Exposures to pesticides and risk of cancer: Evaluation of recent epidemiological evidence in humans and paths forward
Abstract
Knowledge of the role in cancer etiology of environmental exposures as pesticides is a prerequisite for primary prevention. We review 63 epidemiological studies on exposure to pesticides and cancer risk in humans published from 2017 to 2021, with emphasis on new findings, methodological approaches, and gaps in the existing literature. While much of the recent evidence suggests causal relationships between pesticide exposure and cancer, the strongest evidence exists for acute myeloid leukemia (AML) and colorectal cancer (CRC), diseases in which the observed associations were consistent across several studies, including high-quality prospective studies and those using biomarkers for exposure assessment, with some observing dose-response relationships. Though high-quality studies have been published since the IARC monograph on organophosphate insecticides in 2017, there are still gaps in the literature on carcinogenic evidence in humans for a large number of pesticides. To further knowledge, we suggest leveraging new techniques and methods to increase sensitivity and precision of exposure assessment, incorporate multi-omics data, and investigate more thoroughly exposure to chemical mixtures. There is also a strong need for better and larger population-based cohort studies that include younger and nonoccupationally exposed individuals, particularly during developmental periods of susceptibility. Though the existing evidence has limitations, as always in science, there is sufficient evidence to implement policies and regulatory action that limit pesticide exposure in humans and, hence, further prevent a significant burden of cancers.
Abstract
What's new?
Most of the evidence suggesting pesticide carcinogenicity in the 2017 International Agency for Research on Cancer report came from animal and mechanistic studies, as the epidemiologic evidence was insufficient to draw conclusions. Here, the authors provide a unique review of 63 epidemiological studies on exposure to pesticides and cancer risk in humans published from 2017 to 2021, with an emphasis on new findings, methodological approaches, and gaps in the existing literature. The review shows there is sufficient evidence for implementing policies and regulatory action to limit pesticide exposure in humans, and hence further prevent a significant burden of cancers.
Abbreviations
-
- AHS
-
- Agricultural Health Study
-
- ALL
-
- acute lymphoblastic leukemia
-
- AML
-
- acute myeloid leukemia
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- CanCHEC
-
- Canadian Census Health and Environment Cohort
-
- CI
-
- confidence interval
-
- CNAP
-
- Cancer in the Norwegian Agricultural Population
-
- CNS
-
- central nervous system
-
- CRC
-
- colorectal cancer
-
- DDE
-
- dichlorodiphenyldichloroethylene
-
- DDT
-
- dichlorodiphenyltrichloroethane
-
- EFSA
-
- European Food Safety Authority
-
- EPIC
-
- European Prospective Investigation into Cancer and Nutrition
-
- HCB
-
- hexachlorobenzene
-
- HCH
-
- hexachlorocyclohexane HR
-
- IARC
-
- International Agency for Research on Cancer
-
- JEM
-
- job exposure matrix
-
- KMCC
-
- Korean Multi-Center Cancer cohort
-
- MESA
-
- United States Marshfield Epidemiologic Study Area Farm cohort
-
- MHC
-
- Multiphasic Health Checkup Cohort
-
- NB
-
- neuroblastoma
-
- NHL
-
- non-Hodgkin's lymphoma
-
- NOWAC
-
- Norwegian Women and Cancer Cohort
-
- OR
-
- odds ratio
-
- PCA
-
- principal component analysis
-
- PCB
-
- polychlorinated biphenyl
-
- PLCO
-
- Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
-
- RCC
-
- renal cell carcinoma
-
- RR
-
- risk ratio
-
- STROBE-ME
-
- Strengthening the Reporting of Observational studies in Epidemiology - Molecular Epidemiology
-
- SUS
-
- Danish Sund Stald Stud
-
- US EPA
-
- United States Environmental Protection Agency
1 INTRODUCTION
Knowledge of environmental contributions to cancer development is a crucial prerequisite for primary prevention, the only form of prevention that decreases cancer incidence. While inherited genetic susceptibility plays a role in subsets of human cancers, from human and practical perspectives it is vital to acknowledge that carcinogenic exposures can be modified by policies and behaviors, whether collective or individual (eg, government regulation, occupational safety measures, changes in policies and attitudes toward smoking).1-8 Exposure to chemicals such as pesticides and secondhand smoke has proven malleable to modification. This has been demonstrated as urinary concentrations of pesticides and tobacco products decrease rapidly after changing to an organic diet or quitting smoking, respectively.9, 10 Many policies on environmental and occupational conditions have achieved positive effects.1, 12
An extensive literature from research conducted in vitro and in animals demonstrates the biological plausibility of pesticides as carcinogens. It has also shown their action through multiple mechanisms of carcinogenesis. Research has documented the ability of numerous pesticides to induce genotoxicity, hormone disruption, oxidative stress, inflammation, immune modulation and procarcinogen activation.11-14
Certainly, evidence of the carcinogenic effects of pesticides is sparser in humans than in animals. However, in humans, it has modestly but steadily grown and continues evolving, partly because of the progressive introduction of molecular epidemiology approaches and techniques some 40 years ago. Today such techniques are widely used, including sensitive biomarkers to more effectively assess exposures and intermediate events.3, 15, 16 Early studies focused on occupationally exposed populations. Later, as the diversity of chemicals used to combat pests rapidly rose, studies of nonoccupational populations have proven increasingly relevant; notably, as effects on the endocrine system of low concentrations have emerged. Research efforts have also focused on vulnerable populations, long-term effects and critical windows of susceptibility like prenatal and childhood exposure.17-19
Within their long-established program of Monographs to identify environmental factors that are carcinogenic hazards to humans,20 in 2017 the International Agency for Research on Cancer (IARC) published a report that comprehensively evaluated data on five major organophosphate pesticides, some insecticides and one herbicide, with known human exposure: tetrachlorvinphos, parathion, malathion, diazinon and glyphosate. IARC concluded that they were all either “probably carcinogenic to humans” (group 2A) or “possibly carcinogenic to humans” (2B), and suggested genotoxicity and oxidative stress as the most likely modes of action.21 However, debate still surrounds the pesticides covered by IARC, and resistance to regulation of pesticides remains influential.22 Most evidence suggesting pesticide carcinogenicity—in the IARC report and elsewhere—has come from animal and mechanistic studies, as the epidemiology literature was insufficient to draw conclusions. Epidemiologic evidence has since increased in both quantity and quality, and now covers many other pesticides that were not included in IARC's review, or in those of other national and international agencies.23
The present review focuses on all types of epidemiological studies on exposure to pesticides and cancer risk in humans published between 2017 and 2021. We contextualize this new evidence with the most relevant published reviews, address methodological approaches and gaps in the existing literature and suggest new directions to improve evidence potentially useful to act.
2 METHODS
While this review is strictly not systematic, we did implement methods frequently utilized in systematic reviews. Titles and abstracts were screened for all papers produced by the literature search. Studies excluded from the primary analyses during title and abstract screening were reviews,24-26 editorials,27 animal or in vitro studies,28 mechanistic studies (even if they used endpoints potentially related to clinical cancer, as epigenetic markers, telomere length, endocrine response),29-32 papers on study protocols,33 biostatistics methods papers,34, 35 epidemiological profiles and cohort descriptions,36, 37 risk/exposure assessments38 and other studies where human cancer was not a primary outcome.39
The following search terms were used in PubMed to identify relevant journal articles assessing the relationship between human exposure to pesticides and cancer, which were published in 2017 or later: (“pesticide*”[Title/Abstract] OR “organphosphate*”[Title/Abstract] OR “glyphosate”[Title/Abstract] OR “chlorpyrifos”[Title/Abstract] OR “atrazine”[Title/Abstract] OR “acephate”[Title/Abstract] OR “Mancozeb”[Title/Abstract]) AND (“cancer”[Title/Abstract] OR “neoplasm”[Title/Abstract] OR “tumor”[Title/Abstract]) AND (“cohort studies”[MeSH Terms] OR (“cohort”[All Fields] AND “studies”[All Fields]) OR “cohort studies”[All Fields] OR “cohort”[All Fields] OR “cohort s”[All Fields] OR “cohorte”[All Fields] OR “cohorts”[All Fields] OR “case-control”[All Fields] OR “cross-sectional”[All Fields]).
Among the papers deemed potentially relevant during title and abstract screening, full text versions were reviewed. During full-text screening, we extracted information on study design, selection methods and characteristics of the study population, methods for exposure and outcome assessment, statistical analyses, notable strengths and limitations and overall findings. Extracted information was organized into tables for comparison and analysis.
We included cohort, case-control, case-cohort and survival studies40 assessing the relationship between pesticide exposure and cancer incidence, prevalence, survival and markers of severity in humans. Initially, we detected many reviews on various facets of the pesticides and cancer topic that were potentially relevant.24, 41-59 Of them, some41-50, 60, 61 are particularly relevant to complement our analysis and address issues that it is unnecessary to repeat in the present paper.
Additional articles were excluded during full-text screening based on quality, using criteria from IARC and from “Strengthening the Reporting of OBservational studies in Epidemiology—Molecular Epidemiology” (STROBE-ME). Criteria comprised the use of clearly defined methods to assess exposure and disease, consideration of potential confounders, susceptibility to recall and/or selection bias, adequate power and descriptions of statistical models used to generate estimates of association, with further considerations for studies using biological samples.11, 62 For example, given the high susceptibility of recall bias in case control-studies of pesticides and cancer (as recently illustrated again by Crump et al.63), studies were excluded if exposure was self-reported and assessment occurred many years after the time period of interest, and were not supplemented or validated with other data such as crop/job-exposure matrices or pesticide purchasing records. The exception to this was studies of childhood cancer, where the time between recall and exposure is shorter. Additionally, childhood cancer studies are highly underrepresented, and the case-control studies included provide some of the only evidence available on this vulnerable population.
Frequencies and percentages were reported for study characteristics such as study design, biomarker use for exposure assessment, type of exposure (agricultural or household/urban) and study population. Notable empirical findings from high quality studies were summarized, and full tables of study summary information on all included studies can be found in the tables. Gaps in existing research were noted, with emphasis on underrepresented populations.
3 RESULTS
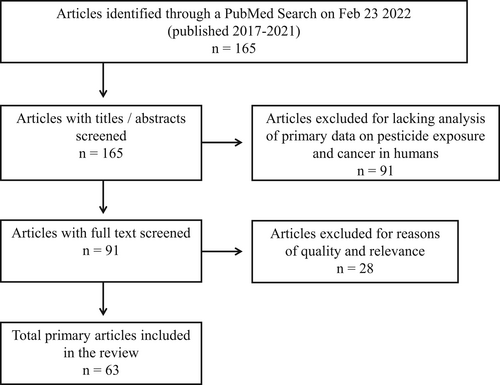
In the initial PubMed searches 165 articles published between 2017 and 2021 were found. After exclusion at various phases as explained above, 63 articles19, 61, 64-124 were included in the final literature sample (Figure 1).
