Exploring the potential of exosomes in diagnosis and drug delivery for pancreatic ductal adenocarcinoma
Biaoming Xu, Yu Chen and Mingjing Peng contributed equally to this study.
Funding information: Science and Technology Bureau, Changsha, Grant/Award Number: KQ2004130; National Natural Science Foundation of China, Grant/Award Number: 82170192
Abstract
Pancreatic cancer (PC) is a cancer of the digestive system, and pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 90% of all PC cases. Exosomes derived from PDAC (PDAC-exosomes) promote PDAC development and metastasis. Exosomes are nanoscale vesicles secreted by most cells, which can carry biologically active molecules and mediate communication and cargo transportation among cells. Recent studies have focused on transforming exosomes into good drug delivery systems (DDSs) to improve the clinical treatment of PDAC. This review considers PDAC as the main research object to introduce the role of PDAC-exosomes in PDAC development and metastasis. This review focuses on the following two themes: (a) the great potential of PDAC-exosomes as new diagnostic markers for PDAC, and (b) the transformation of exosomes into potential DDSs.
Graphical Abstract
Abbreviations
-
- 5-FU
-
- 5-fluorouracil
-
- ACTH
-
- abnormal connective tissue hyperplasia
-
- APCs
-
- antigen-presenting cells
-
- CA19-9
-
- carbohydrate antigen 19-9
-
- CAFs
-
- cancer-associated fibroblasts
-
- CD
-
- cytosine deaminase
-
- CRC
-
- colorectal cancer
-
- CT
-
- computed tomography
-
- CTLs
-
- cytotoxic T lymphocytes
-
- CTNF-α
-
- TNF-α that were fused with cell penetrating peptides
-
- CTNF-α-exosomes
-
- exosomes loaded with TNF-α that are fused with cell-penetrating peptides
-
- DCs
-
- dendritic cells
-
- DDSs
-
- drug delivery systems
-
- ECM
-
- extracellular matrix
-
- EGFR
-
- epidermal growth factor receptor
-
- EM
-
- electron microscopy
-
- ER
-
- endoplasmic reticulum
-
- EUS-FNA
-
- endoscopic ultrasound-guided fine needle aspiration
-
- EVPs
-
- extracellular vesicles and particles
-
- GBM
-
- glioblastoma multiforme
-
- GEM
-
- gemcitabine
-
- GPC1
-
- glypican-1
-
- hMSH2
-
- human DNA MutS homolog 2
-
- IAC
-
- immunoaffinity chromatography
-
- ILVs
-
- intraluminal vesicles
-
- miRNAs
-
- microRNAs
-
- MRI
-
- magnetic resonance imaging
-
- mRNAs
-
- messenger RNAs
-
- MSCs
-
- mesenchymal stem cells
-
- PC
-
- pancreatic cancer
-
- PD-1
-
- programmed cell death-1
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- PDAC-exosomes
-
- exosomes derived from PDAC
-
- PDAC-TME
-
- the TME of PDAC
-
- PD-L1
-
- programmed death-ligand 1
-
- PSCs
-
- pancreatic stellate cells
-
- PTX
-
- paclitaxel
-
- R-Exos
-
- recombinant exosomes
-
- SEC
-
- size-exclusion chromatography
-
- siRNA
-
- small interfering RNA
-
- TME
-
- tumor microenvironment
-
- TMZ
-
- temozolomide
-
- TNF-α
-
- tumor necrosis factor-α
-
- UPRT
-
- uracil phosphoribosyl transferase
1 INTRODUCTION
Pancreatic cancer (PC) is a cancer of the digestive system. It has an insidious onset, rapid progression and extremely poor prognoses.1 As the most common type of PC, pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 90% of PC cases.2 Current radiotherapies and chemotherapies are often ineffective in PDAC treatment. The 5-year survival rate of patients with PDAC is approximately 10%.3
PDAC tumors are composed of cancer cells and tumor microenvironment (TME).4 Cancer cells and TME participate jointly in the development and metastasis of PDAC.
Exosomes are vesicles that are secreted by most cells. Exosomes derived from PDAC (PDAC-exosomes) are important components of the TME of PDAC (PDAC-TME).5 The exosome components are closely related to their donor cells. PDAC-exosomes specifically reflect the physiological status of PDAC.5
This review considers clinically representative PDAC as the main objective to introduce the physiological effects of PDAC-TME and PDAC-exosomes. This review focused on the following two themes: (a) PDAC-exosomes are expected to become new tumor diagnostic markers for PDAC, and (b) exosomes have great potential as drug delivery systems (DDSs) for PDAC.
