Association of antidepressant drug use with outcome of patients with glioblastoma
Funding information: EORTC Brain Tumor Group
Abstract
Depressive symptoms are common among patients with glioblastoma, but patients are often not treated with antidepressants. There is only limited evidence on the association of antidepressant drug use with survival in glioblastoma. We performed a pooled analysis of patients treated within the CENTRIC, CORE, AVAglio and ACT-IV trials to explore the relation of antidepressant drug use with progression-free (PFS) and overall survival (OS) at baseline, at the start of maintenance therapy and at the start of maintenance cycle 4. We further assessed the association of antidepressant drugs with seizure, cognition, fatigue and a diagnosis of depression. Among more than 1700 patients, we found no significant association between the use of antidepressants at baseline or at the start of maintenance therapy and PFS or OS. However, we found OS, but not PFS, to be significantly worse in patients using antidepressants at the start of maintenance cycle 4. After adjustment for antiepileptic drug use and despite showing a trend for increased risk, seizures were not significantly associated with antidepressant drug use, nor was there a change in mini mental state examination (MMSE) scores or fatigue by antidepressant drug use at baseline. However, there was a significant positive association between antidepressant use at the start of maintenance treatment and fatigue during maintenance treatment. The association of antidepressant use at the start of maintenance cycle 4 with inferior OS of glioblastoma patients requires independent confirmation and further study. Further prospective trials should evaluate efficacy, side effects and associations with outcome of antidepressants in glioblastoma.
Graphical Abstract
What's new?
Depressive symptoms are common yet often undertreated in patients with glioblastoma, in part due to limited evidence on the association of antidepressant drug use with survival. Among 1,700 patients with glioblastoma derived from clinical trials, the authors found no significant association between progression-free or overall survival and the use of antidepressant drugs at baseline or at the start of maintenance therapy. While overall survival was worse at the start of maintenance cycle 4 in patients using antidepressant drugs, progression-free survival was not significantly changed. The findings suggest that antidepressants should not be withheld from patients with glioblastoma.
Abbreviations
-
- ADP
-
- antidepressant drug
-
- AED
-
- antiepileptic drug
-
- Akt
-
- protein kinase B
-
- CI
-
- confidence interval
-
- GB
-
- glioblastoma
-
- HR
-
- hazard ratio
-
- IDO
-
- indoleamine 2,3-dioxygenase
-
- MGMT
-
- O-6-methylguanine-DNA methyltransferase
-
- MMSE
-
- mini mental state examination
-
- mTOR
-
- mammalian target of rapamycin
-
- n
-
- number
-
- OS
-
- overall survival
-
- PFS
-
- progression-free survival
-
- PH
-
- proportional hazard
-
- PI3K
-
- Phosphatidylinositol 3-kinase
-
- PS
-
- performance status
-
- SSRI/SNRI
-
- selective serotonin and norepinephrine reuptake inhibitors
-
- TDO
-
- tryptophan-2,3-dioxygenase
-
- TMZ
-
- temozolomide
1 INTRODUCTION
Depressive symptoms are common among patients with gliomas and they have been associated with inferior survival.1 Patients with glioma may receive antidepressants less frequently than the general population according to a study from Norway,2 but it is unclear, if this may be translated to other countries as well. Challenges in the pharmacological treatment of depression in glioma patients may be difficulties to diagnose depression because of tumor symptoms, an uncertainty whether antidepressants are active in these patients, whether their use has adverse effects on seizure frequency, fatigue and cognition, or whether they interact with antiepileptic drugs or chemotherapy.3-6 Conversely, there is also an assumption that these drugs may positively affect outcome.7-9
Glioblastomas are WHO grade 4 gliomas with poor prognosis.10 Despite intensive therapy including surgery, concurrent radio-chemotherapy with temozolomide (TMZ) and maintenance chemotherapy with TMZ with or without the addition of tumor treating fields median overall survival of patients with glioblastoma ranges only between 15 and 26 months in clinical trials, and outcome is almost universally fatal.11, 12
Several preclinical studies have proposed antineoplastic effects of antidepressants, including tricyclic antidepressants, selective monoamine reuptake inhibitors and others. Tricyclic antidepressants and citalopram are proposed to modulate potassium channels in glioblastoma cells.7 Glioblastoma cells express specific subtypes of these channels such as the Kv10.1 subtype8 which may impact proliferation and apoptosis.13 Furthermore, tricyclic antidepressants may inhibit the PI3K (Phosphatidylinositol 3-kinase)/Akt (protein kinase B)/mTOR (mammalian target of rapamycin) signaling pathway14 and reduce mitochondrial respiration,15, 16 leading to increased autophagy.14, 17 Interestingly, interference with tryptophan metabolism, which is one major mode of action of antidepressant drugs,18 can also lead to immune-modulatory effects mediated by tryptophan-2,3-dioxygenase (TDO)19 or indoleamine 2,3-dioxygenase (IDO).20 Furthermore, various other potential mechanisms of antitumor activity have been proposed for particular antidepressants, such as the disruption of actin polymerization for fluvoxamine9 or the blocking of acid sphingomyelinase for fluoxetine.21
Only few previous studies reported on survival of glioblastoma patients adjusted for the use of antidepressants.4, 6 Therefore, this study aimed at exploring the association of antidepressant drug use (at baseline, at the start of maintenance treatment and at the start of maintenance cycle 4) with outcome (progression-free (PFS) and overall survival (OS)) in newly diagnosed glioblastoma patients.