3.1 Design and other characteristics of studies
Included research largely fell into one of four categories: (a) large prospective cohort studies, (b) cohort-nested case-control studies and case-cohort studies, (c) population-based case-control studies and (d) hospital-based case-control studies (Table 1).
| Characteristics | N | (%) |
|---|---|---|
| Study design | ||
| Prospective cohort | 23 | 36.5 |
| Cohort-nested case-control and case-cohort | 10 | 15.9 |
| Population-based case-control | 18 | 28.6 |
| Hospital-based case-control | 8 | 12.7 |
| Survival | 4 | 6.3 |
| Used biomarkers | ||
| Yes | 22 | 34.9 |
| No | 41 | 65.1 |
| Assessed occupational exposures | ||
| Yes | 29 | 46 |
| No | 34 | 54 |
| Timing of the exposures | ||
| Adult | 53 | 84.1 |
| Prenatal and childhood | 10 | 15.9 |
| Type of exposurea | ||
| Agricultural | 31 | 49.2 |
| Residential | 6 | 9.5 |
| Other/not specified | 27 | 42.9 |
- a Total does not add up to 100% because one study was both agricultural and residential.
There were nine large prospective studies.65, 80-85, 125, 126 Three were occupational cohort studies: the Agricultural Health Study (AHS) in the United States,125 the AGRICAN cohort study in France,126 and the AGRICOH Consortium; the latter is a pooled study combining data from ARGRICAN, AHS and other occupational cohorts, including Cancer in the Norwegian Agricultural Population (CNAP), Australian Pesticide Exposed Workers, Victorian Grain Farmers, Korean Multi-Center Cancer cohort (KMCC), United States Marshfield Epidemiologic Study Area Farm cohort (MESA) and Danish Sund Stald Stud (SUS).64, 65 In these cohorts, exposure was assessed at baseline via self-report or through census data; none included biomarkers. The AHS cohort is based in North Carolina and Iowa, and it includes some 57 000 licensed private and commercial pesticide applicators enrolled from 1993 to 1997. Researchers analyzed relationships between binary exposure variables (occupationally exposed vs not exposed), and cumulative and intensity-weighted lifetime days of exposure, a metric that adjusts for factors that influence exposure intensity, for many different pesticides and cancer types.125 The AGRICAN cohort is a prospective cohort study in France that enrolled 180 060 current and retired farmers from 2005 to 2007. Pesticide exposure was assessed at enrollment with a questionnaire, and incident cancer was assessed via linkage to population-based registries.126
Three studies were general population cohorts, with occupation being used to assess exposure through a job exposure matrix (JEM); a JEM is a tool that classifies individuals into likely exposure categories based on time spent in various occupational roles.127 The studies included the International Childhood Cancer Cohort Consortium83 (pooled birth cohorts), a Swiss census-based cohort study,84 and the Canadian Census Health and Environment Cohort.85 In these studies, subjects occupationally exposed to pesticides were compared to those nonoccupationally exposed.83, 84
Two of the prospective cohort studies measured dietary exposure to pesticides using food frequency questionnaires for fruit and vegetable intake, and information on organic status, and probable pesticide residue levels from government agricultural databases on farming practices.80, 81 Only one prospective cohort study used biomarkers for exposure assessment.82
In contrast to the cohort studies, nested case-control and case-cohort studies typically included prospectively measured biomarkers for exposure assessment (eg, biomarkers measured in biological samples collected at study entry, years before cancers occurred). Some such studies used questionnaires as well; questionnaires and biomarkers often complement each other. These studies are a part of larger cohort studies and, per design, were highly efficient (eg, precise) with small to medium sample sizes (median sample size ~300 participants). Examples include case-control studies nested within the Janus Serum Bank Cohort,128 a Norwegian population-based biobank established in 1973, and the Danish National Birth Cohort, a population-based birth cohort out of Denmark established in 1996129 (Table 3).
Population-based case-control studies94-112 had medium to large sample sizes (median sample size ~1800 individuals). They used population-based data sets from census, and cancer and birth registries. In these studies, exposure was typically assessed via existing data sets, using occupation and job-exposure matrices, or via geographical analysis of proximity to agricultural sites as a proxy for exposure; at other times exposure was self-reported using self-administered or interviewer-administered questionnaires (Table 4).
All hospital-based case-control studies used biomarkers for exposure assessment. Pesticides were quantified in blood samples,113, 114, 116-119 or in adipose tissue samples (in breast cancer)115 if surgery was performed. Less than 8% of the studies assessed whether varying levels of pesticide exposure influenced differences in survival among patients with cancer (Tables 5 and 6).121-124
About 10% of the studies assessed prenatal and childhood exposures and the development of cancer in children, while the adult studies typically studied populations in their 50s and 60s. Thirty percent of the studies included only women, 5% of the studies included only men, and 65% of the studies lumped together both genders; several of the latter were occupational studies, which had a large proportion of males.125 Forty-six percent of the studies assessed occupational exposures. A slightly larger proportion of reports looked at agricultural exposures (including residences near farms). Only six (9.5%) of the reviewed studies specifically assessed residential pesticide use.78, 100, 105, 107, 109, 121 About 16% of the studies used prospectively measured biomarkers19, 82, 86-88, 90-93, 124 (Table 1).
3.2 Main empirical findings
The main empirical findings from the included studies are summarized below and in Tables 2-6 grouped by study design. Two studies from the AGRICOH consortium were published during the time period of interest. Togawa and colleagues examined the incidence of primary cancer diagnosis and specific subtypes in the occupational cohorts compared to the general population; in women, they found increased risks for melanoma of the skin (meta-SIR = 1.18, CI: 1.01-1.38) and multiple myeloma (meta-SIR = 1.27, CI: 1.04-1.54), and in men, for prostate cancer (meta-SIR = 1.06, CI: 1.01-1.12). They also observed a deficit for the incidence of cancers of the bladder, breast, colorectum, esophagus, larynx, lung, pancreas, and all cancers combined (meta-SIR for all cancers combined = 0.83, 95% CI: 0.77-0.90).64 The other AGRICOH study specifically investigated non-Hodgkin's lymphoma (NHL), and observed an increased risk for NHL among ever users of terbufos (meta-HR = 1.18, CI: 1.00-1.39); for chronic lymphocytic leukemia/small lymphocytic lymphoma among ever users of deltamethrin (meta-HR = 1.48, CI: 1.06-2.07); and for diffuse large B-cell lymphoma in ever users of glyphosate (meta-HR =1.36, CI: 1.00-1.85; Table 2).65
| Study cohort | Study location | Exposure assessment timing/method | Exposure type | First author, year | Sample size | Pesticides | Outcome | Covariates | Findings |
|---|---|---|---|---|---|---|---|---|---|
| AGRICOH Consortium [pooled study of AGRICAN, Agricultural Health Study (AHS), and Cancer in the Norwegian Agricultural Population (CNAP)] | Iowa, North Carolina, France and Norway | Adolescence and adulthood, self-report and census data | Occupational | Togawa, 202164 | 248 742 | Unspecific | Primary cancer diagnosis and several subtypes | Sex | Increased risks were observed for melanoma of the skin (number of cohorts = 3, meta-SIR = 1.18, CI: 1.01-1.38) and multiple myeloma (n = 4, meta-SIR = 1.27, CI: 1.04-1.54) in women; and for prostate cancer (n = 6, meta-SIR = 1.06, CI: 1.01-1.12); all compared to the general population. A lower incidence of several cancers was observed, including cancers of the bladder, breast, colorectum, esophagus, larynx, lung, pancreas and all cancers combined (n = 7, meta-SIR for all cancers combined = 0.83, 95% CI: 0.77-0.90); likely due to the healthy worker effect, less smoking and higher occupational physical activity, as discussed in the article. |
AGRICOH consortium Same as above and Australian Pesticide Exposed Workers, Victorian Grain Farmers, Korean Multi-Center Cancer cohort (KMCC), United States Marshfield Epidemiologic Study Area Farm cohort (MESA) and Danish Sund Stald Stud (SUS) |
Iowa, North Carolina, Wisconsin, France, Norway, Denmark, Australia, Korea | Leon, 201965 | 316 270 | Manya | Non-Hodgkin's lymphoma (NHL) and subtypes | Animal production, number of crops treated with specific pesticides, retirement status, sex, specific pesticides and state of residence | No observed association for most analyses. Moderately increased risk for NHL observed among ever users of terbufos (meta-HR = 1.18, CI: 1.00-1.39); chronic lymphocytic leukemia/small lymphocytic lymphoma among ever users of deltamethrin (meta-HR = 1.48, CI: 1.06-2.07); and diffuse large B-cell lymphoma and ever users of glyphosate (meta-HR = 1.36, CI: 1.00-1.85); as well decreased risk of NHL with the broader groups of organochlorine insecticides (meta-HR = 0.86, CI: 0.74-0.99) and phenoxy herbicides (meta-HR = 0.81, CI: 0.67-0.98), but not with active ingredients within these groups, after adjusting for exposure to other pesticides. | ||
| AGRICAN (farmers) | France | Adulthood, self-report | Occupational | Tual, 201966 | 155 192 | Unspecific | Multiple myeloma (MM) | (Sensitivity analyses) body mass index (BMI), correlated exposure, gender, personal protective equipment | Increased risk of MM in farmers who started using pesticides on crops in the 1960s, especially among those applying pesticides on corn (≥20 years: HR = 1.73, CI: 1.08-2.78) and using insecticides on animals (HR = 1.48, CI: 1.11-1.98), especially among horse farmers (≥10 years: HR = 2.77, CI: 1.22-6.27) |
| Piel, 201967 | 170 858 | Carbamates | Central nervous system tumors (CNST) | Gender, occupational status, educational, familial status, history of allergic diseases, tobacco smoking/alcohol consumption | Increased risk of CNST with overall exposure to carbamate fungicides (HR = 1.88, CI: 1.27-2.79) carbamate herbicides (HR = 1.44, CI: 0.94-2.22). Positive associations were observed with specific carbamates, including some fungicides (mancozeb, maneb, metiram) and herbicides (chlorpropham, propham, diallate) | ||||
| Boulanger, 201868 | 148 044 | Unspecific | Lung cancer | Cattle and horse farming, gender, smoking status | Increased risk of small cell lung cancer (HR = 2.38, CI: 1.07-5.28 for pesticide use on peas and increased risk of squamous cell carcinoma for pesticide use on beets (HR = 1.47, CI: 0.92-2.34) | ||||