2 THE TME PROVIDES AN IMPORTANT PHYSIOLOGICAL BASIS FOR THE DEVELOPMENT AND METASTASIS OF PDAC
The PDAC-TME is a complex physiological structure inside tumors. It promotes the development and metastasis of cancer cells and helps cancer cells resist clearance by the immune system and drugs. The PDAC-TME is composed of various non-neoplastic cells, extracellular matrix (ECM) and related soluble metabolites (Figure 1).6, 7
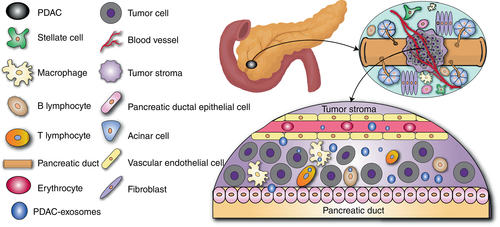
Representative non-neoplastic cells in the PDAC-TME include, but are not limited to, pancreatic stellate cells (PSCs), cancer-associated fibroblasts (CAFs), myeloid-derived suppressor cells and tumor-infiltrating immune cells.1
Generally, ECM is present in most human tissues. It is a dense network mainly composed of proteins, polysaccharides and enzymes.8 Abnormal connective tissue hyperplasia (ACTH) is a histological feature of PDAC.9 Cancer cells stimulate PSCs to upregulate the expression of collagen and fibronectin through the paracrine pathway, thus inducing connective tissue proliferation in the tumor stroma. ACTH causes PDAC-TME to contain abnormally rich ECM.10, 11 ECM poses challenges to PDAC treatment. It is worth noting that PDAC is characterized by a large amount of ECM compared to other solid tumors.12 There are many soluble metabolites in the PDAC-TME. Some soluble metabolites play key regulatory roles in PDAC progression.13, 14
3 PDAC-EXOSOMES PLAY AN IMPORTANT ROLE IN THE DEVELOPMENT AND METASTASIS OF PDAC
Exosomes are a class of vesicle structures secreted by cells ranging in diameter from 50 to 150 nm.15, 16 Furthermore, exosomes may be the most well-defined extracellular vesicles. Figure 2 shows the basic structure and process of cell secretion and absorption of the exosomes.
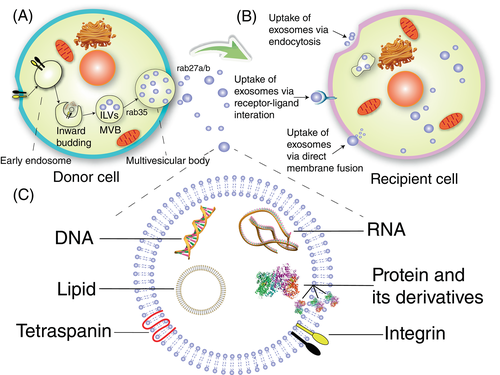
-
PDAC-exosomes contain diverse proteins and microRNAs (miRNAs). After being taken up by other cells via phagocytosis, PDAC-exosomes release proteins and miRNAs inside these cells. The released proteins and miRNAs can interfere with the normal physiological activities of these cells, thereby favoring PDAC progression. A study conducted a comprehensive analysis of the proteome of exosomes derived from human PC cell lines AsPC-1 (RRID: CVCL_0152), BxPC-3 (RRID: CVCL_0186), MIA PaCa-2 (RRID: CVCL_0428), normal pancreatic ductal epithelial cell line hTERT-HPNE (RRID: CVCL_C466) and HPDE (RRID: CVCL_4376). Our study showed that 362 proteins were specifically expressed in exosomes derived from AsPC-1, BxPC-3 and MIA PaCa-2 cells, most of which induced PC progression.23 For example, annexin A1 of exosomes secreted by MIA PaCa-2 cells promoted cancer migration, invasion and angiogenesis.24 In addition, exosomes secreted by the highly aggressive PC cell line PC-1.0 (RRID: CVCL_L901) were rich in miRNA-125b-5p. The miRNA-125b-5p significantly induced PC invasion and metastasis.25
PDAC-exosomes create a suitable microenvironment at metastatic sites for cancer metastasis. Mouse Kupffer cells are a type of macrophage in the liver. After absorbing PDAC-exosomes, Kupffer cells upregulate the expression of transforming growth factor-β to promote fibronectin expression of hepatic stellate cells. Fibronectin enhances the recruitment of bone marrow-derived macrophages to create a microenvironment for liver metastasis of PDAC.26
- 2.
PDAC-exosomes mediate immune escape and suppression in PDAC. Programmed death-ligand 1 (PD-L1) is an immunoglobulin superfamily type I transmembrane protein.27 The combination of PD-L1 of cancer cells and programmed cell death-1 (PD-1) of cytotoxic T lymphocytes (CTLs) can lead to T-lymphocyte exhaustion and tumor immunity escape.27 Metastatic melanoma can release many exosomes that contain PD-L1 in their surface to inhibit the immune response of CTLs and promote tumor progression.28 Although there remains no similar report about PDAC, the PD-L1 in cancer cells of PDAC mediates tumor immune escape.29 Therefore, PDAC-exosomes may also contain PD-L1 to inhibit anticancer CTL effects.
