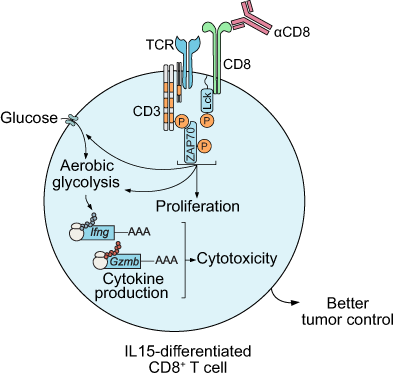
CD8 agonism functionally activates memory T cells and enhances antitumor immunity
Funding information: CRI Lloyd J. Old STAR Award, Grant/Award Number: 3914; Deutsche Forschungsgemeinschaft, Grant/Award Numbers: 259332240/RTG2099, CU375/5-1, CU375/5-2, CU375/7-1, CU375/9-1; EMBO Young Investigator Award; ERC Consolidator Award, Grant/Award Number: #101045416; Helmholtz Young Investigator Award, Grant/Award Number: VH-NG-1113; HI-TRON Kick-Start Seed Funding, Grant/Award Number: HITR-2021-08; The German Cancer Aid Foundation, Grant/Award Numbers: 70113343, 70114224; The Hector Foundation, Grant/Award Number: M20102; The Helmholtz Zukunftsthema Ageing and Metabolic Programming, Grant/Award Number: ZT0026
Abstract
Memory CD8+ T cells mature after antigen clearance and ultimately express CD8 protein at levels higher than those detected in effector CD8+ T cells. However, it is not clear whether engagement of CD8 in the absence of antigenic stimulation will result in the functional activation of T cells. Here, we found that CD8 antibody-mediated activation of memory CD8+ T cells triggered T cell receptor (TCR) downstream signaling, enhanced T cell-mediated cytotoxicity and promoted effector cytokine production in a glucose- and glutamine-dependent manner. Furthermore, pretreatment of memory CD8+ T cells with an agonistic anti-CD8 antibody enhanced their tumoricidal activity in vitro and in vivo. From these studies, we conclude that CD8 agonism activates glucose and glutamine metabolism in memory T cells and enhances the efficacy of memory T cell-based cancer immunotherapy.
Graphical Abstract
What's new?
Memory CD8+ T cells mature after antigen clearance and ultimately express high levels of CD8 protein. However, it is unclear whether engagement of CD8 in the absence of antigenic stimulation could result in the functional activation of T cells. Our study shows that CD8 agonists can activate memory T-cell glucose and glutamine metabolism in the absence of T-cell receptor stimulation. Furthermore, CD8 agonists can enhance the efficacy of memory T cell-based cancer immunotherapy. The authors propose targeting CD8 as a potentially useful, intrinsically specific and importantly, tumor-agnostic approach to antitumor immunotherapy.
Abbreviations
-
- ADCC
-
- antibody-dependent cellular cytotoxicity
-
- Arm
-
- Armstrong
-
- CTV
-
- CellTrace Violet
-
- ECAR
-
- extracellular acidification rate
-
- Fab
-
- antigen-binding fragment
-
- Fc
-
- fragment crystallizable
-
- GzmB
-
- granzyme B
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- Lck
-
- lymphocyte-specific protein tyrosine kinase
-
- LCMV
-
- lymphocytic choriomeningitis virus
-
- mAb
-
- monoclonal antibody
-
- MPEC
-
- memory precursor effector cells
-
- OCR
-
- oxygen consumption rate
-
- pfu
-
- plaque-forming unit
-
- SLEC
-
- short-lived effector cells
-
- SRC
-
- spare respiratory capacity
-
- TCM
-
- central memory T cells
-
- TCR
-
- T cell receptor
-
- TEM
-
- effector memory T cells
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- TNF
-
- tumor necrosis factor
-
- ZAP70
-
- zeta-chain associated protein kinase 70
1 INTRODUCTION
CD8+ T cells are a subset of T lymphocytes that express the transmembrane glycoprotein CD8. The expression of CD8 on the surface of CD8+ T cells is not static.1-3 Instead, longitudinal analysis of naive, effector and memory CD8+ T cells in the course of viral infections reveals a dynamic pattern, with naive and memory CD8+ T cells expressing higher levels of CD8 protein than effector CD8+ T cells.4 The rebound of CD8 protein level that accompanies the transition from the effector to the memory CD8+ T cell phenotype presumably promotes the interaction between T cell receptor (TCR)-peptide-major histocompatibility class I (MHC-I) and increases the avidity of memory CD8+ T cells for recurrent antigens.
