.25, 26 In our cohort, late occurring metachronous CRC (5–10 years after primary CRC) was observed for 3.0% of patients. A bit surprising, the incidence of late occurring metachronous CRC decreased from 2004 to 2013 and affected only 2.1% of patients operated from 2009 to 2013. We speculate if the lowered risk of metachronous CRC is due to increased focus on adenoma removal and updated recommendations to include colonoscopies in standardized postoperative surveillance within 5 years after primary surgery.4, 27, 28 Excluding late metachronous CRC, we report late occurring second primary cancers to affect 7.2% of the patients, and we found second primary cancers to occur at cancer sites similar to what has been reported by others.26 In contrast to late recurrence and late metachronous CRC the risk of late second primary cancers did not change during our study period.
The American Society of Clinical Oncology (ASCO) endorsement of Cancer Care Ontario guidelines29 recommend a surveillance colonoscopy approximately 1 year postoperative and repeated every 5 years to identify metachronous CRC. Similarly, European Society for Medical Oncology (ESMO) guidelines4, 5 recommend a colonoscopy at 1 year postoperative, and thereafter every 3 to 5 years. Consequently, colonoscopy is the only current surveillance modality, which extend beyond 5 years. However, as we show that the risk of late metachronous CRC is only 2.1% for 2009–2013 patients with a flattening cumulative incidence curve from 5 to 10 years, we speculate if long-term endoscopic follow-up is at all justified if the colon and rectum appears normal 5 years after surgery.30 Also given that our survival analysis showed the survival associated with late metachronous CRC was high with a 5-year overall survival of 72%.
Strengths of the current study include its design and the nationwide cohort, reflecting the CRC management at a national level and not the performance of individual sites or clinical trials. A study demanding a minimum of 5 and preferably 10 years of follow-up requires a design with consistent registration of exposures and patient characteristics, and consistent identification of outcomes throughout the study period. This is possible in Denmark due to our extensive health care registries. In this study, we identified recurrence using an algorithm validated on Danish health care registry data from 2001 throughout 2018. Previous validations have shown great performance in identifying recurrence.21 However, the algorithm as originally designed, classifies all events as recurrence and does not distinguish between recurrence and metachronous CRC. Misclassification of recurrence as metachronous CRC and vice versa might therefore impact our findings. We took precautions to limit misclassification by mapping the remaining segments of the colon and rectum for each patient to be able to see if the recurrence (as defined by the algorithm) was in a segment not previously resected at time of recurrence. In addition, if there is any misclassification at all, we expect it to affect the 2004–2008 and the 2009–2013 periods similarly. Another limitation of our approach is our ability to distinguish between distant and local recurrence. We report lower rates of local recurrence compared to other studies,10, 11 and therefore refrained from performing local recurrence specific analyses. Finally, we chose a maximum age of 80 years at surgery to limit the potential detection bias among elderly patients, as they are not always offered recurrence surveillance according to guidelines.
In conclusion, our findings do not advocate for extending CRC-specific recurrence surveillance beyond 5 years. The burden of late recurrence and metachronous CRC has decreased to a low level, while the burden of second primary cancers has remained unchanged. Thus, symptoms or suspicion of a cancer occurring 5–10 years from primary CRC treatment, is more likely to represent a non-CRC cancer (7.1%), than late recurrence (2.9%) or metachronous CRC (2.1%). Furthermore, the survival following second primary cancers were comparable to the post-recurrence survival.
AUTHOR CONTRIBUTIONS
All the authors made significant contributions to the completion of this study. Jesper Nors: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, visualization, project administration. Kåre Andersson Gotschalck: conceptualization, methodology, investigation, writing—review & editing, supervision. Rune Erichsen: conceptualization, methodology, writing—review & editing, supervision. Claus Lindbjerg Andersen: conceptualization, investigation, resources, data curation, writing—review & editing, supervision, project administration, funding acquisition. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission. Jesper Nors and Claus Lindbjerg Andersen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENT
We would like to extend our gratitude to the Danish Colorectal Cancer Group (DCCG) for providing data from the national colorectal cancer database, and to CONNECT at Aarhus University Hospital for assistance in applying for data from the Danish Health Data Authority.
FUNDING INFORMATION
This study was funded by a PhD scholarship from Institute of Clinical Medicine, Aarhus University, Denmark [Nors] and support from the Novo Nordisk Foundation [grant number NNF17OC0025052 (Andersen), NNF22OC0074415 (Andersen)], Innovation Fund Denmark [grant number 9068-00046B (Andersen)] and the Danish Cancer Society [grant numbers R231-A13845, R257-A14700 (Andersen)]. The funding organizations and entities listed above had no influence on the study's design, data collection, analysis, interpretation of the data, manuscript preparation and review, or decision to submit for publication.
CONFLICT OF INTEREST
The author(s) have no affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the study subject or materials discussed in the manuscript.
ETHICS STATEMENT
This study was approved by the Danish Colorectal Cancer Group and the Danish Data Protection Agency (Central Denmark Region, J. no. 1-16-02-274-19) and adheres to the General Data Protection Regulations. The study is based on anonymized registry data and therefore does not require approval from the National Research Ethics Committee.
Open Research
DATA AVAILABILITY STATEMENT
The data and code for data cleaning and analysis that support the findings of this study are available from Danish Health care registries and upon reasonable collaboration with a Danish Research Institution. Please see https://sundhedsdatastyrelsen.dk/da/english/health_data_and_registers/research_services for further details. Further information is available from the corresponding author upon request.