T lymphocytes are the key cells that mediate adaptive immune responses against cancer. BxPC-3 is a PDAC cell line isolated from clinical tumor samples. BxPC-3 cells secrete exosomes vigorously. In vitro co-culture helps to explore how BxPC-3 cells inhibit the activity of T lymphocytes. In the co-culture experiments of exosomes derived from BxPC-3 cells and human T lymphocytes in vitro, exosomes derived from BxPC-3 cells could abnormally induce endoplasmic reticulum (ER) stress and apoptosis of human T lymphocytes by upregulating the phosphorylation level of p38 mitogen-activated protein kinases in human T lymphocytes.30 The ER stress response could reduce the intracellular concentration of unfolded proteins and hinder their aggregation. This is generally a self-protective mechanism of cells. However, the exosomes of BxPC-3 cells induced T-lymphocyte apoptosis through abnormal activation of the ER stress response. Therefore, inducing apoptosis of T lymphocytes by exosomes may be an important way for PDAC cells to resist immune clearance.30
However, exosomes from nonmetastatic melanoma cells effectively can inhibit lung metastasis by stimulating the proliferation of Ly6Clow patrolling monocytes in the bone marrow and promoting cancer cell elimination in the lung.31 Therefore, not all cancer-derived exosomes can promote cancer metastasis.
- 3.
PDAC-exosomes enhance drug resistance in PDAC. Gemcitabine (GEM) is a chemotherapeutic drug typically used for PC treatment. CAFs in the PDAC-TME have endogenous resistance to GEM. Quantitative polymerase chain reaction showed that the transcription level of miRNA-106b in exosomes secreted by CAFs was significantly upregulated following GEM treatment. TP53INP1 is a key regulatory gene of tumor suppressors. The miRNA-106b specifically binds to TP53INP1 and inhibits its regulatory function, increasing CAFs' resistance to GEM. Most importantly, PDAC cells acquire miRNA-106b by absorbing exosomes from CAFs. Exosomal miRNA-106b in CAFs can also inhibit the regulation of TP53INP1 in PDAC cells. Therefore, CAFs can transmit drug resistance to PDAC cells via exosomes in the TME.32
The following strategies may contribute to inhibiting the drug resistance of PDAC: (a) inhibiting the synthesis and secretion of exosomes in PDAC lesions to block the cell-to-cell transmission of drug resistance and (b) lowering the content of miRNAs that promote drug resistance in PDAC lesions.
4 PDAC-EXOSOMES ARE POTENTIAL DIAGNOSTIC INDICATORS FOR PDAC
Exosomes are widely present in body fluids.33 Components of exosomes are mainly regulated and determined by their donor cells. Cancer-derived exosomes are significantly different from those of healthy tissues, reflecting the special physiological status of tumors.34 This particularity is the basis for PDAC-exosomes as potential PDAC diagnostic markers.
4.1 Representative isolation and detection techniques for PDAC-exosomes
Isolation of exosomes is the first step in the diagnosis based on PDAC-exosomes. Ultracentrifugation, ultrafiltration, size-exclusion chromatography (SEC) and immunoaffinity chromatography (IAC) are representative exosome separation techniques.35-42 Electron microscopy (EM) and flow cytometry are classic exosome detection methods.43-47 Microfluidic technology is a novel exosome detection technology (Table 1).
| Item | Fundamental | Main application | Advantage | Limitation | References |
|---|---|---|---|---|---|
| Ultracentrifugation | Centripetal force based on mass and size | Isolation | Large batch processing; Easy operation; low cost | Long processing time; Affect exosome structure | [37, 41, 48] |
| Ultrafiltration | Differences in molecular sizes of substances | Isolation | Large batch processing; Easy operation | The filter membranes are easily clogged or damaged | [37, 39, 41] |
| Size-exclusion chromatography | Differences in molecular sizes of substances | Isolation | Retain the intact structure and natural biological activity of exosomes | Inability to separate substances with similar mass | [35, 38, 41, 42] |
| Immunoaffinity chromatography | Antigen-antibody specific binding | Isolation | High product purity | High cost, especially specific antibodies that adsorb exosomes | [37, 40, 41] |
| Electron microscopy | Interaction of electron beams and samples | Detection | Accurately reflect exosome structures | The equipment is expensive; Sample processing may alter the original properties of exosomes | [43-46] |
| Flow cytometry | Cell sorting and quantification based on optical signals | Detection | Rapid analysis of samples with different characteristics | High requirements for sample processing | [47, 49] |
| Microfluidics technology | Antigen-specific adsorption and computer comprehensive analysis | Isolation and detection | Low dosage requirements for samples; integrated sample separation and analysis; high degree of automation, suitable for large-scale clinical applications | Current technology is immature; High cost; difficulty in integrating multiple disciplines and technologies | [50-52] |
Ultracentrifugation is the most widely used exosome-separation technology. Ultracentrifugation is suitable for the initial purification of exosomes.37 Ultrafiltration places a high demand on membranes with specific pore sizes. Before the discovery of exosomes, SEC was widely used in the high-resolution separation of macromolecules, such as proteins, polymers and various liposome particles.35, 38 However, SEC cannot effectively separate substances with similar molecular weights.35, 38 High-purity exosomes separated by IAC provide convenience for quantitative analysis. Only exosomes containing the target protein can be adsorbed by the corresponding antibodies.40 Different separation and purification methods have different effects on exosomes. Ultracentrifugation may lead to the structural disruption of exosomes and co-precipitation of soluble protein impurities. Moreover, the yield of exosomes obtained by ultracentrifugation is low.48 The efficiency and yield of ultrafiltration to isolate exosomes are higher than those of ultracentrifugation, but ultrafiltration may also lead to the deformation and rupture of exosomes, which may bias the results of downstream analysis.37 Compared to ultracentrifugation and ultrafiltration, SEC not only protects the structure of exosomes, but also effectively removes the contamination of soluble proteins and improves the purity of exosomes to provide the basis for DDSs and biomarkers.41 High cost remains an important factor limiting the large-scale application of IAC.41
EM and flow cytometry are classic techniques used to characterize the nature and structure of exosomes.43-47 Microfluidics technology uses microtubes to analyze trace amounts of samples. Microfluidic technology has the characteristics of high detection sensitivity and fast detection speed that give it good clinical application potential.50, 51 Immunoaffinity-based microfluidic technology uses antibodies immobilized on chips to specifically recognize and adsorb exosomes with corresponding ligands. For example, immunoaffinity-based microfluidic technology can efficiently isolate exosomes from trace serum and cell culture media using anti-CD63 antibodies.52 Microfluidic technology can integrate exosome isolation and analysis to change the paradigm of exosome-based diagnostics.