2 PATIENTS AND METHODS
2.1 Data source and study population
The patient population included randomized patients from the control and experimental arms of the CENTRIC (NCT00689221; n = 545),11 the CORE (NCT00813943; n = 265),22 AVAGlio (NCT00943826; n = 921),23 and ACT-IV (NCT01480479; n = 745)24 trials. All four trials explored the addition of a novel agent added to standard therapy (concurrent radiochemotherapy with TMZ followed by six cycles of maintenance chemotherapy with TMZ). The study was conducted according to the STROBE guidelines.
2.2 Exposures
2.2.1 Antidepressant drug use
Use of antidepressant drugs was classified by pharmacological groups as tricyclic vs selective monoamine reuptake inhibitors including selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, selective serotonin and norepinephrine reuptake inhibitors (SSRI/SNRI/SSNRI) vs others. Tricyclic antidepressants included doxepin, amitriptyline, imipramine, clomipramine, desipramine, nortriptyline, trimipramine and opipramol. Selective monoamine reuptake inhibitors included citalopram, escitalopram, sertraline, paroxetine, fluoxetine, fluvoxamine, venlafaxine, duloxetine, reboxetine and milnacipran. Other antidepressants included tranylcypromine, moclobemide, mianserin, mirtazapine, maprotiline, lithium, agomelatine, bupropion, trazodone or tianeptine.
Baseline antidepressant use was defined as use at randomization or within 14 days prior to randomization and before starting concomitant treatment. It was right truncated at date of the first TMZ treatment dose if its use continued afterwards, and in addition, left truncated at the date of glioblastoma (GB) diagnosis if antidepressant use had started at an earlier date and had not stopped before the date of randomization minus 2 weeks. Antidepressant use at the start of maintenance treatment was defined as antidepressant use within 28 days before the start of maintenance TMZ treatment. This was right truncated at the start of the maintenance TMZ and left truncated at the date of the first concomitant TMZ treatment if its use had started before the start of concomitant TMZ. Correspondingly, antidepressant use at the start of maintenance cycle 4 was defined as antidepressant use within 28 days before the start of maintenance treatment cycle 4. Again, antidepressant use at the start of maintenance cycle 4 was right truncated at the start of maintenance cycle 4 and left truncated at the date of the first maintenance TMZ treatment if its use had started before the start of maintenance TMZ.
2.3 Statistical analysis
Landmark analysis approach25 was used to analyze the association of antidepressant use with outcome of patients with glioblastoma. Thus, antidepressant use was assessed at three different fixed time points—at baseline, at the start of maintenance TMZ treatment and at the start of maintenance cycle 4. Kaplan-Meier survival plots and stratified log-rank tests (by trial) were used to assess the association of antidepressant use at the three fixed time points with PFS and OS. At each time point, patients who were alive and free of progression were included in the PFS analyses, whereas all patients alive at each time point were included in the OS analyses. Patients from ACT-IV were not included in the analysis that assessed the association of baseline antidepressant use (antidepressant use prior to the start of concomitant TMZ treatment) with outcome. This is because ACT-IV enrolled patients that had already completed standard radiotherapy with concomitant TMZ at inclusion, whereas the other three trials enrolled patients prior to the start of standard radiotherapy with concomitant TMZ. PFS was calculated as the number of days from the date of randomization, date of start of maintenance treatment, or date of start of maintenance cycle 4 (for baseline, start of maintenance, or start of cycle 4, respectively) up to the date of progression or death from any cause, whichever comes first. In case a patient was alive and without progression, PFS was censored at the date of last disease assessment. OS was calculated accordingly, but only up to the date of death from any cause and not up to the date of progression. For patients still alive or lost to follow-up, OS was censored at the date last known to be alive.
Cox proportional hazard (PH) models were used to estimate the association of baseline antidepressant use, antidepressant use at the start of maintenance treatment, and antidepressant use at the start of maintenance cycle 4 with PFS or OS. Each model (separate models for PFS and OS) was stratified by trial (CENTRIC and CORE together, AVAglio and ACT-IV) and was adjusted for the following prognostic factors assessed at baseline (for baseline antidepressant use) and at the start of maintenance TMZ treatment assessed within 28 days of the start of maintenance TMZ treatment (for analysis at the start of maintenance treatment and analysis at the start of maintenance cycle 4): age (<55 years vs ≥55 years), sex (male or female), WHO performance status (PS = 0 or PS > 0), steroid use (yes or no), O-6-methylguanine-DNA methyltransferase (MGMT) promotor methylation status (unmethylated, methylated or unknown), and extent of initial resection (biopsy only/partial resection or gross total resection). For each Cox regression model, the PH assumption was assessed for antidepressant use (the variable of interest) using the supremum test. Where the PH assumption was violated, the interaction of antidepressant use with time was included in the model. Missing data was included as a separate category in the multivariate model.