CD8 is traditionally considered to be a co-receptor for the TCR as it binds to the MHC-I and enhances TCR-mediated T cell stimulatory signal.5-9 The role of CD8 beyond assisting TCR-peptide-MHC-I in activating T cells and promoting TCR avidity maturation is unclear. Because the antigen has already been cleared when memory CD8+ T cells functionally mature, the comparatively high expression levels of CD8 on these T cells provide an opportunity to test whether and how agonism of CD8 alone, without TCR stimuli, may regulate the phenotype and function of memory CD8+ T cells.
In the current study, we treated lymphocytic choriomeningitis virus (LCMV)-specific memory T cells with an agonist antibody for CD8. We found that CD8 agonism initiated TCR downstream signaling and T cell metabolic reprogramming independent of antigen recognition. Furthermore, CD8 engagement increased effector cytokine production by CD8+ T cells in a glucose- and glutamine-dependent manner. Finally, we found that CD8 agonism enhanced antigen-specific T cell-mediated killing of cancer cells both in vitro and in vivo. Taken together, our results indicate that agonistic activation of CD8 without concomitant TCR stimulation results in the functional activation of both the metabolic and effector functions of memory T cells. The results of our study provide an insight into an important and independent role for CD8 in promoting memory CD8+ T cell activation.
2 MATERIALS AND METHODS
2.1 Mice
Male and female 6- to 10-week-old mice were used in all experiments. P14 mice10 were maintained in the German cancer research center (DKFZ) specific pathogen-free facility. Wild-type C57BL/6N mice (Janvier) were used for LCMV infection and tumor implantation experiments. For LCMV infection, mice received 1 × 104 naive P14 cells and were injected intraperitoneally with 2 × 105 plaque-forming units (pfu) of LCMV Armstrong. For the melanoma model, 2 × 105 B16-GP33 cells (kindly provided by Professor Hanspeter Pircher, Max Planck Institut für Immunbiologie und Epigenetik)11 were injected subcutaneously. Eleven days after tumor implantation, mice received 3 × 105 in vitro differentiated P14 cells (as described in the following section) and the mice were sacrificed 9 days later (or earlier if termination criteria were reached). In some experiments, 1 × 106 B16-GP33 cells were implanted, 1 × 106 in vitro differentiated P14 cells were adoptively transferred on day 11, and the mice were sacrificed 2 days later.
2.2 CD8+ T cells treatments in vitro
For analysis of CD8 signaling, naive P14 spleen cells were incubated with either 10 μg/mL anti-CD8α clone 53-6.7 (BioLegend, 100763) or 0.5 μg/mL anti-CD3ε (Biolegend, 100314) for 15 minutes before staining of anti-phospho-Lck (Cell Signaling Technology [CST], 2751) or anti-phospho-Zap70 (CST, 2717). Briefly, cells were fixed for 10 minutes in fixation buffer (Biolegend, 420801) at room temperature followed by 1 hour in 70% methanol at 4°C. Cells were washed twice with FACS buffer (phosphate-buffered saline [PBS] with 2% fetal calf serum [FCS]) before staining for phosphorylated antigens. Alexa-Fluor-647-labeled donkey-anti-rabbit IgG was used for secondary staining (Biolegend, 406414).
In vitro differentiation of P14 cells was performed using previously published protocols.12 Briefly, spleen cells were incubated with 10 ng/mL each of GP33 (GenScript, RP20091) and IL-2 (PeproTech, 200-02) for 3 days at 37°C. Cells were then washed extensively before gradient centrifugation using Histopaque-1077 (Sigma-Aldrich, 10771). Living cells obtained by this method were incubated for 1 day with 10 ng/mL of IL-15 (PeproTech, 210-15) in the presence or absence of 10 μg/mL anti-CD8α clone 53-6.7. Unless otherwise specified, the cells were cultured in complete Roswell Park Memorial Institute (RPMI) medium (RPMI supplemented with 10% FCS, 1% penicillin/streptomycin, 1% nonessential amino acids and β-mercaptoethanol [β-ME]). In some experiments, the cells were treated with 10 μM cyclosporin A (Santa Cruz, sc-3503), or with anti-IL-2 antibody (1 μM or 5 μM) during differentiation. Alternatively, the cells were differentiated under limited nutrient conditions in minimal RPMI (Biological Industries, 01-101-1A) supplemented with 10% dialyzed FCS (Sigma, F0392), β-ME, 2 mM glutamine (Sigma, G7513) and/or 6.7 mM glucose (Sigma, G8644).