4.2 The feasibility of exosomes as indicators for PDAC detection
Current diagnostic tools for PDAC are computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and blood tests (Table 2).53-55, 64
| Item | Principle | Advantage | Application | Limitation | Maturity | References |
|---|---|---|---|---|---|---|
| Computed tomography | Imaging detection based on X-ray | Tissue imaging and localization with high-resolution; short detection time | Main diagnostic and analytical tool | Radiation exposure; inability to detect biochemical information. | Mature | [53, 54] |
| Magnetic resonance imaging | Imaging detection based on signals generated by atomic nuclei within magnetic fields | Tissue imaging and localization with high-resolution; no radiation exposure | Main diagnostic and analytical tool | High cost of detection; inability to detect biochemical information; strictly confined metal implants; long detection time | Mature | [53] |
| Endoscopic ultrasound-guided fine needle aspiration | Imaging detection based on ultrasound | If necessary, imaging and sampling can be performed at the same time | Main diagnostic and analytical tool | Invasive testing may cause infection; high operating and learning costs | Mature | [53, 55] |
| Traditional blood test | Biochemical analysis based on tumor-specific markers other than PDAC-exosomes, especially CA19-9 | Reflect the biochemical information; low testing cost | Auxiliary tools for diagnosis and assessment | No imaging information;the sensitivity and specificity of detection need to be further improved | Mature | [56] |
| Exosome detection | Biochemical analysis based on tumor-specific markers of PDAC-exosomes | Reflecting biochemical information more accurately compared to traditional blood test | Auxiliary tools for diagnosis and assessment | The technology is not yet mature; the cost needs to be further reduced; no imaging information | Immature | [49, 57-63] |
Carbohydrate antigen 19-9 (CA19-9) is the most commonly used blood test marker for PDAC. In addition to PDAC, other intestinal diseases may also lead to the upregulation of CA19-9 markers in the blood.56 CA19-9 does not have sufficient specificity for early PDAC. The development of new auxiliary detection markers will contribute to improving the early PDAC diagnosis. Detecting PDAC by PDAC-exosomes has enough feasibility as follows: (a) MRI requires patients not to carry any metal objects to ensure safety, which means that people with metal grafts in their bodies cannot be tested by MRI. Contrast agents used for CT may cause serious adverse effects. EUS-FNA can lead to pathogenic infections and severe discomfort. In contrast, body fluid detection based on exosomes has lower requirements and side effects for patients.53-55, 65, 66 (b) Real-time accurate tracking of tumor physiological status can be realized via PDAC-exosomes, providing a reliable basis for the diagnosis and PDAC treatment.51, 67, 68 (c) In some clinical trials, the diagnostic sensitivity and specificity of miRNA detection indicators in PDAC-exosomes extracted from plasma were better than those of CA19-9. Although more clinical data are needed for further verification, PDAC-exosomes can be reliable diagnostic markers for PDAC.69 Compared to other solid tumors, the early symptoms of PDAC are not obvious. It is difficult to detect early PDAC by using conventional methods. PDAC-exosomes carry various biological information about tumor cells and the detection of PDAC-exosomes is helpful for the early diagnosis of PDAC. Therefore, exosome diagnosis is an attractive option for the detection of PDAC.