| Piel, 201769 | 146 745 | Unspecific | CNST | Age, alcohol consumption, education, gender, smoking status | Increased risks of CNST in farmers, especially in pesticide users (HR = 1.96, CI: 1.11-3.47). Associations varied with tumor subtypes and kinds of crop and animal farming. Increases in risk were observed for meningiomas in pig farmers and in farmers growing sunflowers, beets and potatoes and for gliomas in farmers growing grasslands | ||||
| Lemarchand, 201770 | 181 842 | Unspecific | Primary cancer diagnosis and several subtypes | N/A | Overall cancer incidence in the cohort and the general population were the same. SIRs were significantly higher for prostate cancer (SIR = 1.07, CI: 1.03-1.11) and non-Hodgkin lymphoma (SIR = 1.09, CI: 1.01-1.18) among men, skin melanoma among women (SIR = 1.23, CI: 1.05-1.43) and multiple myeloma (men: SIR = 1.38, CI: 1.18-1.62; women: SIR = 1.26, CI: 1.02-1.54). In contrast, SIRs were lower for upper aero-digestive tract and respiratory cancers. | ||||
| Boulanger, 201771 | 148 051 | Unspecific | Bladder cancer | Gender, smoking status | Increased risk among field-grown vegetable workers (HR = 1.89, CI: 1.20-0.99), with an exposure-response relationship with duration of work (≥30 years HR = 2.54, CI: (1.11-5.83), and higher risk among women (HR = 3.82, CI: (1.58-9.25). Nonsignificantly increased risks were also observed in greenhouse farmers (HR = 1.95), pea sowing (HR = 1.84), rape sowing (HR = 1.64); seed treatment (HR = 1.24); and in activities and tasks potentially exposing to arsenic compounds via pesticide use (HR = 1.49) or re-entry tasks (HR = 1.63). | ||||
| Agricultural Health Study (pesticide applicators) | Iowa, North Carolina | Adulthood, self-report | Occupational | Lerro, 202172 | 53 096 | Manya | Thyroid cancer | Age, applicator type, BMI, correlated pesticides smoking status, state | Increased risk of thyroid cancer with exposure to fungicide metalaxyl (HR = 2.03, CI:1.16-3.52) and organochlorine insecticide lindane (HR = 1.74, CI: 1.06-2.84) Decreased thyroid cancer risk was observed with exposure to herbicide chlorimuron-ethyl when analysis restricted to papillary thyroid cancer, the most common subtype (HR = 0.52, CI: 0.28-0.96). Decrease risk of thyroid cancer was observed with o high use of the insecticide carbaryl (HR = 0.20, CI: 0.08-0.53) |
| Andreotti, 202073 | 55 837 | Manya | RCC | Age, BMI, 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) use, smoking status, state | Increased risk of RCC among the highest users of 2,4,5-T compared to never users unlagged rate ratio for intensity-weighted days (RRIWD,T3 = 2.92, CI: 1.65-5.17), with similar risk estimates for lagged exposure (20-year lag RRIWD,T3 = 3.37, CI: 1.83-6.22). In 20-year lagged analyses, there were exposure-response associations with chlorpyrifos (RRIWD,Q4 = 1.68 CI: 1.05-2.70), chlordane (RRIWD,T3 = 2.06, CI: 1.10-3.87), atrazine (RRIWD,Q4 = 1.43, CI: 1.00-2.03), cyanazine (RRIWD Q4 = 1.61, CI: 1.03, 2.50), and paraquat (RRIWD > Median = 1.95, CI: 1.03-3.70) |
||||
| Lerro, 202074 | 49 922 | Dicamba | Primary cancer diagnosis and several subtypes | Age, applicator type, education, imazethapyr use, family history of cancer, ethnic, sex, smoking history, state Cancer-specific models had additional adjustments |
Increased risk of liver and intrahepatic bile duct cancer for highest quartile of diacamba exposure (RRQ4 = 1.80, CI: 1.26-2.56) and chronic lymphocytic leukemia (CLL) (RRQ4 = 1.20, CI: 0.96-1.50) Decrease risk of myeloid leukemia for highest quartile of diacamba exposure (RRQ4 = 0.73, CI: 0.51-1.03) |
||||
| Pardo, 202075 | 8933 | Manya | Aggressive prostate cancer (PCa) | Family history of prostate cancer, ethnic, smoking status, state, year of birth | Increased risk of PCa among ever users of the organodithioate insecticide dimethoate (HR = 1.37, CI: 1.04-1.80) compared to never users Inverse association between aggressive PCa and the herbicide triclopyr (HR = 0.68, CI: 0.48-0.95) |
||||
| Lerro, 201976 | 89 656 | Unspecific | Primary cancer diagnosis and several subtypes | N/A | AHS cancer incidence was lower than the general population (standardized incidence ratio [SIR] private = 0.91, CI:0.89-0.93; SIR spouse = 0.89, CI:0.86-0.92; SIR commercial =0.83, CI:0.76-0.92), with deficits across applicators and spouses for oral cavity, pancreas and lung cancers. Cancer excesses included prostate cancer, lip cancer, certain B-cell lymphomas, AML, thyroid cancer, testicular cancer and peritoneal cancer | ||||
| Andreotti, 201861 | 54 251 | Glyphosate | Any cancer, leukemia, central nervous system tumors (CNST), lymphoma, non-CNS solid tumors | Age, alcohol consumption, cigarette smoking status, family history of cancer, the five pesticides most highly correlated with glyphosate, state | Increased risk, though not statistically significant, of AML among applicators in the highest exposure quartile, compared to never users (RR = 2.44, CI: 0.94-6.32) Results for AML were similar with a 5-year (RRQ4 = 2.32, CI: 0.98-5.51) A statistically significant association was observed for 20-year exposure lag, (RRT3 = 2.04, CI: 1.05-3.97) | ||||
Engel, 201777 Note: AHS spouses are the population for our study |
30 594 | Manya | Breast cancer | All other pesticides associated with breast cancer, combined parity/age at first birth, ethnic, state | No evidence of association between insecticides and breast cancer risk. Increased risk among women who had ever used the organophosphates chlorpyrifos (HR = 1:4, CI: 1.0-2.0) or terbufos (HR = 1:5, CI: 1.0-2.1) | ||||
Louis, 201778 Note: AHS spouses are the population for our study |
28 909 | Organocholorine pesticides: aldrin, chlordane, dieldrin, DDT, heptachlor, lindane and toxaphene | Primary cancer diagnosis and several subtypes | N/A | Most cancers were not associated with OC use. Increased risk of glioma was observed among users of at least one OC (RR = 3.52, CI: 1.72-7.21) and among lindane users (RR = 4.45, CI: 1.36-14.55). Increased risk of multiple myeloma was associated with chlordane (RR = 2.71, CI: 1.12-6.55). | ||||
| Bonner, 201779 | 49 812 | Manya | Lung cancer | Age, sex, smoking status, total lifetime pesticide use | Increased incidence in the highest exposure category of lifetime days of use for pendimethalin (HR = 1.50, CI: 0.98-2.31), dieldrin (1.93; 95% CI: 0.70, 5.30), and chlorimuron ethyl (HR = 1.74, CI: 1.02-2.96). The HRs for intensity-weighted lifetime days of use of these pesticides were similar. | ||||
| Nurses' Health Study, Nurses' Health Study II, Health Professionals Follow-up Study | United States | Adult, food frequency questionnaire | Dietary | Sandoval-Insausti, 202180 | 180 316 | Unspecific | Primary cancer diagnosis and several subtypes | Age, alcohol consumption, Alternate Healthy Eating Index score, BMI, colonoscopy in the past 2 years, ethnicity, family history of cancer, height, mammography/prostate-specific antigen testing in the past 2 years, multivitamin use, physical activity, physical examination in the past 2 years, postmenopausal hormone use, regular aspirin use, smoking status, total energy intake | No observed association between high-pesticide-residue FV intake and cancer overall, or risk of specific sites, including malignancies previously linked to occupational pesticide exposure |
| NutriNet-Sant e ́ | France | Adult, food frequency questionnaire | Dietary | Rebouillat, 202181 | 13 149 | Manyb | Breast cancer | Alcohol consumption, BMI, education, family history of cancer, height, menopausal treatment, overall quality of diet, parity, physical activity, smoking status | Negative associations between Component 3, reflecting low exposure to synthetic pesticides, and postmenopausal BC risk were found (HRQ5 = 0.57, CI: 0.34-0.93). Positive association between Component 1 score (highly correlated to chlorpyrifos, imazalil, malathion, thiabendazole) and postmenopausal BC risk was found specifically among overweight and obese women (HRQ5 = 4.13, CI: 1.50-11.44). No associations were detected for the other components. |
| GraMo Cohort | Granada Province, Southern Spain | Adult, gas chromatography in adipose tissue | Biomarker | Mustieles, 202182 | 348 | p,p′-DDE, HCB, dicofol, α- and β-HCH | Primary cancer diagnosis and some subtypes | Age, alcohol consumption, BMI, education, place of residence, sex, smoking status | Increased risk of nonhormone-dependent cancers (NHDC) for higher concentrations of β-HCH (HR = 1.70; CI: 1.09-2.64), and HCB (HR 1.54, CI: 1.02-2.33). The enzymes superoxide dismutase (SOD) and glutathione reductase (GRd) were also positively associated with the risk of NHDC. Possible mediation effect of SOD and GRd on associations between some pesticides and NHDC. |
| International Childhood Cancer Cohort Consortium (birth cohorts) | Australia, Denmark, Israel, Norway and United Kingdom | Prenatal, job-exposure matrix | Occupational | Patel, 202083 | 329 658 | Unspecific | ALL, AML, CNS tumors | Stratified by: cohort, Adjusted for child's sex, paternal age | Increased risk, though not statistically significant, of childhood AML for paternal exposures to pesticides (herbicides HR = 3.22, CI: 0.97-10.68; insecticides HR = 2.86, CI: 0.99-8.23, but not ALL or CNS tumors. |
| Switzerland (census-based, general population) | Switzerland | Childhood, job-exposure matrix | Occupational | Coste, 202084 | 2 100 548 | Unspecific | Any cancer, leukemia, CNST, lymphoma, non-CNS solid tumors | Education, maternal age at birth, parental occupational exposure to benzene, modeled air concentration of NO2, modeled dose rate of ionizing background radiation, SES | No evidence of an association was found with maternal or paternal exposure for any of the outcomes, except for non-CNS solid tumors (High vs None; Father: adjusted HR = 1.84, CI:1.31-2.58, Mother: adjusted HR = 1.79, CI: 1.13-2.84) |
| Canadian Census Health and Environment Cohort (CanCHEC; census-based, general population) | Canada | Adult, occupation records | Occupational | Kachuri, 201785 | 2 051 315 | Unspecific | Primary cancer diagnosis and several subtypes | Age, education, province | Among men, increased risks were observed for NHL (HR = 1.10, CI: 1.00-1.21), prostate (HR = 1.11, CI:1.06-1.16), melanoma (HR = 1.15, CI: 1.02-1.31) and lip cancer (HR = 2.14, CI: 1.70-2.70). Decreased risks in males were observed for lung, larynx and liver cancers. Among females, increased risks were observed for pancreatic cancer (HR = 1.36, CI:1.07-1.72). Increased risks of melanoma (HR = 1.79, CI: 1.17-2.73), leukemia (HR = 2.01, CI:1.24-3.25) and MM (HR = 2.25, 95% CI = 1.16-4.37) were observed in a subset of female crop farmers |
- Abbreviations: CI, confidence interval; HR, hazard ratio; IWD, intensity-weighted days; Q4, Quartile 4; Q5, Quantile 5; RR, risk ratio; SIR, standardized incidence ratio; T2, tertile 2; T3, tertile 3.