2.3 Purification of naive CD8+ T cells
CD8+ T cells were negatively selected from the spleens of naive mice using the CD8+ T cell isolation kit (Miltenyi Biotec, 130-104-075) following the manufacturer's protocol.
2.4 Metabolic analyses
For a quantitative determination of glucose and fatty acid uptake, cells were starved in PBS for 10 minutes at 37°C before incubation with 10 μg/mL 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxyglucose (2-NBDG; Cayman, 11 046) or with 1 μM 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-hexadecanoic acid (C16-Bodipy; Thermo Fisher, D3821) in PBS for 15 to 20 minutes at 37°C. Cells were extensively washed with FACS buffer and stained for flow cytometry.
For Seahorse analysis, 0.5 × 106 live cells were plated in XF medium supplemented with 2 mM l-glutamine (GIBCO, 25030-024) and 1 mM sodium pyruvate (Sigma-Aldrich, P2256-5G). Extracellular acidification rates (ECAR) and oxygen consumption rates (OCR) were measured using the XF-96 Extracellular Flux Analyzer (Seahorse Bioscience).
To analyze metabolite abundance, IL-15-treated cells (skewed in the presence or absence of 10 μg/mL anti-CD8 antibody) were used for gradient centrifugation to enrich live cells. Ten million cells were snap-frozen and stored at −80°C. Pellets were extracted in absolute methanol at 70°C for 15 minutes, mixed with chloroform and shaken for 5 minutes at 37°C before adding water followed by centrifugation to separate the polar and organic phases. The polar phase was collected and dehydrated in a vacuum concentrator. For metabolite derivatization, on-line methoximation (20 μL of 20 mg/mL methoxyamine hydrochloride [Sigma, 226 904] in pyridine [Sigma, 270 970] for 90 minutes at 37°C) and silylation (30 μL N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide [MTBSTFA; Sigma, 394 882] for 30 minutes at 37°C) were performed in a multipurpose autosampler (MPS; Gerstel) with agitation. After derivatization, samples were held at room temperature before further analysis. For gas chromatography (GC)/mass spectrometry (MS) analysis, a GC-time of flight (ToF) system was used that consisted of an Agilent 7890 GC (Agilent, Santa Clara) fitted with a Rxi-5Sil MS column (30 m × 0.25 mm × 0.25 μm; Restek Corporation, Bellefonte) coupled to a Pegasus Bt (LECO Corporation, Michigan). The GC was operated with an injection temperature of 250°C. One microliter of each sample was injected in splitless mode. Helium at constant linear velocity was used as a carrier gas. The ToF was operated with ion source and interface temperatures of 250°C, a solvent cut time of 9 minutes and a scan range (m/z) of 50 to 600 with an acquisition rate of 17 spectra/second. Data were processed with DExSI software.13
2.5 Microscopy
Cell clusters that formed after cytokine and antibody incubation were imaged using a microscope fitted with the Oxion Inverso Camera (Euromax).
2.6 B16 cell and T cell co-cultures
B16 cells were maintained in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% FCS, 1% penicillin/streptomycin and 1% nonessential amino acids. Cells were cultured in round-bottom, 96-well plate for 1 day before incubation with 10 ng/mL GP33 peptide for 2 hour at 37°C. B16 cells were then washed twice with PBS and incubated for one more day with T cells at an effector-to-target ratio of 5:1. T cells were prelabeled with Cell Trace Violet (CTV; Invitrogen, C34557) for 15 minutes at 37°C. In subsequent flow cytometry analysis, B16 cells were gated as CTV-negative cells. All experiments were performed with mycoplasma-free cells.