Proteins and RNAs are the main indicators of PDAC-exosomes. The proteins in PDAC-exosomes can be divided into two categories: (a) proteins that are ubiquitous in most exosomes from different cells, such as CD9, CD63, CD81 and heat shock proteins57, 58; and (b) proteins whose contents are specifically upregulated in PDAC-exosomes.57, 58 These proteins can be used as reliable detection indicators. For example, Glypican-1 (GPC1) is specifically enriched in PDAC-exosomes. GPC1 detection accurately distinguished healthy individuals from patients with early or advanced PDAC.59
Circular RNAs in exosomes can be stored for a long time at room temperature and remain stable.60 The expression of circular RNA-IARS was upregulated in PDAC tumors and plasma exosomes of patients with metastatic disease.68 Circular RNA-IARS accessed human microvascular vein endothelial cells via PDAC-exosomes, followed by increased endothelial monolayer permeability to promote cancer invasion and metastasis.68 Circular RNA-IARS could be a reliable indicator for early diagnosis and prognostic prediction of PDAC.68 In addition, miRNAs are another candidate detection indicator. A clinical trial showed that miRNA-200b and miRNA-200c were overexpressed in serum PDAC-exosomes. The combined diagnosis with miRNA-200b and miRNA-200c made the diagnostic accuracy of CA19-9 increase to 97%.61 In another clinical trial, miRNA-1246, miRNA-4644, miRNA-3976 and miRNA-4306 were significantly upregulated in 83% of PC-exosomes of serum. The levels of these miRNAs in exosome-depleted serum were also upregulated. Their levels in exosome-depleted serum were still much lower than those in exosomes. This clinical trial combined miRNA detection with flow cytometry to provide reliable references for the early diagnosis of PDAC.49 A recent clinical trial showed that the combination of CA19-9 detection and miRNA sequencing analysis of exosomes in blood both increased the sensitivity and specificity of distinguishing PDAC and pancreatitis to approximately 90%.62 The serum-based exosome detection is expected to become an auxiliary detection method for PDAC screening in the future.
A recent clinical trial demonstrated the feasibility of screening for early-stage cancer by detecting tumor-specific substances in extracellular vesicles and particles (EVPs) in body fluids.63 More than 400 samples of human tissues, blood and other bodily fluids were collected. These samples covered 18 different stages of cancer, such as breast, colon, lung, pancreatic and so forth.63 The results showed that (a) there were large differences in EVPs proteins among humans and mice, which meant that we cannot rely solely on mouse models to simulate human liquid biopsy markers; (b) there was a group of multiple proteins in EVPs. The levels of these proteins were significantly higher in EVPs derived from patients with cancer. They could be used as tumor diagnostic markers, and (c) cancer types corresponding to EVPs could be analyzed and judged using reasonable statistical methods.
Compared to classical imaging methods such as CT, MRI and EUS-FNA, PDAC diagnostic methods based on exosome detection are better at providing case analysis based on biochemical information rather than imaging information. In fact, for small lesions in the early stage of solid tumors, classical imaging methods may not provide sufficient information. PDAC diagnostic methods based on exosome detection may be more important in the early stages of a cancer diagnosis.
The following points can help improve the early diagnosis of PDAC: (a) middle-aged and elderly people are at higher risk of developing PC. They should receive regular early screening and diagnosis. (b) The comprehensive application of different diagnostic methods can help promote the early diagnosis of PC. CT and ultrasonography are noninvasive imaging methods. The combined detection of CT and ultrasonography is suitable for diagnosing the underlying lesion structures. Serum exosome screening can be used to detect tumor-specific markers of PDAC. The combined detection of CA19-9 and exosomal diagnostic markers in serum can help to improve the accuracy of diagnosis; and (c) the development of more efficient automated detection systems can cover more populations and facilitate an early diagnosis of PDAC.
5 THE FEASIBILITY OF EXOSOMES AS DRUG DELIVERY SYSTEMS AGAINST PDAC
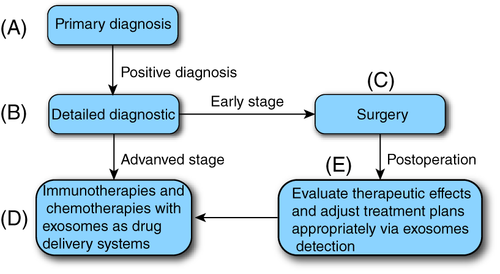
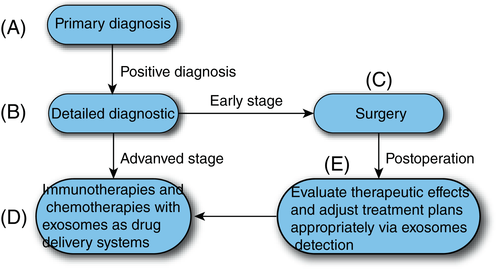
Currently, the development of ideal DDSs for improving PDAC treatment is of great interest. We proposed a blueprint for the diagnosis and treatment of PDAC (Figure 3). First, early diagnosis can be achieved by detecting PDAC-exosomes in body fluids. Then, accurate diagnosis and staging of patients with a positive diagnosis of PDAC- exosomes were performed. Early PDAC can be effectively removed surgically. Advanced patients receive immunotherapy or chemotherapy with exosomes as DDSs. In addition, the physiological status of PDAC can be tracked continuously using the PDAC-exosome detection.
5.1 Tumor targeting of exosomes
Tumor targeting is an important DDSs indicator. Studies have shown that unmodified tumor-derived exosomes are poorly targeted to tumors.70 Unmodified tumor-derived exosomes were quickly cleared after intravenous injection in mouse models, after which tumor-derived exosomes showed affinity for tumors, and it was related to the similar cell membrane composition of tumor-derived exosomes and intratumoral cells.70 Therefore, it is important to enhance the tumor targeting of native exosomes.