- a Methyl bromide, aluminum phosphide, metalaxyl, captan, benomyl, chlorothalonil, maneb/mancozeb, chloroacetanilide, alachlor, metolachlor, dinitroaniline, trifluralin, pendimethalin, phenoxy, 2,4-D, 2,4,5 T, 2,4,5-TP, thiocarbamate, butylate, EPTC, triazine, atrazine, cyanazine, glyphosate, dicamba, metribuzin, imazethapyr, petroleum oil, chlorimuron ethyl, carbamate, carbaryl, carbofuran, aldicarb, organochlorine, lindane, DDT, chlordane, heptachlor, aldrin, toxaphene, organophosphate, malathion, chlorpyrifos, diazinon, phorate, terbufos, fonofos, parathion, dichlorvos, coumaphos, pyrethroid, permethrin.
| Study cohort | Study location | Exposure assessment timing/method | Exposure type | First author, year | Sample size | Pesticides | Outcome | Covariates | Findings |
|---|---|---|---|---|---|---|---|---|---|
| European Prospective Investigation into Cancer and Nutrition (EPIC) cohort | Denmark, Sweden, Germany, the UK, the Netherlands, Italy, Spain, Greece, France, and Norway | Adult, gas chromatography with plasma (blood drawn years before cancer occurred) | Biomarker | Porta, 202186 | 1533 | p,p′-DDT, p,p′-DDE, α-HCH, β-HCH, γ-HCH, pentachlorobenzene (PeCB), HCB, trans-nonachlor and oxychlordane | Pancreatic cancer | Study center, sex, age at blood collection, date and time of blood collection, fasting status, total lipids; for women, use of exogenous hormones; BMI, smoking. | Increased risk was observed at higher concentrations of p,p′-DDT, trans-nonachlor (OR upper quartile = 1.55, CI: 1.06-2.26, p-trend = .025), β-HCH and the sum of six organochlorine pesticides (OR upper quartile = 1.48, CI: 1.00-2.20, p-trend = .045). Associations were stronger in the groups predefined as most valid (participants having fasted >6 hours, with microscopic diagnostic confirmation, normal weight, and never smokers). Among participants with a follow-up ≥10 years, estimates were higher than in participants with a shorter follow-up (for trans-nonachlor: OR upper quartile = 2.14, CI: 1.01-4.53, p-trend = .035). |
| Korean National Cancer Center Community (KNCCC) cohort | Haman, Sancheong, Changwon, Chungju and Chuncheon, Korea | Adult, gas chromatography with serum | Biomarker | Park, 202187 | 339 | Manya | Colorectal cancer (CRC) | Age, alcohol consumption, BMI, education, ever use of pesticides, metabolic health status, physical activity red meat consumption, sex, smoking status, vegetable/fruit consumption | Increased risk of CRC for those exposed to cis-heptachlor epoxide (HRT3 = 2.76, CI:1.25-6.07) Dose-response relationships observed for those exposed to trans-nonachlor: (HRT2 = 3.90, CI:1.56-9.75, HRT3 = 4.86, CI: 1.95-12.16); and p,p′-DDD (HRT3 = 6.02, CI: 2.05-17.70, HRT3 = 7.43, CI: 2.42-22.84) |
| Multiethnic Cohort | Hawaii | Adult, liquid chromatography with urine | Biomarker | Franke, 202188 | 250 | Aminomethyphosphonic acid (AMPA) (glysophate metabolite) | Breast cancer | Age first live birth, age at menarche, alcohol consumption, BMI, education, family history of breast cancer, hormone use, mammography screening menopausal status, physical activity, smoking status | Increased risk for highest vs lowest quintile of AMPA excretion observed (odds ratio (ORQ5 = 4.49, CI: 1.46-13.77) |
| Danish National Birth Cohort | Denmark | Pregnancy, geospatial methods survey | Proximity to pesticide using facilities/farmland | Patel, 202089 | 9506 | Unspecific | Leukemia and Central Nervous System Tumors (CNST) | Animals within 1000 m child gender, maternal age | Increased risk of childhood leukemia among offspring of mothers with increasing crop area near their home (<500 m) compared to no crops (HRT3: 2.6, CI:1.02-6.8) Crops within 500 m of the home were not associated with CNS tumors |
| Kaiser Permanente Multiphasic Health Checkup Cohort (MHC) and Janus Serum Bank Cohort | Northern California, Norway | Adult, gas chromatography with serum | Biomarker | Engel, 201990 | 880 | o,p′-DDT, p,p′-DDE, p,p′-DDT, β-HCH, γ-HCH, dieldrin, HCB, mirex, heptachlor epoxide, oxychlordane and trans-nonachlor | Liver cancer | Hepatitis B and C status, smoking status, additional adjustment for BMI in the MHC | MHC participants: nonsignificant exposure-response trend for trans-nonachlor (ORT2 = 1.63, CI: 0.87-3.06, ORT3 = 1.95, CI: 0.98-3.86) and a nonsignificant elevated risk for the highest tertile of oxychlordane (ORT3 = 1.87, CI: 0.94-3.72). Janus participants: nonsignificant exposure-response trend for p,p′-DDT (ORT3 = 1.70, CI: 0.66-4.38, ORT3 = 2.14, CI: 0.79-5.75) |
| Janus Serum Bank Cohort | Norway | Adult, gas chromatography with serum | Biomarker | Bassig, 201991 | 344 | o,p′-DDT, p,p′-DDE, p,p′-DDT, β-HCH, γ-HCH, dieldrin, HCB, mirex, heptachlor epoxide, oxychlordane, and trans-nonachlor | Acute myeloid leukemia (AML) | Matching factors: age at blood draw, county of residence, date of blood draw, sex Adjusted for: smoking status |
Increased risk of AML, though not statistically significant, among those with higher serum levels of total chlordane/heptachlor metabolites (ORT3 = 2.26, CI:0.91-5.63) Significant exposure-response associations were observed for levels of heptachlor epoxide (ORT3 = 2.85, CI: 1.05-7.73) and dieldrin (ORT3 = 2.71, CI: 1.07-6.83) |
| Adult, gas chromatography with serum | Biomarker | Lerro, 201892 | 324 | DDT, chlordane, HCH, HCB | Thyroid cancer | BMI and smoking status | Increased risk of thyroid cancer among participants born from 1943 to 1957 with higher levels of chlordane metabolites (OR per 10 ng/g = 1.78, CI:1.09-2.93) For individuals born before 1943, associations were generally null or in the inverse direction. Inverse association between DDT metabolites and thyroid cancer (OR per 1000 ng/g = 0.80, CI: 0.66-0.98) |
||
| Child Health and Development Studies | Berkely, CA | Adult, gas chromatography with serum | Biomarker | Cohn, 201919 | 266 | p,p′-DDT, p,p′-DDE and o,p′-DDT | Breast cancer | Age, parity, ethnic | Increased risk of early postmenopausal breast cancer was associated with p,p′-DDT for all women (ORDDT 50-54 = 1.99, 95% CI = 1.48-2.67). This association was accounted for by women first exposed to DDT after infancy (ORDDT 50-54 for first exposure after infancy = 2.83, 95% CI = 1.96-4.10. In contrast, for premenopausal breast cancer, p,p′-DDT was associated with risk among women first exposed during infancy through puberty, but not after (ORDDT < 50 for first exposure during infancy = 3.70, 95% CI = 1.22-11.26) |
| Korean Cancer Prevention Study | Korea | Adult, gas chromatography | Biomarker | Lim, 201793 | 366 | Oxychlordane, nonachlor (trans-, cis-), chlordane (trans-, cis-), heptachlor, hepatachlor epoxide (trans-, cis-), HCB, HCH (α-, β-, γ-, δ-), p,p′-DDT, o,p′-DDT, p,p′-DDD, o,p′-DDD, p,p′-DDE, o,p′-DDE | Prostate cancer | Age, BMI, smoking status | No evidence of association between organochlorine pesticides and prostate cancer |
- Abbreviations: CI, confidence interval; HR, hazard ratio; OR, odds ratio; Q4, Quantile 4; T2, tertile 2; T3, tertile 3.
- a HCB, HCH, β-HCH, g-HCH, d-HCH, heptachlor, cis-heptachlor epoxide, trans-heptachlor epoxide, cis-chlordane, trans-chlordane, oxychlordane, cis-nonachlor, trans-nonachlor, p,p′-DDT, o,p′-DDT, p,p′-DDE, o,p′-DDE.