Overall statistical significance was established at a level of 5%, which was split into three for the main analysis: 1.7% for the baseline antidepressant analysis, 1.7% for the analysis at the start of maintenance treatment and 1.7% for the analysis at the start of maintenance cycle 4. For other association analyses, an exploratory 5% significance level was used.
The association of baseline antidepressant use with seizures (yes vs no) throughout the whole concomitant treatment phase plus 4 weeks was explored, while stratifying by baseline antiepileptic drugs (AEDs; levetiracetam, lacosamide, valproate, lamotrigine, others and combinations), using the generalized Cochran-Mantel-Haenszel Test. Chi-squared/Fishers' exact test was used to explore the association of baseline antidepressant use with fatigue (yes vs no) and with change in neurocognitive function (ie, change in Mini-Mental State Examination [MMSE] from baseline to the end of concomitant treatment, clinically significant increase in score [>3 points increase], stable score, that is [−3,3], clinically significant decrease in score [>3 points decrease]) throughout the whole concomitant treatment phase +4 weeks washout period. The association of antidepressant use at the start of maintenance TMZ treatment and time to first seizure or time to first fatigue during maintenance treatment was assessed using Fine and Gray's competing risk model,26 with progression or death before seizure or fatigue as competing events. The competing risk model for seizure was stratified by AED use at the start of maintenance treatment.
SAS version 9.4 (2002-2012 per SAS Institute Inc., Cary, NC) was used for the analysis.
3 RESULTS
3.1 Patient characteristics and antidepressant use
The study population consisted of 1731 patients at baseline and included patients enrolled in the CENTRIC (n = 545), CORE (n = 265) and AVAglio (n = 921) trial. For the analysis of OS and PFS at the start of maintenance treatment 2131 were alive and 2010 patients were alive and without progression, including 718 and 706 patients from the ACT-IV trial. At the start of maintenance cycle 4, 1600 and 1501 patients, respectively, from all four trials were included for the analysis of OS and PFS. The distribution of baseline demographic variables was similar over the three trials at baseline, and four trials at the start of maintenance treatment and at the start of maintenance treatment cycle 4 except that fewer patients in AVAglio had a performance status above 0 and that the number of patients with gross total resections was higher in ACT-IV than in the other trials. The CENTRIC trial only included patients with tumors with a methylated MGMT promoter, whereas the CORE trial only included patients with tumors with an unmethylated MGMT promoter (Tables 1 and S1).
| Trial | Total (N = 1731) | |||
|---|---|---|---|---|
| Centric (N = 545) | Core (N = 265) | Avaglio (N = 921) | ||
| N (%) | N (%) | N (%) | N (%) | |
| Sex | ||||
| Male | 291 (53.4) | 155 (58.5) | 580 (63.0) | 1026 (59.3) |
| Female | 254 (46.6) | 110 (41.5) | 341 (37.0) | 705 (40.7) |
| Age group | ||||
| <55 years | 225 (41.3) | 117 (44.2) | 372 (40.4) | 714 (41.2) |
| ≥55 years | 320 (58.7) | 148 (55.8) | 549 (59.6) | 1017 (58.8) |
| WHO performance status | ||||
| PS 0 | 309 (56.7) | 131 (49.4) | 630 (68.4) | 1070 (61.8) |
| PS >0 | 236 (43.3) | 132 (49.8) | 289 (31.4) | 657 (38.0) |
| Missing | 0 (0.0) | 2 (0.8) | 2 (0.2) | 4 (0.2) |
| MGMT promoter | ||||
| Unmethylated | 0 (0.0) | 265 (100.0) | 462 (50.2) | 727 (42.0) |
| Methylated | 545 (100.0) | 0 (0.0) | 237 (25.7) | 782 (45.2) |
| Unknown | 0 (0.0) | 0 (0.0) | 222 (24.1) | 222 (12.8) |
| Extent of surgery | ||||
| Partial resection or biopsy | 274 (50.3) | 128 (48.3) | 537 (58.3) | 939 (54.2) |
| Gross total resection | 269 (49.4) | 136 (51.3) | 384 (41.7) | 789 (45.6) |
| Missing | 2 (0.4) | 1 (0.4) | 0 (0.0) | 3 (0.2) |
| MMSE | ||||
| <27 | 106 (19.4) | 65 (24.5) | 213 (23.1) | 384 (22.2) |
| ≥27 | 432 (79.3) | 197 (74.3) | 696 (75.6) | 1325 (76.5) |
| Missing | 7 (1.3) | 3 (1.1) | 12 (1.3) | 22 (1.3) |
| Steroid use at baseline | ||||
| No | 329 (60.4) | 168 (63.4) | 522 (56.7) | 1019 (58.9) |
| Yes | 216 (39.6) | 97 (36.6) | 395 (42.9) | 708 (40.9) |