Displaying molecules that enhance tumor targeting on the cell membrane of tumor-derived exosomes is an important way to enhance tumor targeting. For example, the expression level of the epidermal growth factor receptor (EGFR) is significantly upregulated in human breast cancer cells and exosomes. EGFR can serve as a receptor target for DDSs. Epidermal growth factors significantly promote mitosis and angiogenesis in cancer cells. Therefore, Ge11 peptides with higher biosafety were screened.71 Ge11 peptides were found to specifically bind to EGFR in breast cancer.71 Most importantly, Ge11 peptides did not significantly promote mitosis and neovascularization in breast cancer. The Ge11 peptide gene was then integrated into HEK293 cells (RRID: CVCL_0045). Exosomes secreted by HEK293 cells could efficiently target breast cancer cells in mouse models. Considering that the expression of EGFR is significantly increased in most PDACs, similar tumor-targeting enhancement strategies may also be applicable to PDACs in the future.72
5.2 Advantages of exosomes as drug delivery systems for PDAC
Chemotherapies generally have defects such as a high circulation clearance rate and serious side effects. As novel DDSs, liposomes, nanomaterials, antibody-drug conjugates and exosomes are expected to improve the PDAC prognosis.73-78
Exosomes have the following characteristics required for ideal DDSs: (a) they generally have low levels of immunogenicity and cytotoxicity79; and (b) they can improve drug stability to reduce dosages and side effects. For example, CD47 is ubiquitous in most exosomes derived from human tissue. CD47 significantly delay the exosome clearance by monocytes and macrophages80; and (c) exosomes efficiently penetrate biological membranes to improve drug delivery efficiency. Exosomes generally have good biocompatibility and affinity with corresponding donor cells or tissues.81 Exosomes, nanomaterials, liposomes and antibody-drug conjugates have been compared from the perspective of their properties as DDSs (Table 3).
| Item | Size | Ingredient | Natural tumor targeting | Administration | Drug delivery capacity | Side effect | Immune boosting | Production complexity | References |
|---|---|---|---|---|---|---|---|---|---|
| Exosome | 50-150 nm | RNA, lipids, proteins and their derivatives, cytokines | Tumor-derived exosomes show tumor-targeting/affinity | Transdermal drug delivery, intravenous, intratumor | Good capacity | Generally weak | Generally weak | Immature | [16, 82-86] |
| Nanomaterial | 1-100 nm | Carbon, metal, etc. | Generally weak before necessary modification | Oral, intravenous, intratumor | Good capacity with necessary modification | Weak or strong, depending on composition and structure of materials | uncertain, depending on specific nanomaterial | More mature than exosomes | [74-76] |
| Liposome | 25-1000 nm | Natural phospholipids, synthetic phospholipids, cholesterol | Generally weak before necessary modification | Oral, intravenous, intratumor | Good capacity with necessary modification | Generally have lipid-toxic | Depending on the similarity between liposomes and cell membranes | More mature than exosomes | [73, 74] |
| Drug alone | Wide range, no strict scope | Depending on specific situations | Generally weak before necessary modification | Oral, intravenous, intratumor, skin smear | Generally weak | Generally strong | Uncertain, depending on specific drugs | Different drugs have different process difficulties | [77, 78, 87] |
| Antibody-drug conjugate | Wide range, no strict scope | Antibodies and drug molecules | Depending on antibodies | Oral, intravenous | Mainly depending on antibodies | Antibodies can reduce side effects by improving drug delivery efficiency | Uncertain, depending on specific Antibodies | More mature than exosomes | [77, 78] |
Most studies have transformed exosomes derived from healthy cells into DDSs for the PDAC treatment. Exosomes rich in miRNA-1231 extracted from mesenchymal stem cells (MSCs) significantly inhibited the PDAC progression in mouse models.88 The miRNA-3607-3p in exosomes derived from NK cells significantly inhibited PDAC metastasis by directly targeting interleukin-26 in PANC-1 cells (RRID: CVCL_0480) in mouse models.89 In summary, some exosomes derived from healthy cells are not only good drug delivery vehicles, but also contain natural anticancer molecules.
The methods for loading therapeutic molecules into exosomes can generally be divided into endogenous and exogenous modes (Figure 4). The endogenous mode means that therapeutic molecules are first incubated with donor cells. When released from donor cells, the drug-loaded exosomes are separated and purified. The endogenous mode is simple to operate. However, it cannot precisely control drug loading efficiency and may destroy the natural physiological functions of membrane proteins.82-84 Exosomes are first separated and purified from donor cells and then loaded with therapeutic molecules using methods such as electroporation. The exogenous mode may cause problems such as exosome aggregation and membrane damage.82-84
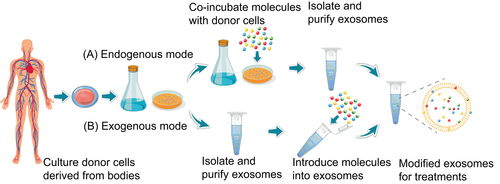
5.3 Exosomes carry multiple types of anticancer drug molecules
Safe delivery of therapeutic molecules into PDAC tumors represents a bottleneck in clinical treatment. Studies have shown that exosomes have great potential to carry different therapeutic molecules, including proteins, cytokines, chemical drugs, RNAs, tumor antigens and immune adjuvants.