2.7 Flow cytometry
Cells were resuspended in FACS buffer and stained with antibodies specific for surface antigens. For intracellular staining, cells were incubated in fixation buffer or eBioscience fixation/permeabilization concentrate (ThermoFisher Scientific, 00-5123-43) before washing with permeabilization buffer (ThermoFisher Scientific, 00-8333-56). Anti-mouse anti-CD8α (53-6.7), CD8β (YTS156.7.7), CD44 (IM7), CD62L (MEL-14), KLRG1 (2F17KLRG1), IL-7R (SB/199), PD-1 (29F.1A12), TIGIT (1G9), Lag3 (C9B7W), T-bet (4B10), IFNγ (XMG1.2), TNFα (MP6-XT22), GzmB (GB11), Perforin (S16009A), Ki67 (16A8) and anti-rabbit (poly4064) were purchased from Biolegend. Anti-TNFα (MP6-XT22), CD98 (REA861), CD71 (REA627) and Bcl2 (REA356) were purchased from Miltenyi Biotec. Anti-TCF1 (C63D9) antibody was from CST. Anti-human CD8α (HTT8a), CD3 (OKT3), CD45RO (UCHL1) and CD62L (DREG-56) were from Biolegend. Samples were acquired using Canto II or LSR II instruments and analyzed using FlowJo software (Tree Star).
2.8 RNA-seq analysis
Single cell RNA-sequencing (RNA-seq) data were downloaded from GEO (GSE120575)14 and analyzed using the Python-based implementation of SCANPY.15 CD8+ T cells were defined based on the co-expression of CD8A and CD3E (log(CD8A) > 1.5 and log(CD3E) > 1.5), thus selecting 4850 cells for downstream analysis. No distinction was made between patients receiving anti-PD1, anti-CTLA4 or the combination therapy. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test was used to determine the statistical significance of differences observed between the groups.
2.9 Statistical analysis
Unless otherwise indicated, statistical significance was assessed using a two-tailed Student's t test. P-values <.05 were considered significant. Analysis and data visualization were done using GraphPad Prism version 7.05 for Windows (GraphPad Software, La Jolla, California, www.graphpad.com).
3 RESULTS
3.1 Memory CD8+ T cells express high levels of CD8 protein
A previous longitudinal analysis of naive, effector and memory CD8+ T cells revealed the kinetics of CD8 protein expression on antigen-restimulated CD8+ T cells.4 Because antigen stimulation influences the cell surface level of CD8 protein, we isolated CD8+ T cells from mice infected with LCMV Armstrong (Arm) and examined the CD8 protein expression level without antigen restimulation. Naive CD8+ T cells steadily downregulated cell surface expression of CD8 as they differentiated into effector T cells 8 days after infection (D8) (Figure 1A). While most of these effectors will disappear shortly after viral clearance, higher levels of surface CD8 were detected in long-lived memory cells, examined 30 days postinfection (D30) (Figure 1A). We used equal numbers of splenocytes for flow cytometry staining to account for the much higher CD8+ T cell numbers per spleen at the effector time point.
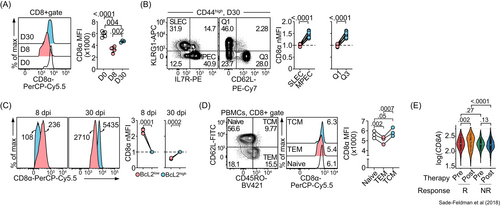
In addition to a longitudinal analysis of CD8 protein dynamics over the course of a viral infection, we also performed a cross-sectional examination of CD8 expression in different CD8+ T cell subsets on day 30 after LCMV Arm infection. Memory CD8+ T cells and their precursors express high levels of IL-7Rα and CD62L and low levels of KLRG1.16, 17 We found that KLRG1−IL-7Rα+ (memory precursor effector cells, MPECs) expressed higher CD8 levels compared to their short-term effector cell (SLEC) counterparts (Figure 1B). Similarly, CD8 levels were higher in KLRG1−CD62L+ cells compared to levels in terminal effector memory (KLRG1+CD62L−) cells.
One of the cardinal features of memory T cells is longevity, which is associated with the expression of the antiapoptotic protein Bcl-2.18 To examine the relationship between CD8 and Bcl-2 protein expression levels, we performed a flow cytometric analysis of CD8 protein in Bcl-2high and Bcl-2low memory CD8+ T cell populations. We found that CD8 and Bcl-2 protein levels were positively correlated only at the memory time point (D30) (Figure 1C).
Building on the aforementioned mouse studies, we examined CD8 expression in effector memory T (TEM) cells, central memory T (TCM) cells and naive T cells in human peripheral blood mononuclear cells (PBMCs). We found that the stem cell-like CD62L+CD45RO+ TCM cells expressed significantly higher levels of CD8 than CD45RO+CD62L− TEM cells (Figure 1D). Collectively, these results suggest that both human and mouse memory CD8+ T cells, especially those with stem cell features, express high levels of CD8 protein.