| Study location | Exposure assessment timing/method | Exposure type | First author, year | Sample size | Pesticides | Outcome | Covariates | Findings |
|---|---|---|---|---|---|---|---|---|
| Connecticut | Adult, gas chromatography, serum | Biomarker (all exposure routes) | Deziel, 202194 | 500 | HCB, oxychlordane, trans-nonachlor, HCB, β-HCH, p,p′-DDT, p,p′-DDE and o,p′-DDT | Papillary thyroid cancer | Age, alcohol consumption, BMI, education, family history of cancer | No significant associations between measured pesticides and papillary thyroid cancer risk |
| British Columbia, Canada | Adult, gas chromatography with plasma | Biomarker (all exposure routes) | Darvishian, 202195 | 587 | aldrin, β-HCH, α-, γ-chlordane, cis-, trans-nonachlor, p,p′-DDT, p,p′-DDE, HCB, mirex and oxychlordane | Cutaneous malignant melanoma (CMM) | Age, education, hair color, moles, skin color, skin reaction to first sun exposure without sunscreen, skin reaction to repeated sun exposure without sunscreen, sun exposure | Increased CMM risk associated with increasing plasma concentrations of several OC pesticides (β-HCH, HCB, Mirex, oxychlordane and trans-nonachlor). For example, compared to lowest plasma concentration quartile of β-HCH, the second, third and fourth quartiles were associated with 1.3 (CI: 0.7-2.3), 2.1 (CI: 1.2-3.7) and 2.3(CI: 1.2-4.4) -fold increased risks of CMM, respectively |
| Perugia, Florence, Novara, Verona, Cagliari and Nuoro in Sardinia, and Bari and Taranto in Apulia | Adult, survey | Occupational | Meloni, 202196 | 1641 | Glyphosate | Lymphoma and all subtypes | Age, education, gender, study center | Increased risk of follicular lymphoma for those exposed to glyphosate (OR = 7.1, CI: 1.57-31.9). Ever glyphosate use was not associated with lymphoma (with all subtypes combined or with any other subtype). |
| Gironde, Calvados, Manche and Héraul, France | Adult, survey | Occupational | Baldi, 202197 | 1788 | Unspecific | CNST | NA | For overall agricultural exposure, we observed no increase in risk for all brain tumors (OR = 1.04, CI: 0.69-1.57) and a slight increase for gliomas (OR = 1.37, CI: 0.79-2.39). Risks for gliomas were higher when considering agricultural exposure for more than 10 years (OR = 2.22, CI: 0.94-2.24) and significantly trebled in open field agriculture (OR = 3.58, CI: 1.20-0.70). Increases in risk were also observed in nonagricultural exposures, especially in greenspace workers who were directly exposed (OR = 1.89, 0.82-4.39), and these were statistically significant for those exposed for over 10 years (OR = 2.84, CI: 1.15-6.99). |
| Andalusia, Spain | Adult, geospatial methods | Proximity to pesticide using facilities/farmland | Requena-Mullor, 202198 | 15 963 | Unspecific | Ovarian and testicular cancer | Age, environmental pesticide exposure | Increased risk of both ovarian and testicular cancer for those living in higher compared to lower pesticide use region (OR ovarian = 1.41, CI: 1.24-1.60, OR testicular = 1.59, CI: 1.37-1.85) |
| Kansas, Nebraska, and six Canadian provinces | Adult, survey | Occupational | Latifovic, 202099 | 4392 | Manya | Hodgkin's lymphoma | Age group, province or state of residence, respondent type (proxy, self), sex | HL cases ≤40 years old were three times more likely to report ever using dimethoate (OR = 3.76, CI: 1.02-33.84) and almost twice as likely to have ever used malathion (OR = 1.86, CI: 1.00-3.47). Those ≤40 years of age reporting use of 5 + organophosphate insecticides had triple the odds of HL (OR: 3.00, CI: 1.28-7.03). Longer use of 2,4-D, ≥6 vs 0 years, was associated with increased odds of HL (OR = 2.59, CI 1.34-4.97) |
| France | Prenatal, survey | Residential | Rios, 2020100 | 1217 | Unspecific | Wilm's tumor | Matching factors: child's age and gender Adjusted for: maternal age, urban status of the area of residence |
Increased risk of Wilms' tumor in children for those reporting maternal pesticide use during pregnancy (OR = 1.6, CI: 1.1-2.3). Among insecticides specifically, the association was stronger (OR = 1.7, CI: 1.1-2.6) and even more so when they were used more often than once a month (OR = 1.9, CI: 1.2-3.0) |
| California | Prenatal, geospatial methods | Proximity to pesticide using facilities/farmland | Park, 2020101 | 9967 | Manya | Acute lymphoblastic leukemia (ALL) and AML | Matching factors: birth year, Adjusted for: mother's ethnic, neighborhood, other carcinogenic pesticide exposure, socioeconomic status (SES) | Increased risks of ALL with exposure to any carcinogenic pesticide (adjusted odds ratio (aOR) = 2.83, CI: 1.67-4.82), diuron (Single-pesticide model, aOR = 2.38, CI: 1.57-3.60), phosmet (OR = 2.10, CI: 1.46-3.02), kresoxim-methyl (OR = 1.77, CI: 1.14-2.75), and propanil (OR = 2.58, CI: 1.44-4.63). Analyses based on chemical classes showed elevated risks for the group of 2,6-dinitroanilines (OR = 2.50, CI: 1.56-3.99), anilides (OR = 2.16, CI: 1.38-3.36) and ureas (OR = 2.18, CI: 1.42-3.34) |
| California′s Central Valley | Adult, geospatial methods | Proximity to pesticide using facilities/farmland | Tayour, 2019102 | 305 | Organochlorines, chlorpyrifos, diazinon, 1,3-dichloropropene | Breast cancer | Age, age at menarche, age at menopause, alcohol consumption, BMI, menopausal hormone therapy use, number of births, number of years lived in counties, oral contraceptive use, SES | Increased breast cancer risk among women exposed to chlorpyrifos (OR = 3.22, CI: 1.38-7.53) |
| Great Britain | Prenatal, job-exposure matrix | Occupational | Bunch, 2019103 | 5033 | Unspecific | Lymphoma (Hodgkin's, Burkitt and non-Hodgkin's) | Matching factors: birth registration subdistrict Adjusted for SES |
No one exposure was significantly associated with increased risk within all subgroups and for total lymphoma |
| San Joaquin Valley of California | Childhood, job-exposure matrix | Occupational | Mills, 2019104 | 189 | Manyc | Breast cancer | Age, age at first full-term pregnancy, family history of breast cancer, number of pregnancies, number of live births, protective clothing used during farm work | Increased odds of breast cancer for those with low compared to medium methyl parathion use compared to no use was significantly associated with (OR = 5.28, CI: 1.17-23.7). This relationship was not significant when comparing high use to no use. No other chemicals were significantly associated with breast cancer. |
| Costa Rica | Prenatal, survey | Residential | Hyland, 2018105 | 828 | Unspecific | ALL | Birth year, child sex, SES | Significant associations between maternal insecticide use inside the home in the year before pregnancy, during pregnancy and while breastfeeding; use during such periods was associated with increased risk of ALL among boys: (aOR = 1.63, CI: 1.05-2.53, 1.75, 1.13-2.73 and 1.75, 1.12-2.73, respectively). Observed dose-response relationships between the frequency of pesticide use in the home in the year before pregnancy, during pregnancy, and while breastfeeding and ALL among boys and girls: OR comparing high to low = 1.56, CI: 1.07-2.27, 1.58, 1.08-2.31, 1.56, 1.07-2.29, respectively) |
| Spain (Asturias, Barcelona, Cantabria, Girona, Gipuzkoa, Huelva, Leon, Madrid, Navarre, and Valencia) | Adult, geospatial methods | Proximity to pesticide using facilities/farmland | García-Pérez, 2018106 | 1963 | Unspecific | Breast cancer | Matching factors: age, and province of residence Adjusted for: age at menarche, age at first birth, BMI, family history of breast cancer, education, menopausal status, previous biopsies |
Increased risk for individuals within 2 km of industries releasing pesticides (OR = 2.09, CI: 1.14-3.82) |
| France | Prenatal, survey | Residential | Vidart d'Egurbide Bagazgoïtia, 2018107 | 35 539 | Unspecific | Brain tumors | Matching factors: age gender Adjusted for child's birth year, size of the urban unit of residence, type of housing |
Increased risk of childhood brain tumor for those reporting maternal home use of pesticides during pregnancy (OR = 1.4, CI 1.2-1.8) and specifically with insecticide use (OR = 1.4, CI: 1.2-1.8) |
| France | Adult, geospatial methods | Proximity to pesticide using facilities/farmland | Carles, 2017108 | 1470 | Unspecific | Brain tumors | Age, alcohol consumption, education, gender, mobile phone use, occupational exposure to pesticides, tobacco consumption | Increased risk of meningioma for those with proximity to open field crops above the 75th percentile (OR = 2.30, CI 1.04-5.10). No significant association was found between glioma and proximity to agricultural land. |
| France | Prenatal, survey | Residential | Rios, 2017109 | 2140 | Unspecific | Neuroblastoma (NB) | Matching factors: age gender Adjusted for: child's birth year, child's sex, size of the urban unit of residence, type of housing |
Increased risk of NB observed for those with prenatal use of any type of pesticide (OR = 1.5, CI: 1.2-1.9). The most commonly used type of pesticides were insecticides and an increased risk of NB was associated with their use alone (OR = 1.4, CI: 1.1-1.9) or with other pesticides (OR = 2.0, CI: 1.1-3.4) |
| Connecticut | Adult, job-exposure matrix | Occupational | Zeng, 2017110 | 960 | Unspecific | Thyroid cancer | Age, alcohol consumption, benign thyroids disease, BMI, family history of thyroid cancer, gender, physical activity ethnic, smoking, | Increased risk of thyroid cancer for individuals ever occupationally exposed to biocides(OR = 1.65, CI: 1.16-2.35) and the highest risk was observed for high cumulative probability of exposure (OR = 2.18, CI: 1.28-3.73). The observed observations were similar when restricted to papillary thyroid cancer and well-differentiated thyroid cancer. |
| South of Brazil | Adult, occupational records | Occupational | Boccolini, 2017111 | 3951 | Unspecified | Death from non-Hodgkin's lymphoma (NHL) | Education, ethnic, sex, state | Increased risk of death by NHL was observed for agricultural workers (vs nonagricultural workers) aged 20-39 years (OR = 2.06, CI: 1.20-3.14) |
| Minnesota | Adult, survey | Occupational | Poynter, 2017112 | 2073 | Unspecified | AML and myelodysplastic syndromes | Age, exposure to chemotherapy, lived on farm or in rural area for ≥1 year, sex | Pesticides and agricultural chemicals were not significantly associated with AML or MDS. |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
- a Organophosphate, chlorpyrifos, diazinon, dimethoate, famphur, fonofos, malathion, phorate, terbufos, organochlorine, aldrin, chlordane, dieldrin, DDT2, lindane, methoxychlor, carbamate, carbaryl, carbofuran.
- b Pendimethalin, trifluralin, oryzalin, ethalfluralin, benefin, propyzamide, isoxaben anilide, boscalid, propanil, propiconazole, tebuconazole, fenbuconazole, triadimefon, S-metolachlor, metolachlor, iprodione, ziram, maneb, mancozeb, metam-sodium, methyl-bromide, 1,3-dichloropropene, carbaryl, thiodicarb, dicofol, chlorpyrifos, dimethoate, malathion diazinond, phosmet, acephate, methidathion, S,S,S-tributyl phosphoro trithioate, permethrin, bifenthrin, S-cyper methrin, chlorothalonil dicloran, simazined, pymetrozine, diuron linuron, glyphosate, oxyfluorfen, paraquat dichloride, propargite norflurazon thiophanate methyl captan, clofentezine, buprofezin, kresoxim, methyl hexythiazox, pyrithiobac, sodium metaldehyde, pyraflufen ethyl chlorthal, dimethyl.