5.3.1 Protein
Currently, there are no reliable clinical cases of the delivery of therapeutic proteins into PDAC tumors via exosomes. However, a study has successfully delivered therapeutic cytosine deaminase (CD) proteins fused with uracil phosphoribosyl transferase (UPRT) into schwannomas in mouse models via exosome-based DDSs, providing valuable references for PDAC.85 The process of protein loading into exosomes was through overexpression of CD-UPRT proteins in donor cells. CD-UPRT proteins convert inactive 5-fluorocytosine into 5-fluorouracil (5-FU) with anticancer activity, thereby effectively regressing schwannomas in mouse models.
5.3.2 Cytokine
Tumor necrosis factor-α (TNF-α) is a cytokine that is mainly secreted by macrophages. Due to serious side effects and the lack of natural tumor targeting, TNF-α, which can induce tumor necrosis, has long been severely restricted in clinical cancer treatment.86 A study safely and efficiently promoted the binding of TNF-α with corresponding tumor necrosis factor receptor I on membrane surfaces of cancer cells and then induced melanoma necrosis in mouse models via tumor-targeted DDSs based on exosomes.86 First, MSCs secreted exosomes (CTNF-α-exosomes) loaded with TNF-α were fused with cell-penetrating peptides (CTNF-α). CTNF-α was anchored to the outer surfaces of CTNF-α-exosomes. To achieve tumor targeting, membrane-targeted superparamagnetic iron oxide nanoparticles were combined with CTNF-α-exosomes through transferrin receptors. Guided by an external magnetic field, this novel drug carrier has a significant therapeutic effect on melanoma with fewer side effects. Although the therapeutic target is not PDAC, our study provides an attractive idea to enhance the tumor targeting of exosomes by magnetic nanomaterials for PDAC.
5.3.3 Chemical
Paclitaxel (PTX) is a well-known broad-spectrum chemotherapeutic molecule that promotes the apoptosis of cancer cells by inhibiting mitosis.87 However, the PTX does not have natural tumor-targeting properties. Direct administration of PTX often leads to severe loss of delivery and side effects.
MSCs are easy to isolate and proliferate in vitro. MSCs exist in various mammalian tissues.90 MSCs are enriched in tumors after systemic injection.91 Natural tumor targeting makes MSCs a common cell model for the delivery of anticancer drugs.92 MSCs can release exosomes rich in PTX after being stimulated by PTX in vitro assays.90 This phenomenon might be a MSCs self-protection mechanism: the PTX was transported outside cells via exosomes to avoid the PTX cytotoxicity. Exosomes rich in PTX significantly inhibited the proliferation of CFPAC-1 (RRID: CVCL_1119), a human PC cell isolated from PDAC, indicating that PTX did not lose its anticancer activity after being encapsulated by MSC-derived exosomes. In summary, exosomes secreted by MSCs may help achieve effective chemotherapeutic drug delivery to PDAC tumors. Under the stimulation of chemotherapeutic drugs, MSCs may secrete exosomes rich in other classic chemotherapeutic drugs against PDAC, such as GEM.
5.3.4 RNA
MiRNAs are endogenous noncoding RNAs with regulatory functions in eukaryotes. Mature miRNAs recognize target messenger RNAs (mRNAs) through complementary base pairing and guide silencing complexes to degrade or inhibit the translation of targeted mRNAs.93
Studies have found that CD133(+) glioblastoma multiforme (GBM) cells highly express miRNA-9. miRNA-9 mediates the CD133(+) GBM resistance to temozolomide (TMZ).94 Blocking miRNA-9 relieves the TMZ resistance in GBM. MSCs communicate with cancer cells via gap junctions and exosomes. Therefore, MSCs could transport exosomes rich in anti-miR-9 into GBM to neutralize miRNA-9 and restore the sensitivity of CBM to TMZ in vitro assays.95 Recently, exosomes have been used to deliver small interfering RNA (siRNA) and oxaliplatin into PDAC tumors to recruit more CTLs within tumors in mouse models.96
5.3.5 Tumor antigen and immune adjuvant
Endogenous tumor antigens contained in tumor cell-derived exosomes can be presented to antigen-presenting cells (APCs) to enhance anticancer immune effects, especially in dendritic cells (DCs).97 Therefore, exosomes derived from tumor cells can simultaneously deliver natural tumor antigens and exogenous immune adjuvants to APCs to enhance the anticancer immune response. For example, exosomes from mouse melanoma B16-BL6 cells (RRID: CVCL_0157) effectively delivered the immune agonist CpG-DNA and tumor antigens to DCs in vitro.98
In addition, exosomes of the human PDAC cell line MIA PaCa-2 not only retained tumor targeting, but also reduced the risk of inducing cancer metastasis after removing the contents.99 Content-free exosomes derived from MIA PaCa-2 cells were stably loaded with the photosensitizer chlorin e6 to form recombinant exosomes (R-Exos). R-Exos inhibited the proliferation of MIA PaCa-2 cells by generating reactive oxygen species through laser irradiation. Moreover, tumor antigens in exosomes can activate antitumor immunity.
5.4 Key clinical applications of exosomes in PDAC
Diagnosis and treatment are two important applications of exosomes in the clinical treatment of PDAC.