| Missing | 0 (0.0) | 0 (0.0) | 4 (0.4) | 4 (0.2) |
| Antidepressant use | ||||
| No | 503 (92.3) | 244 (92.1) | 838 (91.0) | 1585 (91.6) |
| Yes | 42 (7.7) | 21 (7.9) | 83 (9.0) | 146 (8.4) |
| Duration of antidepressant use | ||||
| Median (days) | 28 | 34 | 36 | 34 |
| Range | 1-48 | 3-53 | 1-65 | 1–65 |
| Type of antidepressant | ||||
| No antidepressants | 503 (92.3) | 244 (92.1) | 838 (91.0) | 1585 (91.6) |
| SSRI alone | 27 (5.0) | 14 (5.3) | 55 (6.0) | 96 (5.5) |
| Tricyclic alone | 4 (0.7) | 3 (1.1) | 10 (1.1) | 17 (1.0) |
| Others alone | 6 (1.1) | 4 (1.5) | 7 (0.8) | 17 (1.0) |
| Combination of antidepressants | 5 (0.9) | 0 (0.0) | 11 (1.2) | 16 (0.9) |
| Tricyclic antidepressant use | ||||
| Other antidepressants | 38 (7.0) | 18 (6.8) | 71 (7.7) | 127 (7.3) |
| Tricyclic alone or in combination | 4 (0.7) | 3 (1.1) | 12 (1.3) | 19 (1.1) |
At baseline, at the start of maintenance treatment and at the start of maintenance treatment cycle 4, the percentage of patients that used antidepressants were for both OS and PFS populations 8.4%, 9.9% and 13.6% (OS population), and 8.4%, 9.9% and 13.4% (PFS population), respectively. SSRI were the most commonly used drugs at all three timepoints (OS population: 5.5%, 6.9% and 8.8%; PFS population: 5.5%, 6.7% and 8.5%). The median duration of antidepressant use was 34 days at baseline, 74 and 71 days for the OS and PFS population, respectively, at the start of maintenance treatment and 85 days at the start of maintenance treatment cycle 4 (Tables 1 and S1). Patient characteristics by antidepressant use were comparable at all three timepoints (Tables 2 and S2).
| Antidepressant use at baseline | Antidepressant use at the start of maintenance treatment | Antidepressant use at the start of maintenance cycle 4 | ||||
|---|---|---|---|---|---|---|
| No (N = 1585) N (column %) | Yes (N = 146) N (column %) | No (N = 1919) N (column %) | Yes (N = 212) N (column %) | No (N = 1382) N (column %) | Yes (N = 218) N (column %) | |
| Sex | ||||||
| Male | 955 (60.3) | 71 (48.6) | 1220 (63.6) | 113 (53.3) | 880 (63.7) | 124 (56.9) |
| Female | 630 (39.7) | 75 (51.4) | 699 (36.4) | 99 (46.7) | 502 (36.3) | 94 (43.1) |
| Age | ||||||
| <55 years | 664 (41.9) | 50 (34.2) | 794 (41.4) | 80 (37.7) | 606 (43.8) | 91 (41.7) |
| ≥55 years | 921 (58.1) | 96 (65.8) | 1125 (58.6) | 132 (62.3) | 776 (56.2) | 127 (58.3) |
| WHO performance status | ||||||
| PS 0 | 986 (62.2) | 84 (57.5) | 1073 (55.9) | 81 (38.2) | 834 (60.3) | 89 (40.8) |
| PS >0 | 595 (37.5) | 62 (42.5) | 808 (42.1) | 124 (58.5) | 531 (38.4) | 123 (56.4) |
| Missing | 4 (0.3) | 0 (0.0) | 38 (2.0) | 7 (3.3) | 17 (1.2) | 6 (2.8) |
| MGMT promoter | ||||||
| Unmethylated | 672 (42.4) | 55 (37.7) | 927 (48.3) | 97 (45.8) | 630 (45.6) | 106 (48.6) |
| Methylated | 717 (45.2) | 65 (44.5) | 786 (41.0) | 90 (42.5) | 600 (43.4) | 91 (41.7) |
| Unknown | 196 (12.4) | 26 (17.8) | 206 (10.7) | 25 (11.8) | 152 (11.0) | 21 (9.6) |
| Extent of surgery | ||||||
| Partial resection or biopsy | 857 (54.1) | 82 (56.2) | 912 (47.5) | 107 (50.5) | 618 (44.7) | 106 (48.6) |
| Gross total resection | 725 (45.7) | 64 (43.8) | 1004 (52.3) | 105 (49.5) | 762 (55.1) | 112 (51.4) |
| Missing | 3 (0.2) | 0 (0.0) | 3 (0.2) | 0 (0.0) | 2 (0.1) | 0 (0.0) |
| MMSE | ||||||
| <27 | 348 (22.0) | 36 (24.7) | 356 (18.6) | 38 (17.9) | 236 (17.1) | 41 (18.8) |
| ≥27 | 1216 (76.7) | 109 (74.7) | 1391 (72.5) | 145 (68.4) | 1037 (75.0) | 154 (70.6) |
| Missing | 21 (1.3) | 1 (0.7) | 172 (9.0) | 29 (13.7) | 109 (7.9) | 23 (10.6) |
| Steroid use | ||||||
| No | 955 (60.3) | 64 (43.8) | 996 (51.9) | 99 (46.7) | 768 (55.6) | 99 (45.4) |
| Yes | 626 (39.5) | 82 (56.2) | 923 (48.1) | 113 (53.3) | 614 (44.4) | 119 (54.6) |
| Missing | 4 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
In all three datasets included in the baseline analysis (CENTRIC, CORE, AVAglio), depression was reported in four of 1731 patients (0.2%), compared with 146 of 1731 patients (8.4%) using antidepressants at baseline. The association of a diagnosis of depression at baseline, at the start of maintenance treatment and at the start of maintenance treatment cycle 4 with antidepressant use is presented in Table S3.