- c Endosulfan, captan, dicofol, malathion, mancozeb, maneb, methyl bromide, methyl parathion, 1,3-dichloropropene, propargite, parathion, simazine, triflurin, 2,4-D, toxaphene, chlordane, aldrin, dieldrin, lindane.
| Study location | Exposure assessment timing/method | Exposure type | First author, year | Sample size | Pesticides | Outcome | Covariates | Findings |
|---|---|---|---|---|---|---|---|---|
| Addis Ababa, Ethiopia | Adult, gas chromatography with serum | Biomarker (all exposure routes) | Mekonen, 2021113 | 100 | o,p′-DDT, p,p′-DDD, p,p′-DDE, p,p′-DDT, total DDT, heptachlor, aldrin, heptachlor-epoxide, γ-chlordane, endrin, endosulfan, dieldrin, methoxychlor, dibutyl-chlorendate | Breast cancer | Age, alcohol consumption, contraceptive use, smoking status and others | Mean serum (blood of cases drawn at diagnosis) level of p,p′-DDE, p,p′-DDT, heptachlor, gamma-chlordane, endosulfan, and dibutyl-chlorendate were significantly higher in the serum of breast cancer patients than the controls. An increased risk was observed for each unit increment of the concentration of p,p′-DDT (aOR = 2.03, CI: 1.041-3.969) and γ-chlordane (aOR = 3.12, CI; 1.186-8.203). |
| Delhi, India | Adult, gas chromatography with whole blood | Biomarker (all exposure routes) | Sharma, 2019114 | 200 | α-HCH, β-HCH, γ-HCH, dieldrin, endosulfan-I, endosulfan-II, p,p′-DDT, p,p′-DDE, heptachlor | CA-125, endothelial ovarian cancer | Age, drinking water source family history, parity | Significantly higher levels of β-HCH, endosulfan-I, endosulfan-II, p,p′-DDT, p,p′-DDE and heptachlor were found in cases than controls |
| Shantou city, China | Adult, gas chromatography with adipose tissue | Biomarker (all exposure routes) | Huang, 2019115 | 374 | DDT and DDE | Breast cancer | Age, age at menarche, menopausal status | Significantly increased risk of breast cancer for women in higher tertiles of DDE (OR = 1.63, CI: 1.15-2.85). No association between breast cancer and DDT |
| Kerman, Iran | Adult, gas chromatography with serum | Biomarker (all exposure routes) | Mortazavi, 2019116 | 87 | α-HCH, β-HCH, γ-HCH, [2,4-DDT], 4,4-DDT, [2,4-DDE] and 4,4-DDE | Bladder cancer | Matching factors: age, BMI, gender, living area | α-HCH, γ-HCH, 4,4-DDE, 2,4-DDT and 4,4-DDT were significantly higher in BC patients than controls |
| Kerman, Iran | Adult, gas chromatography with serum | Biomarker (all exposure routes) | Abolhassani, 2019117 | 72 | α-HCH, β-HCH, γ-HCH, [2,4-DDT], 4,4-DDT, [2,4-DDE] and 4,4-DDE | CRC | NA | Serum concentrations of all pesticides were higher in CRC patients than in controls |
| Daegu, Korea, | Adult, gas chromatography with serum | Biomarker (all exposure routes) | Lee, 2018118 | 277 | HCH, o,p′-DDE, p,p′-DDE, o,p′-DDT, p,p′-DDT, trans-chlordane, oxychlordane, trans-nonachlor, cis-nonachlor, heptachlor epoxide and heptachlor | Colorectal polyps and cancer | Age, alcohol consumption, BMI, cigarette smoking status, diabetes, family history of CRC, fiber intake physical activity, red meat consumption, sex, weight change | Increased risk of both polyps and cancer (aOR polyps = 2.3, CI: 0.9-5.7, aOR cancer = 3.6, CI:1.1-11.8) |
| Greenland | Adult, gas chromatography with serum | Biomarker (all exposure routes) | Wielsoe, 2017119 | 164 | p,p′-DDT, p,p′-DDE, mirex β-HCH, HCB, cis- and trans-nonachlor, aldrin, α-, γ-chlordane and oxychlordane | Breast cancer | Age, breastfeeding, BMI, cotinine, parity | The serum levels were significantly higher in cases compared to controls for the majority of the compounds, and after adjusting for age the difference was maintained for the sum of all organochlorine pesticides, and p,p′-DDE |
| Qingdao, China | Adult, gas chromatography with adipose tissue | Biomarker (all exposure routes) | He, 2017120 | 102 | β-HCH, γ-HCH, PCB28, PCB52, pentachlorothioanisole (PCTA) and p,p′-DDE | Breast cancer | NA | Increased risk of breast cancer among those with high concentrations of p,p′-DDE (OR = 0.129, CI: 0.0310-0.542) and PCB52 (OR = 0.656, CI: 0.441-0.976). The other five potential risk factors were not significantly related to breast cancer. |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
| Study location | Exposure assessment timing/method | Exposure type | First author, year | Sample size | Pesticides | Outcome | Covariates | Findings |
|---|---|---|---|---|---|---|---|---|
| Long Island, NY | Adult, survey | Residential | Niehoff, 2020121 | 1505 | Unspecific | Survival after breast cancer diagnosis | Age, education, marital status, parity, ethnic | Inverse association between all-cause mortality of the lawn and garden group of pesticides, for both ever use compared to never use (HR = 0.77, CI: 0.63-0.95) and higher lifetime applications (HRQ4 = 0.62, CI: 0.47-0.81). Nuisance-pest pesticides, and all groups combined, were not associated with all-cause or breast cancer-specific mortality. |
| North Carolina | Adult, gas chromatography, plasma samples | Biomarker (all exposure routes) | Parada, 2019122 | 748 | DDT, DDE | Survival after breast cancer diagnosis | Age at diagnosis, BMI, cancer stage, education, ER status, parity/lactation history, ethnic, smoking status | Increased risk of all-cause mortality for those with highest vs lowest DDE tertile and the highest vs nondetectable DDT (HR = 1.95, CI:1.31-2.92 and 1.64, CI: 1.10-2.44), respectively, for 20-year conditional all-cause mortality. Increased risk of 20-year conditional breast cancer-specific mortality among women overall, black women, and white women (HR = 1.69, CI:1.06-2.68, 2.36, 1.03-5.42, and 1.57, 0.86-2.89, respectively). |
| Copenhagen or Aarhus area, Denmark | Adult, gas chromatography, adipose tissue | Biomarker (all exposure routes) | Roswall, 2018123 | 399 | HCH, p,p′-DDE, p,p′-DDT, oxychlordane, trans-nonachlor, cis-nonachlor, heptachlor, dieldrin, HCB | Survival after breast cancer diagnosis, women with adverse prognostic factors. | NA | Inverse association between mortality and all pesticides, except β-HHC, which was not associated with mortality (mortality rate ratio [MRR] = 1.02, CI: 0.87-1.18 per IQR), and dieldrin, which was associated with an increased risk of death (MRR = 1.22, CI: 1.05-1.41 per IQR) |
| Copenhagen or Aarhus area, Denmark | Adult, gas chromatography, adipose tissue | Biomarker (all exposure routes) | Roswall, 2018124 | 232 | HCH, p,p′-DDE, p,p′-DDT, oxychlordane, trans-nonachlor, cis-nonachlor, heptachlor, dieldrin, HCB, PCBs | Survival after NHL diagnosis | Stratified by sex and age. Adjusted for alcohol consumption, physical activity, education, smoking status, waist circumference |
Concentrations in adipose tissue of the polychlorinated biphenyls (PCBs) and organochlorine pesticides were not associated with NHL survival. |
- Abbreviations: CI, confidence interval; HR, hazard ratio; IQR, inner-quartile range; Q4, quantile 4f.
In an analysis from the AHS focused on thyroid cancer, an increased risk of this cancer was found with exposure to metalaxyl (hazard ratio (HR) = 2.03, confidence interval (CI): 1.16-3.52), and lindane (HR = 1.74, CI: 1.06-2.84). High usage of the insecticide carbaryl was inversely associated with thyroid cancer (HR = 0.20, CI: 0.08-00.53).72 Another AHS analysis that examined renal cell carcinoma (RCC) found, in an unlagged analysis, an increased risk of RCC among ever users of 2,4,5-T compared to never users (RR = 2.92, CI: 1.65-5.17).73 In a 20-year lagged analysis, exposure-response relationships were observed between RCC risk and chlorpyrifos (RR = 1.68, CI: 1.05-2.70), chlordane (RR = 2.06, CI: 1.10-3.87), atrazine (RR = 1.43, CI: 1.00-2.03), cyanazine (RR = 1.61, CI: 1.03-2.50), and paraquat (RR = 1.95, CI: 1.03-3.70).73 No associations were observed with glyphosate exposure and solid tumors or lymphoid malignancies overall, nor with non-Hodgkin's lymphoma. An increased risk of acute myeloid leukemia (AML) was observed among the group with highest exposure to glyphosate (RR = 2.44, CI: 0.94-6.32).61 Other analyses from the AHS suggest that the risk of aggressive prostate cancer is increased by dimethoate use (HR = 1.37, CI: 1.04-1.8) and decreased with triclopyr use (HR = 0.68, CI: 0.48-0.95; Table 2).75
The GraMo cohort from Southern Spain was the only prospective cohort study included in this review that used biomarkers for exposure assessment. It found an increased risk of nonhormone-dependent cancer for individuals with increased exposure to β-hexachlorocyclohexane (β-HCH) (HR = 1.70; CI: 1.09-2.64) and hexachlorobenzene (HCB; HR 1.54, CI: 1.02-2.33).82
The prospective cohort studies that looked at potential dietary exposures to pesticides in relation to cancer report conflicting findings. Sandovali-Insuati and colleagues found no association between high-pesticide food intake and risk of cancer overall or for specific subtypes.80 However, Reboulliat et al., in a study looking at pesticide mixtures using Principal Component Analysis (PCA) reported increased risk of breast cancer for overweight and obese women with a higher component 1 score, reflecting high exposures to chlorpyrifos, imazalil, malathion, and thiabendazole (HRQ5 = 4.13, CI: 1.50-11.44). They also reported an inverse association between breast cancer risk and component 3 score, reflecting low exposure to synthetic pesticides, and [HRQ5 = 0.57, CI: (0.34-0.93); Table 2].81
As mentioned, two large, prospective studies looked at the relationship between parental exposure to pesticides, assessed through job-exposure matrices and childhood cancer. In the census-based cohort study in Switzerland, maternal and paternal pesticide exposure increased noncentral nervous system (CNS) solid tumors (high vs none, father: adjusted HR = 1.84, CI: 1.31-2.58; mother: HR = 1.79, CI: 1.13-2.84).84 No associations were found between parental exposure and leukemia, lymphoma or CNS tumors (Table 2).84
The similarly designed analysis of pooled data on more than 329 000 participants from the International Childhood Cancer Cohort Consortium indicated that paternal exposure to pesticides increased the risk of childhood AML (herbicides, HR = 3.22, CI: 0.97-10.68; insecticides, HR = 2.86, CI: 0.99-8.23), but not of acute lymphoblastic leukemia (ALL) or CNS tumors (Table 2).83
Several nested case-control studies analyzed data from the aforementioned Janus cohort, a population-based biobank with sera samples prospectively collected from 1972 to 2004 from more than 300 000 Norwegian men and women.90 In a study assessing the relationship between organochlorine pesticides and thyroid cancer, an inverse relationship between DDT metabolites and thyroid cancer risk was observed (OR = 0.80, CI: 0.66-0.98).92 Additionally, significant positive exposure-response relationships were seen between chlordane metabolites and thyroid cancer among participants born from 1943to 1957. These findings are compatible with birth cohort effects and with effects specific to certain historic periods.92 Investigating AML, researchers observed significant exposure-response relationships for heptachlor epoxide (third vs first tertile OR = 2.85, CI: 1.05-7.73) and dieldrin (third vs first tertile OR = 2.71, CI: 1.07-6.83) with AML risk.91 No statistically significant exposure-response associations with AML risk were observed for other pesticides, although the magnitude of the estimates was substantial for chlordane/heptachlor metabolites (third vs first tertile OR = 2.26, CI: 0.91-5.63) and p,p'-DDT (OR = 2.09, CI: 0.83-5.26; Table 3).91
Another study included analyses from two case-control studies nested in different cohorts. Relationships between serum concentrations of organochlorine pesticides and primary liver cancer were assessed separately among the Janus cohort and a Northern Californian Kaiser Permanente cohort in one report.90 Among Janus participants, an exposure-response relationship was observed for p,p′-DDT (second and third tertile ORs = 1.70, CI: 0.66-4.38, and 2.14, CI: 0.79-5.75, respectively). Among the Californian participants, there was an exposure-response relationship for trans-nonachlor (second and third tertile ORs = 1.63, CI: 0.87-3.06 and OR = 1.95, CI: 0.98-3.86), as well as an elevated risk for the highest tertile of oxychlordane (OR = 1.87). None of these relationships were statistically significant (Table 3).90