Recently, two mRNAs (WASF2 and ARF6) and two small nucleolar RNAs (SNORA74A and SNORA25) of exosomes in serum have been proved to be effective in distinguishing patients with PDAC from control participants in clinical trials.100 The sensitivity of this assay exceeds that of conventional CA19-9. The detection of tumor diagnostic markers in PDAC-exosomes is expected to become an important clinical auxiliary detection index in the future.
In terms of diagnosis, the isolation of high-purity PDAC-exosomes from clinical samples at a low cost is a key issue. Recently, microfluidic technology has become more effective in the separation and detection of exosomes in clinical samples. As early as 2010, a microfluidic immunoaffinity device was able to rapidly isolate exosomes using anti-CD63 antibodies.52 The device could effectively separate exosomes from the cell culture medium and serum for subsequent RNA detection and analysis. In the future, effective early screening of PDAC may be realized using trace amounts of blood extracted from fingertips.
In terms of treatment, the formulation of a scientific and effective manufacturing practice for medical exosomes remains controversial. The evaluation of the conditions under which patients are suitable for exosome-based treatment involves ethics and laws. In addition, it may not be realistic to extract sufficient amounts of exosomes from patients under poor conditions. In fact, PDAC-exosomes are at risk of promoting cancer development and metastasis. The potential risks of cancer-derived exosomes require further exploration. Recently, exosomes extracted from normal somatic cells, which are convenient for large-scale production, have been shown to be biologically safe. However, tumor-targeting of exosomes derived from healthy cells is usually insufficient. Modification of exosomes with materials such as nanoparticles and antibodies against cancer cells is an important method for enhancing the tumor targeting of exosomes. Modification and transformation based on tumor-targeting molecules and magnetic nanomaterials can effectively improve the tumor-targeting ability of exosomes derived from healthy cells.101-104
Clinical trials on DDSs based on exosomes are ongoing. A phase I clinical trial of MSC-derived exosomes loaded with siRNA for the treatment of metastatic PDAC with KrasG12D mutations is currently underway (ClinicalTrials.gov identifier: NCT03608631). This phase I trial mainly studied the optimal dose and side effects of siRNA-loaded exosomes. Engineered exosomes may be effective in treating PC in related reports.
5.5 Engineered exosomes reverse drug resistance of PDAC
Colorectal cancer (CRC) is the third most common cancer in the world. The chemotherapy based on 5-FU is a typical treatment regimen for CRC. Long-term 5-FU treatment can lead to drug resistance. Deregulation of miRNAs in cells is an important factor in inducing CRC resistance to 5-FU. CRC upregulates intracellular miRNA-21 to inhibit the expression of human DNA MutS homolog 2 (hMSH2) and enhances drug resistance to 5-FU.105
The resistance of colon cancer cells to 5-FU could be significantly reversed by exosomes loaded with the miRNA-21 inhibitor and 5-FU in mouse models.106 First, engineered exosomes containing Her2 proteins were cultured and extracted from the genetically modified HEK293T cell line (RRID: CVCL_0063).106 Her2 was a membrane protein and a specific tumor-homing polypeptide. Her2 enhanced the targeting of engineered exosomes to HCT 116 (RRID: CVCL_0291) colorectal cancer cells. The inhibitor of miRNA-21 and 5-FU were then co-loaded into engineered exosomes by electroporation.106 After systemic administration, engineered exosomes significantly downregulated miRNA-21 levels and promoted 5-FU uptake in the 5-FU-resistant cell line HCT 116. The downregulation of miRNA-21 promoted the apoptosis of malignant cells and restored the expression of miRNA-21 regulatory targets PTEN and hMSH2. Compared to the administration of miRNA-21 inhibitor or 5-FU alone, the combination of miRNA-21 inhibitor and 5-FU via engineered exosomes effectively reversed the resistance of HCT 116 cells to 5-FU and significantly inhibited tumor progression in mouse models. In fact, the level of miRNA-21 was also significantly upregulated in malignant PDAC cells.107 Therefore, the combined administration of miRNA-21 inhibitors and drug molecules via engineered exosomes could reverse PDAC resistance and improve prognosis.
6 DISCUSSION AND CONCLUSION
Recently, exosomes have shown great potential in the clinical diagnosis and treatment of PDAC. PDAC-exosomes may be novel early diagnostic markers for PDAC. Exosomes have good biocompatibility and drug-loading capacity to improve drug delivery efficiency and reduce side effects.
Engineering modifications of exosomes are expected to improve the PDAC targeting. In addition, engineered exosomes have great potential to reverse PDAC drug resistance and improve clinical therapeutic effects. Diagnosis and treatment solutions based on exosomes may help reduce the threat and burden of PDAC on human health in the future.
AUTHOR CONTRIBUTIONS
Biaoming Xu, Yu Chen and Mingjing Peng participated in the writing of articles and the preparation of charts to the same extent. Jinhai Zheng participated in the collection and collation of literature. Above all, Chaohui Zuo, as correspondent author, provided guidance and revision throughout the whole process. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
Our study was supported by the National Natural Science Foundation of China (grant No. 82170192) awarded to Dr Chaohui Zuo and by the Science and Technology Bureau, Changsha (grant No. KQ2004130) awarded to Chaohui Zuo.
CONFLICT OF INTEREST
All the authors of this article have no conflicts of interest.