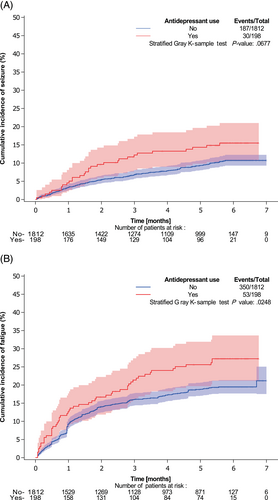
At 5% significance level, no statistically significant association was found between baseline antidepressant use and seizures during concomitant treatment, controlling for baseline antiepileptic use (Cochran-Mantel-Haenszel test: P = .177 [for type of antidepressant] and P = .052 [for antidepressant use], Table S4A). Accordingly, no significant association was found between antidepressant use at the start of maintenance treatment and seizures during maintenance treatment (P = .068, Table S4B and Figure 1A). No significant association was found between baseline antidepressant use and fatigue during concomitant treatment (Chi-square/Fishers' exact test: P = .228 [for type of antidepressant] and P = .090 [for antidepressant use, Table S5A]). The risk of fatigue during maintenance treatment was higher (P = .025) in patients who used antidepressants at the start of maintenance treatment compared with patients who did not use antidepressants (Table S5B and Figure 1B). No significant association was found between baseline antidepressant use and change in MMSE (Fishers' exact test: P = .989 [for type of antidepressant] and P = .194 [for antidepressant use], Table S6).
3.2 Antidepressant use and outcome
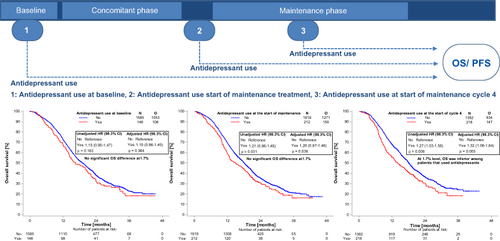
No significant association at the 1.7% level was found between baseline antidepressant use and PFS (P = .040) or OS (P = .163) in unadjusted analyses and following adjustment for important prognostic factors (PFS [P = .111] and OS [P = .364] Figure 2A, B and Table 3).
| N = 1731 | Unadjusted analysis | N = 1720 | Adjusted analysisa | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 98.3% CI | p value | HR | 98.3% CI | p value | ||
| PFS and baseline use of antidepressants | ||||||||
| ADP use | ||||||||
| No | 1585 (91.6) | 1 | 1574 (91.5) | 1 | ||||
| Yes | 146 (8.4) | 1.21 | 0.97-1.51 | 0.040 | 146 (8.5) | 1.16 | 0.93-1.45 | 0.111 |
| OS and baseline use of antidepressants | ||||||||
| ADP use | ||||||||
| No | 1585 (91.6) | 1 | 1574 (91.5) | 1 | ||||
| Yes | 146 (8.4) | 1.15 | 0.90-1.47 | 0.163 | 146 (8.5) | 1.10 | 0.86-1.40 | 0.364 |
| N = 2010 | Unadjusted analysis | N = 1965 | Adjusted analysisa | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 98.3% CI | p value | HR | 98.3% CI | p value | ||
| PFS and antidepressant use at the start of maintenance treatment | ||||||||
| ADP use | ||||||||
| No | 1812 (90.1) | 1 | 1774 (90.3) | 1 | ||||
| Yes | 198 (9.9) | 1.11 | 0.92-1.36 | 0.182 | 191 (9.7) | 1.09 | 0.90-1.33 | 0.289 |
| N = 2131 | Unadjusted analysis | N = 2083 | Adjusted analysisa | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 98.3% CI | p value | HR | 98.3% CI | p value | ||
| OS and antidepressant use at the start of maintenance treatment | ||||||||
| ADP use | ||||||||
| No | 1919 (90.1) | 1 | 1878 (90.2) | 1 | ||||
| Yes | 212 (9.9) | 1.21 | 0.98-1.48 | 0.031 | 205 (9.8) | 1.20 | 0.97-1.48 | 0.038 |
| N = 1501 | Unadjusted analysis | N = 1476 | Adjusted analysisa | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 98.3% CI | p value | HR | 98.3% CI | p value | ||
| PFS and antidepressant use at the start of maintenance cycle 4 | ||||||||
| ADP use | ||||||||
| No | 1300 (86.6) | 1 | 1281 (86.8) | 1 | ||||
| Yes | 201 (13.4) | 1.16 | 0.95-1.42 | 0.072 | 195 (13.2) | 1.16 | 0.94-1.42 | 0.085 |
| N = 1600 | Unadjusted analysis | N = 1575 | Adjusted analysisa | |||||
|---|---|---|---|---|---|---|---|---|
| N (%) | HR | 98.3% CI | p value | HR | 98.3% CI | p value | ||
| OS and antidepressant use at the start of maintenance cycle 4 | ||||||||
| ADP use | ||||||||
| No | 1382 (86.4) | 1 | 1363 (86.5) | 1 | ||||
| Yes | 218 (13.6) | 1.97 | 1.25-3.09 | <0.001 | 212 (13.5) | 1.32 | 1.06–1.64 | 0.003 |