A case-control study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort assessed the relationship between pesticide exposure and pancreatic cancer risk. Increased risk was observed at higher concentrations of p,p′-DDT, trans-nonachlor (OR upper quartile = 1.55, CI: 1.06-2.26), β-HCB and the sum of six organochlorine pesticides (OR upper quartile = 1.48, CI: 1.00-2.20; Table 3).86
In a case-cohort study nested within the Korean National Cancer Center Community (KNCCC) cohort, a significantly increased risk of colorectal cancer (CRC) was associated with serum concentrations of cis-heptachlor epoxide (third tertile HR = 2.76, CI: 1.25-6.07); and significant exposure-response relationships were observed for trans-nonachlor (second tertile HR = 3.90, CI: 1.56-9.75, third tertile HR= 4.86, CI: 1.95-12.16) and p,p′-DDD (second tertile HR = 6.02, CI: 2.05-17.70, third tertile HR = 7.43, CI: 2.42-22.84).87 A different case-cohort study out of Korea looking at prostate cancer found evidence of association between any measured pesticides and prostate cancer.93 A pilot case-control study including Hawaiian participants that provided urine samples from the Multiethnic Cohort study showed a nearly 4.5-fold increased risk of developing breast cancer in the highest vs lowest quintile of AMPA (a glyphosate metabolite) excretion: Q5 vs Q1 OR = 4.49, CI: 1.46-13.77 (Table 3).88
Four population-based case-control studies investigated the relationship between self-reported use of pesticides at home among mothers, and childhood cancer. The Costa Rican Childhood Leukemia study observed significant associations between maternal insecticide use inside the home in the year before pregnancy, during pregnancy, and while breastfeeding; use during such three periods was associated with increased risk of ALL among boys: adjusted OR = 1.63 (CI: 1.05, 2.53), 1.75 (1.13, 2.73) and 1.75 (1.12, 2.73), respectively.105 They also observed dose-response relationships between the frequency of pesticide use in the home in the year before pregnancy, during pregnancy, and while breastfeeding and ALL among boys and girls: OR comparing high to low = 1.56 (CI 1.07-2.27), 1.58 (1.08-2.31) and 1.56 (1.07-2.29), respectively (Table 4).105 In the ESTELLE study, a French nationwide population-based case-control study, maternal use of pesticides during pregnancy was associated with the risk of Wilms' tumor in children (OR = 1.6, CI: 1.1-2.3).100 Among insecticides, specifically, the association was similar: OR = 1.7, CI: 1.1-2.6; when insecticides were used more than once a month, the OR was 1.9 (CI: 1.2-3.0).100 The two other studies report pooled data from the ESTELLE study and the ESCALE study, another French nationwide population-based case-control study. One showed an association between maternal residential use of pesticides during pregnancy and childhood brain tumors (OR = 1.4, CI: 1.2-1.8). The other reported more specifically on neuroblastoma (NB), and found an increased risk of NB among children prenatally exposed to any type of pesticide (OR = 1.5, CI: 1.2-1.9; Table 4).107, 109
All eight hospital-based case-control studies used biomarkers (Table 5). Sample sizes were small, especially in two instances.116, 117 All such studies reported more than one positive association. Notably, both Lee et al118 and Abolhassani et al117 found relationships between several serum concentrations of pesticides and colorectal cancer. Also Huang,115 Wielsoe,119 Mekonen113 and He,120 all report statistically significant associations between dichlorodiphenyldichloroethylene (DDE) concentrations and breast cancer.
Three survival studies assessed the survival of patients after a diagnosis of breast cancer, while one assessed survival after a diagnosis of NHL (Table 6). The only of the breast cancer studies to use a biomarker for exposure assessment, and to control for confounding variables122 found an increased risk of all-cause mortality among the highest vs lowest DDE tertile and the highest tertile vs nondetectable DDT (HR = 1.95 and 1.64, respectively). It also reported an increased risk of 20-year conditional breast cancer-specific mortality among women overall (HR = 1.69), higher for black women (2.36), than for white women (1.57; Table 6). Concentrations in adipose tissue of polychlorinated biphenyls (PCBs) and organochlorine pesticides were not associated with NHL survival.121
4 DISCUSSION
In the years between 2017 and 2021, we found 63 empirical studies of sufficient quality and relevance, which seems a low number compared to other areas of research in cancer and epidemiology (and even lower if compared to studies from clinical specialties as oncology). These studies, in addition to others identified by this review, suffer from some limitations with exposure assessment imprecision, as they rely on crude extrapolations from geographical data or self-report of exposures that may have occurred in the distant past. Clearly, there remain serious gaps in our understanding of exposure-response relationships, structures of confounding, pathways of exposure, and mechanisms of action.
Three of four survival studies assessed patients after a diagnosis of breast cancer; with one studying survival after an NHL diagnoses, hence, there is a clear need for studies to assess the influence of pesticides on clinical progression of cancer, including treatment response.130
We next assess the studies main findings adapting some of Austin B. Hill's criteria for causal inference; we applied criteria that are relevant to the aims of this review131, 132 among the criteria he proposed more than 50 years ago during debates about environmental carcinogens.133 While not all relevant causal criteria can be fully addressed by the research included in this review, we use Hill's framework to contextualize findings and identify gaps hindering causal inference.
4.1 Temporality
Thirty-three of the 63 studies identified for this review assessed exposure to pesticides prospectively, prior to cancer development.19, 61, 64-93 These studies provide the strongest evidence due not only to their prospective and precise exposure assessment, but also due to large sample sizes. Additionally, conversely to case-control and cross-sectional studies, these studies are less susceptible to recall bias, a differential misclassification that tends to bias these studies away from the null.63 Among these 33 studies, including studies reporting consistent associations of increased risk of AML61, 87, 91 and CRC, the causal condition of temporality is fulfilled. Most of these studies report positive associations between pesticide use and cancer. The largest of these studies are from the AGRICOH consortium; pooled analyses of several prospective cohorts. The studies describe increased risk of NHL associated with terbufos, an organophosphate pesticide with similar chemical properties to glyphosate, and increased incidence of MM and CNST compared to the general population.64, 65 Additionally, studies that used biomarkers prospectively were also likely to report positive associations. The EPIC cohort, with robust covariate adjustment and sensitivity analyses, reported increased risk of pancreatic cancer with exposure to summed organochlorine pesticides, β-HCH, DDT, and trans-nonachlor.86
4.2 Consistency
The existing literature is not well suited to address this consistency criterium, as the studies are highly variable in exposure assessment, dose, timing, latency periods and so forth. Thus, here we describe patterns observed that provide some evidence of consistency and discuss potential reasons for heterogenous findings. Though all studies tested multiple hypotheses and thus some are likely due to chance, all but 6 of 63 studies reported at least one statistically significant positive association between some measure of exposure to pesticides and cancer. Several studies report positive relationships that are robust, with consistent results in sensitivity analyses.19, 61, 66, 67, 72-74, 83, 84, 86, 89-92 Further, these studies often assess exposure to several, highly correlated, pesticides. Some analyses were conducted for individual chemicals, sometimes with adjustment for others.66, 125 Sometimes this may be a form of overadjustment, with little significance both from mechanistic and practical standpoints. Adjusting for exposure to correlated pesticides may also introduce multicollinearity, and result in potentially biased and unstable estimates of association.134
While study conclusions varied, four out of six studies assessing AML reported evidence of increased risk for subjects with higher exposure to specific pesticides; particularly glyphosate, hepatachlor-epoxide, dieldrin and chlorpyrifos and carbamates.61, 76, 83, 91, 101, 112 In Andreotti et al, this risk is insignificant for unlagged models, but significant when considering a 20-year lagged analysis.61 The studies reporting positive associations include large, prospective studies that measured several individual pesticides; one included prospectively collected biomarkers for exposure ascertainment. In the two exceptions, pesticides overall (rather than specific pesticides) were analyzed; this approach is documented to have weaker associations with AML.112 Additionally, Coste et al discuss limitations in their analysis, as only three AML cases were in the high exposure category.84 Results were also consistent across studies looking at CRC: all three studies observed an increased risk of CRC with higher exposure to pesticides.87, 117, 118 It is also worthwhile to note that (a) these studies provide evidence of causal relationships between pesticides and different types of cancer, (b) these studies look at pesticides with different mechanisms of action, and (c) to fully conclude that the consistency criteria was fulfilled, more research would be needed on specific individual pesticide-cancer relationships.
Although the body of evidence has increased recently, there are still issues of reproducibility and conflicting findings. One well-known reason for these inconsistencies is the existence of a long latency period for cancer development. IARC states that “experience with human cancer indicates that the period from first exposure to the development of clinical cancer is sometimes longer than 20 years; latent periods substantially shorter than 30 years cannot provide evidence for lack of carcinogenicity”.11 Even cohort studies with the longest follow-up periods, like the AHS, have a roughly 20-year follow-up period; they may thus fail to capture longer-term effects. They may also fail to assess the effects of exposure during critical windows.125
Another possible explanation of the apparently conflicting evidence is heterogeneity in unmeasured co-exposures and in exposure (mis)classification.135 While some studies measured specific pesticides, many measured exposures generally or used various classification schemes to operationalize exposure variables for statistical models. Examples of exposure variables include ever/never use of specific pesticides, cumulative intensity-weighted lifetime days, occupationally exposed vs not, proximity to facilities using pesticides, and serum/tissue levels. While all such practices are acceptable, they complicate comparisons of findings across studies; and such difficulties should not be equated with lack of consistency.135 However, to fully address this criterium, more research replication efforts are needed.
4.3 Strength
As cancer is multifactorial, it is not expected for the association between cancer and any individual environmental exposure to be large in magnitude. However, we can assess the strength of an association in response to increasing dosage to assist in inferring causality. Several of the studies identified exposure-response relationships between pesticide exposure and cancer risk in humans of a substantial strength or magnitude.73, 86, 87, 90-92 These relationships show that increased exposure granularly increases risk, strengthening the claim that the relationship is in fact causal.