Finally, we examined CD8 expression under conditions of chronic antigen exposure. Stem cell-like CD8+ T cells that selectively expand in response to the immune checkpoint inhibitor anti-PD1 have been associated with an enhanced immune response.19 We analyzed CD8A expression in melanoma-infiltrating CD8+ T cells before and after immune checkpoint blockade.14 Although we found no difference in the levels of CD8A expressed by responders and nonresponders before therapy, our analysis revealed a significant upregulation of CD8A in responders (but not nonresponders) after treatment (Figure 1E). Consistently, cells from responding patients were enriched for genes encoding stem cell markers, including IL7R and TCF7 (Figure S1). These findings provide further support for the positive correlation between CD8 expression and CD8+ T cell stemness.
Taken together, our results show that CD8 expression is selectively upregulated in cells with stem cell-like features under conditions of acute or chronic antigen exposure, implying a potential link between CD8 expression and effector/memory T cell function.
3.2 CD8 engagement triggers proximal TCR signaling and induces the expression of effector cell markers in memory-like T cells
Because CD8 protein marks the identity of CD8+ T cells, it is technically challenging to use Cd8-deficient mice to study how loss-of-function influences memory T cell phenotype. To circumvent this issue, we treated T cells with an agonistic monoclonal anti-CD8 antibody (clone 53-6.7, CD8 mAb),20, 21 in order to examine whether the increased expression of CD8 protein on memory T cells is only a biomarker, or is functionally relevant. CD8 mAb treatment alone induced the phosphorylation and activation of TCR proximal signaling pathway components Lck and ZAP70 in polyclonal memory T cells formed after LCMV Arm infection (Figure 2A,B). Because both naive and memory CD8+ T cells expressed higher levels of CD8 than effector CD8+ T cells (Figure 1A), we repeated the experiment with naive, freshly collected CD8+ T cells. We found that CD8 mAb also triggered Lck and ZAP70 phosphorylation in naive CD8+ T cells (Figure 2C). The CD8 mAb-induced phosphorylation of Lck and ZAP70 supports the hypothesis that the engagement of CD8 protein without cognate antigen recognition can lead to T cell activation.
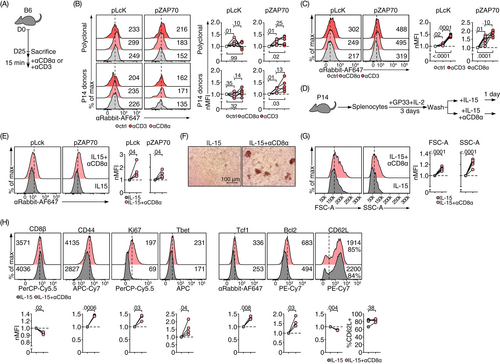
Similar to the LCMV-specific memory CD8+ T cells, memory-like CD8+ T cells differentiated in vitro as previously described,12 responded to agonistic CD8 mAb treatment with rapid phosphorylation of Lck and ZAP70 (Figure 2D,E). These results suggest that this in vitro culture system can be used as a surrogate for bona fide memory CD8+ T cells to functionally characterize the CD8 protein engagement in CD8+ T cells. Using this in vitro culture system, we found that CD8 mAb treatment increased the number as well as the size of blasting cell clusters (Figure 2F). CD8+ T cells were bigger and more granular (as assessed by forward scatter [FSC] and side scatter [SSC], respectively) than cells treated with IL-15 only (Figure 2G). Phenotypically, CD8 ligation increased the expression of proteins typically expressed by activated effector T cells including CD44, Ki-67 and T-bet. However, this did not compromise the stemness of these memory-like T cells. While the percentage of CD62L+ cells was not significantly influenced by CD8 ligation, expression of the antiapoptosis protein Bcl-2 and the stemness transcription factor Tcf1 were induced under these conditions (Figure 2H). Together, these results suggest that the engagement of CD8 with an agonistic mAb activates memory-like CD8+ T cells and supports cell survival.