| ADP use × time | – | 0.96b | 0.93-1.00 | 0.012 | ||||
- a Adjusted for age, sex, WHO performance status, steroid use, MGMT and extent of surgery.
- b 1 month effect.
Correspondingly, no significant association was found between antidepressant use at the start of maintenance treatment and PFS (P = .182) or OS (P = .031) in unadjusted analyses and following adjustment for important prognostic factors (PFS [P = .289] and OS [P = .038], Figure 2C, D and Table 3).
At the start of maintenance cycle 4, no significant association was found between antidepressant use and PFS in unadjusted analyses (P = .072) and adjusted analyses (P = .085). OS was inferior among patients who used antidepressants compared with those who did not use antidepressants in unadjusted analyses (P = .008, median OS in months for use vs non-use of antidepressants = 14.03; 98.3% CI = 10.94-17.58 vs 18.17; 98.3% CI = 17.18-19.19). The adjusted HR was 1.32 (98.3% CI = 1.06-1.64, P = .003) indicating a significant detrimental effect of antidepressant use (Figure 2E, F and Table 3).
Following the significant association observed between antidepressant use at the start of maintenance cycle 4 and OS, the history of antidepressant use was compared between patients who used antidepressants at the start of maintenance cycle 4 and those who also used antidepressants at baseline or at the start of maintenance treatment (Table S7A, B). The adjusted HR for OS for patients who used antidepressants at the start of maintenance cycle 4, but not at the start of maintenance therapy was 1.37 (98.3% CI = 0.96-1.95) and similarly it was 1.29 (98.3% CI = 0.99-1.68) for patients who used antidepressants both at the start of maintenance cycle 4 and at the start of maintenance treatment (Table S7C). Patients, who used antidepressants at the start of cycle 4 but not at baseline had significantly poorer OS compared with patients who did not use antidepressants at the start of cycle 4 (adjusted HR = 1.81; 98.3% CI = 1.16-2.83). In patients who used antidepressants at the start of maintenance cycle 4 and at baseline, no significant OS differences were found (HR = 1.15; 98.3% CI = 0.78-1.68) (Table S7C and Figure S1).
When comparing use of SSRI alone or in combination to other antidepressants, there was no significant difference in PFS or OS at the 1.7% level (Table S8 and Figure S2).
At the 1.7% level, there was no statistically significant survival difference in patients diagnosed with depression at the start of maintenance therapy (P = .048 for PFS; P = .023 for OS) and at the start of maintenance cycle 4 (P = .050 for PFS; P = .022 for OS) in unadjusted analyses (Table S9 and Figure S3). Given that only 4 (0.2%) patients had documented depression at baseline, the association of a diagnosis of depression at baseline with PFS/OS was not explored.
4 DISCUSSION
Among more than 1700 patients with newly diagnosed glioblastoma treated within randomized clinical trials, we found no significant association between PFS and the use of antidepressant drugs at baseline, at the start of maintenance therapy or at the start of maintenance cycle 4. However, we found OS to be significantly worse in patients using antidepressant drugs at the start of maintenance cycle 4, although not at baseline or at the start of maintenance therapy (Figure 2). Despite adjustment for antiepileptic drug use, there was a trend for an increased risk of seizures, but they were not significantly associated with antidepressant drug use, nor was there a change in MMSE or fatigue according to antidepressant drug use at baseline. However, there was a significant association between fatigue during maintenance treatment and antidepressant drug use at the start of maintenance treatment (Figure 1).