4.4 Plausibility
Several mechanisms have been proposed to explain the observed relationships between cancer and pesticides. Among these are genotoxicity, hormone disruption (particularly acetylcholine esterase inhibition),136 oxidative stress, inflammation, immune modulation and procarcinogen activation.11-14 Oxidative stress, a main proposed mechanism, has been connected to cytochrome P-450 induction, resulting in the formation of reactive oxygen species with the ability to cause DNA damage, genome instability and cell proliferation.137
4.5 Coherence
The relationship between exposure to many pesticides and the occurrence of cancer in animal and in vitro studies is well established; it is often summarized in the IARC monographs, for instance.11, 40, 136, 138-140 In the primary human studies we reviewed here, there is modest coherence, partly due to the difficulties with exposure imprecision. A major conclusion of this article is that newer, biomonitoring-based designs can resolve some of the issues with coherence in the literature.
4.6 Specificity
Studies have identified some stronger effects in aging populations, but this is not what is generally considered a specific population. Indeed, in cancer research, specificity is an uncommon causal property: few exposures have unique effects and few cancers are due to exclusive exposures. Accordingly, few studies on pesticides and cancer have assessed specificity directly, such as by identifying control conditions not associated with pesticide exposure. Nevertheless, future studies could sometimes consider specificity; for example, ask whether their findings suggest a specific population with the effects or a specific site of the disease with no other likely explanation. Studies could also assess “falsifier exposures” (exposures tested to confirm specificity of effect of pesticides vs an exposure without biological plausible carcinogenicity), and use “negative controls”40; for example, examine associations of pesticides with conditions known to be unrelated to the pesticides. There may also be specific routes of pesticide exposure (eg, dermal, inhalational) that may be most problematic.
4.7 Analogy
The existence of causal relationships between similar chemicals and cancer increases the likelihood that observed associations are causal. There are several instances of similar environmental chemicals being carcinogenic. For example, formaldehyde, a substance commonly used as a germicide, is known to cause myeloid leukemia, and rare cancers including cancers of the nasal cavity, and nasopharynx.141 Also, and most notably, ethylene oxide, a chemical used as a pesticide, is recognized as a cause of lymphoma and leukemia.142
4.8 Further suggestions for future work
To improve the validity and relevance of the available knowledge regarding exposure to pesticides and cancer risk in humans, studies must increase in number, diversify and improve in several respects. We need more large population cohort studies that allow for longer latency periods. These cohorts also enable to conduct efficient nested case-control studies using biological samples (collected at baseline) for exposure assessment. More hypotheses need to be tested in younger and nonoccupational cohorts, with emphasis on nonoccupational sources of exposure (as diet) and on developmental periods of susceptibility.
The strengths of the main study designs (large prospective cohort studies, cohort-nested case-control studies with long follow-up) hence deserve greater allocation of funds and wider recognition in regulatory institutions, companies, clinical organizations and the media.
Tools from molecular epidemiology (including environmental, epigenetic, metabolomic, exposomic and proteomic measurements used in population-based studies) can be leveraged in cancer cohorts,16 as shown, for instance, in recent papers from the European Prospective Investigation into Cancer and Nutrition (EPIC) study,86, 143 or in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO).144 Also recent is the integration of multi-omics analysis in cohorts as the Norwegian Women and Cancer (NOWAC),145 and the EPICURE study.146 Additionally, advances in biomonitoring will also contribute to progress.147 Such technologies include wearable personal sensors, location trackers and nontargeted methods.148, 149 As cohorts incorporating such technologies mature, evidence will increase in quality and strength as exposure assessment will be a more accurate reflection of real-world exposure. Additionally, multi-omics data will provide rich biological context for mechanisms of environmental carcinogens. Successful adaptation of these techniques and tools remains both a challenge and an avenue for future research.150-152
Furthermore, humans are rarely subjected to individual environmental exposures, but rather to complex chemical mixtures, and novel methods have been developed to allow for analysis of these mixtures.153 Low doses of even noncarcinogenic chemicals can act synergistically to contribute to carcinogenesis, and mixtures analysis has long been a concern for researchers.154, 155 In this review there were only three studies that examined interactions of pesticides and other factors in cancer, and no studies that employed statistical methods designed to assess effects of environmental chemical mixtures. More research on chemical-chemical, chemical-(epi)genetic and other interactions and mixtures156 must be pursued.
Existing evidence is largely from older, occupationally or agriculturally exposed populations; sometimes, studies also used exposure assessment methods with important limitations. While some large prospective cohort studies were conducted, these studies were entirely occupational, not population-based, and relied on self-report of specific pesticide use or crop management to assess exposure, often years after exposure took place.66, 72 These studies are more susceptible to measurement error than studies using biomarkers, and the occupational cohorts cannot be generalized to much of the population at large. Studies that used biomarkers to assess exposure were mostly small hospital-based case-control studies, which collect biological specimens (where exposures are later determined) at the time of diagnosis, often months after the disease is progressing subclinically; hence, they are susceptible to disease progression bias, a form of reverse causality (concentrations of biomarkers may be a result of the disease, not a cause).40, 86 Such studies may also suffer from bias in control selection, particularly when studying gene-environment interactions.135, 157, 158
Of the existing study designs, the design that often balances best exposure assessment methods with sample size and bias control is the cohort-nested case-control study. Unfortunately, only a small number of studies were published in the time period of interest and represent only 15% of the included studies. These studies benefit from prospectively collected biological samples that cannot be influenced by disease status and integrate all sources of exposure (including food, water or air if appropriate). They are also a part of a larger cohort, which contributes to cost and logistical efficiency.135 Cases and controls are matched on follow-up time and some potential confounders to efficiently control for confounding and biomarker storage/batch effects (eg, cases and controls are matched on fasting status at blood collection).86, 159 Such study design, while currently underrepresented in the literature, is growing in popularity and is a good option to strengthen the current body of evidence on the relationship between cancer and pesticides.135
The current body of research is also lacking representation of urban cohorts and data on residential and food exposure to pesticides. Only 10.5% of the reviewed studies looked at residential pesticide use, and there were no explicit investigations of urban pesticide exposure. While frequently overlooked, cities are an important landscape of pesticide use. The NYC Department of Health and Mental Hygiene released a report in 2017 documenting a total of 6711 gal of liquid and 163 182 pounds of solid pesticide products across city agencies the previous year. This large quantity only represents only a minority of urban pesticide use; comparisons to state commercial pesticide use data revealed that city agency use only accounts for an estimated 3% of liquid pesticides, and 21% of the solid pesticides applied in the city.160 The level of pesticide use in cities is thus nontrivial, and different soil properties, environmental fate of pesticides and exposure profiles in urban environments warrant further investigation of the unique health burden of urban pesticide use.161
4.9 When is enough evidence enough?
While further research is always needed, the question ultimately is whether the evidence is sufficient to better regulate pesticides on the basis of carcinogenicity. Debates about causality and the sufficiency of evidence to act not new; for instance, causal inference was addressed by Immanuel Kant and other philosophers centuries ago. It was also raised decades ago by Hulka and others in the context of carcinogenesis.162 It applies both to sufficient evidence to act, when a causal relationship is likely, and to not act, when a causal relationship is unlikely. Hill also emphasized that “all scientific work is incomplete… [and] liable to be upset or modified by advancing knowledge.”133 He added: “That does not confer upon us a freedom to ignore the knowledge we already have or postpone the action that it appears to demand at a given time.”133 Although there are limitations and gaps in the research as well as some discordant findings, there is sufficient evidence for action to regulate pesticides based on their carcinogenicity.
Science has also evolved greatly over the near 60 years since Hill gave his landmark lecture, and scientific studies and institutions have addressed crucial flaws in the risk assessment paradigm leveraged in the European Union, the United States and elsewhere to manage environmental exposures. The conventional paradigm sometimes assumes, in particular, a linear dose-response, which has been shown not to occur in many causal relationships.40, 163 Indeed, the endocrine system, in particular, has revealed U-shaped (nonmonotonic) and nonlinear exposure-response functions, including for pesticides and developmental neurotoxicity.164-166
Conflicting findings and gaps in data have tangible consequences. In 2016, the United States Environmental Protection Agency (US EPA) published an issue paper stating that glyphosate (which, as mentioned earlier, is classified by IARC as probably carcinogenic), is not a carcinogen; and it decided to allow for its continued and increasing use, a decision EPA reaffirmed in 2020.167, 168 The European Food Safety Authority came to a similar conclusion as the EPA in a 2015 report, where they claim glyphosate is “unlikely to pose a carcinogenic threat to humans”.169 The main reason for the opposed conclusions from these authorities is that IARC studied the genotoxic and carcinogenic properties of not only pure glyphosate, but also of glyphosate-based formulations, pesticides where glyphosate is the active ingredient.170 The evidence of carcinogenicity for glyphosate-based formulations and metabolites is stronger than for technical glyphosate, as co-formulants affect absorption, distribution, metabolism and excretion of glyphosate.170, 171 Additionally, the IARC committee only considers peer-reviewed literature, while the assessments done by EPA and EFSA also considered several industry studies conducted by private companies that register the chemicals in question. In response to the ongoing debate, calls have been made within the scientific community for more independently conducted epidemiological research, particularly among the highest exposed and the most vulnerable populations, as well as for more comprehensive and transparent evaluations by independent agencies.170, 172, 173
5 CONCLUSIONS
Though high-quality studies have been published since the IARC monograph on organophosphate insecticides in 2017, there are still gaps in the literature for carcinogenic evidence in humans, in addition to a large number of potentially carcinogenic pesticides that were not included in the IARC assessment. Rather consistent and high-quality evidence of carcinogenicity of pesticides exists for AML and CRC. To further knowledge, suggested next steps include leveraging new techniques and methods developed to increase sensitivity and precision of exposure assessment, incorporating multi-omics data and investigating exposures to complex chemical mixtures. Additionally, we identify a need for more large and longer population-based cohort studies, which should include younger, nonoccupationally exposed individuals during developmental periods of susceptibility. Though improved and diversified research is necessary to better understand environmental contributions to cancer, the existing literature provides enough evidence to justify regulatory action that would reduce human exposure to pesticides.
AUTHOR CONTRIBUTIONS
Haleigh Cavalier: Conceptualization, Data curation, Formal analysis, Project administration, Visualization, Writing - Original draft preparation, Writing - review & editing. Leonardo Trasande: Conceptualization, Supervision, Writing - review & editing. Miquel Porta: Conceptualization, Project administration, Supervision, Writing - Original draft preparation, Writing - review & editing. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
The work was supported in part by research grants from the National Institutes of Health (P30ES000260); Instituto de Salud Carlos III - FEDER, Ministry of Health, Government of Spain (FIS PI17/00088, FIS PI21/00052 and CIBER de Epidemiología y Salud Pública - CIBERESP); the Hospital del Mar Medical Research Institute (IMIM), Barcelona; Fundació La Marató de TV3 (20132910); the CRUE-Santander Fondo Supera Covid-19 (15072020); and the Government of Catalonia (2017 SGR 439). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other institutions.
CONFLICT OF INTEREST
The authors have no conflicts of interest in connection with the paper, and declare no competing financial interests.
Open Research
DATA AVAILABILITY STATEMENT
Only publicly available data were used in our study, and data sources and handling of these data are described in the Materials and Methods and in Tables 2-6. Further information is available from the corresponding author upon request.




