Circulating tumor-associated autoantibodies as novel diagnostic biomarkers in pancreatic adenocarcinoma
Liping Zhuang, Changjing Huang and Zhouyu Ning contributed equally to this study.
Funding information: Shanghai Municipal Health Commission, Grant/Award Number: ZXBZ1201
Abstract
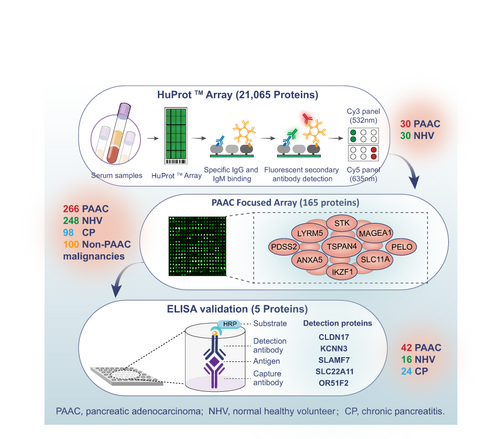
To develop a superior diagnostic approach for pancreatic adenocarcinoma (PAAC), the present study prospectively included 338 PAAC patients, 294 normal healthy volunteers (NHV), 122 chronic pancreatitis (CP) patients and 100 patients with non-PAAC malignancies. In the identification phase, HuProt Human Proteome Microarray, comprising 21 065 proteins, was used to identify serum tumor-associated autoantibodies (TAAbs) candidates differentiating PAAC (n = 30) from NHV (n = 30). A PAAC-focused array containing 165 differentially expressed TAAbs identified was subsequently adopted in the validation phase (n = 712) for specificity and sensitivities. The multivariate TAAbs signature for differentiation PAAC from controls (NHV + CP) identified five candidates, namely the IgG-type TAAbs against CLDN17, KCNN3, SLAMF7, SLC22A11 and OR51F2. Multivariate logistic performance model of y = (22.893 × CA19-9 + 0.68 × CLDN17 − 4.012) showed a significant better diagnostic accuracy than that of CA19-9 and CLDN17 in differentiating PAAC from controls (NHV + CP) (AUC = 0.97, 0.92 and 0.82, respectively, P-value < .0001). We further tested the autoantigen level of CLDN17 by ELISA in 82 sera samples from PAAC (n = 42), CP (n = 24) and NHV (n = 16). Similarly, the model showed superior diagnostic performance than that of CA19-9 and CLDN17 (AUC = 0.93, 0.83 and 0.81, respectively, P-value < .0001) in differentiating PAAC from controls. In conclusion, our study is the first to characterize the circulating TAAbs signatures in PAAC. The results showed that CLDN17 combined with CA19-9 provided potentially clinical value and may serve as noninvasive novel biomarkers for PAAC diagnosis.
Graphical Abstract
What's new?
Pancreatic adenocarcinoma (PAAC) readily evades diagnosis, to the extent that about 85% of cases are not identified until advanced stages of disease, contributing to exceptionally poor 5-year survival. Moreover, PAAC incidence and mortality rates continue to rise, heightening the need to improve diagnostic strategies. Here, the authors examined the diagnostic potential of circulating tumor-associated autoantibodies (TAAbs), which are known to propagate early in tumorigenesis. High-throughout screening resulted in the identification of five candidate TAAbs. Additional analyses revealed that a panel combining two of the candidates, namely TAAbs against CLDN17 and CA19-9, was highly effective in distinguishing PAAC from controls.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Individual data of the patients used in the study were shown in Table S6. Further information is available from the corresponding author upon reasonable request.