A diagnosis of depression was documented in 0.2% of patients at baseline, 3.6% of patients at the start of maintenance treatment and 5.8% of patients at the start of maintenance cycle 4. In contrast, antidepressants were used in 8.4%, 9.9% and 13.6% of patients, respectively. Published studies reported a prevalence of depression among patients with glioma ranging from 15% to 93%.27-30 There is a significant difference between self-reported and physician-reported prevalence of depression in glioma patients as shown in a systematic review of observational studies.31
Although antidepressants may have also been prescribed for reasons other than depression such as anxiety, pain or sleep disorders, depression was most likely underreported in our pooled analysis of clinical trials. Reasons for not reporting a diagnosis of depression may include fear of stigmatization or that depressive symptoms are considered as a natural reaction to the diagnosis of deadly cancer and not a mental disorder.32 Depression was not an exclusion criterium in any of the trials, however, it may still be that patients with depression are less likely to participate in trials. In the CENTRIC and CORE trials, the “presence of any psychological, familial, sociological, or geographical condition potentially hampering compliance with the study protocol and follow-up schedule” was an exclusion criterium (ClinicalTrials.gov; NCT00813943). Correspondingly, in the ACT-IV trial, patients with a “severe acute or chronic medical or psychiatric condition or laboratory abnormality that could increase the risk associated with participating in a clinical trial” were excluded (ClinicalTrials.gov; NCT01480479). Besides physicians being less likely to include patients with depression in clinical trials, depressed patients themselves may be less willing to participate in clinical trials and experimental therapies.33
The proportion of antidepressant use in our study was lower than in the general population at baseline.34 Antidepressant use among patients with glioma varies by geographic region, with reduced prescribing for example in Norway,2 which does not hold true for Sweden.35 Patients with glioma have been reported to have up to 4-fold increased rates of depression compared with the general population.28 Reasons why patients with glioblastoma may be treated with antidepressants less frequently than other patients with depression are manifold. They include a perceived increased risk of seizure since antidepressants may lower the convulsive threshold.36 In our analysis, there was no statistically significant association of seizure risk and use of antidepressant drugs in analyses adjusted for anticonvulsant drug use (Table S4). However, and especially for some antidepressants known to be related to seizures, such as bupropion,37 numbers of patients may have been insufficient to detect significant associations. Furthermore, SSRI antidepressants may increase the risk of bleedings, especially in patients taking anticoagulants.38 Although based on only few patients there was no significant difference between all patients with antidepressants and patients with SSRI antidepressants regarding their association with OS and PFS.
A number of preclinical studies have proposed anti-tumor effects of antidepressant drugs on glioma cells or mouse models with different potential modes of action such as the interaction with potassium channels,7 cellular respiration,15, 16 tryptophan metabolism,19 IDO,20 the disruption of actin polymerization9 or the induction of autophagy.14, 17 Yet, data from relevant animal models are sparse,17 and our data do not support beneficial effects of antidepressant drugs on the outcome for patients with glioblastoma. However, numbers of patients in distinct subgroups of specific antidepressant drugs were low and smaller effects of specific substances may therefore have been missed.
The fact that use of antidepressants at the start of maintenance cycle 4, but not at baseline or at the start of maintenance therapy, is associated with decreased OS may have several reasons. Our results revealed that later use of antidepressant drugs is significantly associated with OS, but not longer use, that is, use already from baseline or from the start of maintenance therapy. Therefore, the significant results for OS at the start of maintenance cycle 4 may indicate noncausal associations as we did not observe an association of shorter survival with longer duration of drug use. Of note, a trend for an association between use of antidepressants at the start of maintenance and OS might reinforce the relationship between these factors. Depressive symptoms that evolve over the course of primary therapy may be due to worsening symptoms and tumor progression (ie, reverse causation). In a systematic review of observational studies, depression was consistently associated with reduced physical function, cognitive impairment and reduced quality of life.31 Common medications used in glioblastoma patients including levetiracetam or dexamethasone may additionally lead to depressive symptoms and may also be associated with an unfavorable clinical course.2, 31 Another reason of inferior OS in patients receiving antidepressants at the start of maintenance cycle 4 may be that antidepressants are used for other indications than depression, which may also be associated with an unfavorable clinical course such as pain or fatigue (ie, confounding by indication).39, 40
In general, in case of reverse causation or confounding by indication, one would also expect a worsened PFS in patients with use of antidepressants, which was not the case. In analyses exploring the association of a diagnosis of depression and OS as compared with the association of antidepressant use and OS there was a statistically nonsignificant trend for worsened survival, which may not have reached significance due to lower patient numbers as compared with the analysis on antidepressants (5.8% of patients vs 13.6% of patients). Therefore, a worse OS of users of antidepressant drugs at the start of maintenance cycle 4 may not be a mechanistic group effect of antidepressant drugs, but may rather reflect the association of depression with patient survival. In general, there is very limited data evaluating the benefits of any pharmacological treatment of depression in patients with glioma.41 Patients able to receive maintenance cycle 4 are a selected population with overall a more favorable outcome, that is, it may be possible to detect associations that are otherwise blunted by the aggressiveness of GB.
Smaller studies also found no statistically significant association between use of antidepressants and survival.4, 6 However, depression was associated with shorter survival in several studies and meta-analyses on glioma patients.1, 42 Patients with depression, severe pain or anxieties may decide more often than patients without those conditions to refuse tumor specific therapies at tumor recurrence.43 Furthermore, several studies proposed potential mechanisms how concurrent depression may influence cancer44 and especially glioma cell biology. Fu et al. found latent transforming growth factor-beta binding proteins (LTBPs) to be a possible link between depression and glioma in their bioinformatics and cellular models derived from glioma patients with concurrent depression.45 Furthermore, an altered level of inflammation,46 metabolism of neurotransmitters,47 cytokines or chemokines,48 or neuroendocrine function49 can be observed in patients with depression and may also affect glioma development and progression,50-53 but exact mechanisms of interaction still have to be elucidated.
We used a large dataset of newly diagnosed glioblastoma patients within prospective clinical trials and adjusted our analysis for important confounding factors. Adherence to drugs and documentation of medications and comorbidities was likely accurate in our analysis, because all patients were treated within clinical trials. Our study is not prone to selection or recall bias due to the pooling of patient data from prospective clinical trials.
Our study is, however, limited by several factors: the analyses were retrospective and the subgroups of patients using antidepressants were small representing <15% of patients. A diagnosis of depression was most likely underreported in our patient population and therefore the analyses on the association of depression and survival were likely underpowered. In addition, some results, for example those on the association of antidepressant use with seizure risk, narrowly missed significance and we can therefore not exclude associations as the data were derived from underpowered exploratory analyses. Furthermore, the percentage of patients receiving antidepressants was slightly higher in patients taking steroids and in patients with lower performance status, but the differences were <10%. We adjusted our analyses for these two factors. We could not analyze the association of a diagnosis of depression with outcome of glioma patients stratified by use vs nonuse of antidepressant drugs and we were not able to perform in-depth dose-response analyses, that is, exploring increasing doses and durations of antidepressant drugs in relation to glioblastoma survival. We could not analyze the association of specific substances of antidepressants with glioma survival as patient numbers were too low.
In summary, we observed no significant association between the use of antidepressant drugs and PFS or OS at baseline or at the start of maintenance therapy. At the start of maintenance cycle 4, we found no relation of antidepressant drug use with PFS, but a significantly worsened OS. Given the missing correlation of dose and response, the results are most likely not causal and may reflect the influence of the tumor-associated clinical status on the patient's mental well-being. Our study does therefore not justify to withhold antidepressant drugs from patients with glioblastoma.
Additional prospective studies should evaluate the effects of antidepressant drugs on depression and any association with survival of depressed patients with glioblastoma with close monitoring for specific tumor-related side-effects. The possible underreporting of depression observed in this large subset of patients underlines the necessity of a multidisciplinary diagnosis and management of depression in patients with glioma.
AUTHOR CONTRIBUTIONS
Conceptualization (Corinna Seliger, Felix Boakye Oppong, Florence Lefranc, Olivier Chinot, Roger Stupp, Burt Nabors, Thierry Gorlia, Michael Weller); Methodology (Felix Boakye Oppong, Thierry Gorlia); Software (Felix Boakye Oppong, Thierry Gorlia); Validation (Corinna Seliger, Thierry Gorlia, Michael Weller); Formal analysis (Felix Boakye Oppong, Thierry Gorlia); Investigation (Corinna Seliger, Felix Boakye Oppong, Thierry Gorlia); Resources (Michael Weller); Data Curation (Corinna Seliger, Felix Boakye Oppong); Writing - Original Draft (Corinna Seliger); Writing - Review & Editing (Felix Boakye Oppong, Florence Lefranc, Olivier Chinot, Roger Stupp, Burt Nabors, Thierry Gorlia, Michael Weller); Visualization (Felix Boakye Oppong, Corinna Seliger); Supervision (Thierry Gorlia, Michael Weller); Project administration (Corinna Seliger); Funding acquisition (Michael Weller). The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENT
Felix Boakye Oppong's fellowship at EORTC (Brussels, Belgium) was supported by a grant from the EORTC Brain Tumor Group.
FUNDING INFORMATION
This work was funded by the EORTC Brain Tumor Group.
CONFLICT OF INTEREST
The authors report no conflicts of interest related to this study.
ETHICS STATEMENT
All trials (NCT00689221, NCT00813943, NCT00943826 and NCT01480479) were approved by the respective institutional review boards. Patient data were protected according to European Union General Data Protection Regulation. All patients gave written informed consent.
Open Research
DATA AVAILABILITY STATEMENT
Qualified researchers may ask the investigators of the CENTRIC, CORE, AVAGlio and ACT-IV trials for access to anonymized individual patient data and redacted trial documents, which may be shared according to the regulations of the respective trials. Further information is available from the corresponding author upon request.