3.3 CD8 mAb treatment functionally activates memory-like T cells
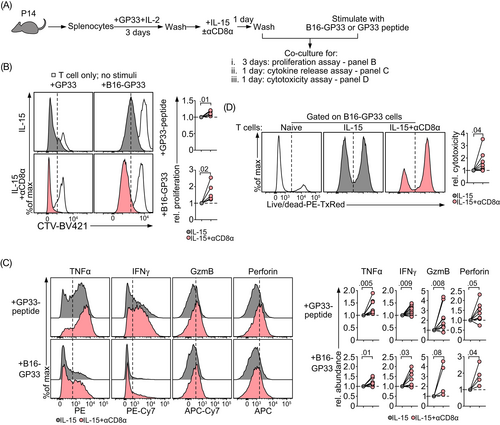
We then asked whether CD8 ligation-induced effector features had functional consequences. The functionality of CD8 mAb-treated, IL-15-differentiated memory-like T cells was evaluated in experiments with B16.F10 (B16) melanoma cells that were precoated with their cognate GP33−41 peptide (B16-GP33). As a positive control, we stimulated the treated T cells with the GP33−41 (GP33) peptide alone to eliminate any potential contribution of cancer cell-induced immune suppression (Figure 3A). In the presence of either B16-GP33 cells or the GP33 peptide, CD8 mAb-treated cells proliferated better than untreated memory-like cells (Figure 3B). Furthermore, CD8 ligated cells produced higher amounts of effector cytokines, including tumor necrosis factor α (TNFα), interferon γ (IFNγ), granzyme B (GzmB) and perforin (Figure 3C), eventually leading to better cytotoxicity (Figure 3D). Collectively, these findings reveal that agonistic CD8 mAb treatment results in functional activation of memory-like CD8+ T cells.
3.4 CD8 mAb-mediated activation of memory-like T cells relies on glucose and glutamine metabolism
A concerted series of metabolic events occur as T cells transition from naive to effector to memory.22 In our next set of experiments, we investigated whether a specific metabolic shift accompanies the functional effects of CD8 mAb treatment. Elevated levels of glucose uptake (as assessed by retention of the glucose analog 2-NBDG) were detected in CD8 mAb-treated memory-like T cells (Figure 4A). CD8 mAb treatment resulted in the upregulation of the neutral amino acid transporter CD98 and downregulation the transferrin receptor CD71. These results suggest an enhanced capacity for amino acid uptake together with a diminished uptake of transferrin-iron complexes, respectively. CD8 mAb ligation did not influence fatty acid uptake, as demonstrated by the comparable levels of Bodipy C16 fluorescence exhibited by CD8 mAb-treated and -untreated cells. In line with the elevated expression of CD98, CD8-ligated cells accumulated higher levels of amino acids and nitrogenous bases that serve as building blocks for cell proliferation (Figure 4B).
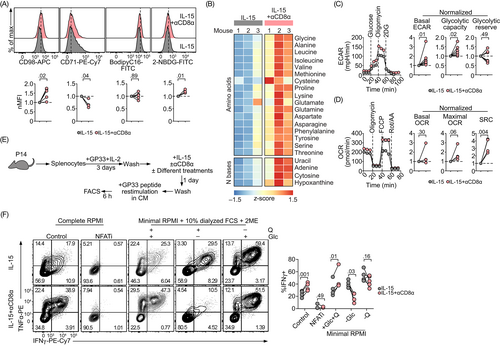
T cells rapidly engage aerobic glycolysis after TCR activation23 while infection-induced memory T cells as well as IL-15 differentiated memory-like T cells rely on mitochondrial oxidative metabolism (measured as oxygen consumption rate, OCR) to support their longevity.12, 24 We asked whether the enhanced glucose uptake induced by CD8 agonism was channeled towards glycolysis. We found that CD8-ligated cells increased their rate of glycolysis, as shown by a higher basal and maximal extracellular acidification rate (ECAR) detected in extracellular metabolic flux experiments (Figure 4C). These glycolytic changes did not compromise the glycolytic reserve (the difference between maximal ECAR [also known as glycolytic capacity] and ECAR levels after the addition of glucose), suggesting that CD8 ligation pushes the limit of the theoretical maximal glycolysis. Importantly, mitochondrial fitness remained intact and was even promoted following CD8 ligation as shown by the similar basal OCR but enhanced maximal OCR as well as spare respiratory capacity (SRC) (Figure 4D).
Several groups have shown that metabolic conditioning of CD8+ T cells plays an important role in their differentiation status.25-27 Thus, we performed experiments to determine whether the metabolic adaptations we observed were the cause or the result of phenotypic and functional changes induced by CD8 ligation. Using GP33 peptide-induced cytokine production as a read-out, we cultured CD8+ T cells in a glucose-free or glutamine-free medium to investigate whether glucose and/or glutamine were required for CD8 mAb to enhance effector cytokine production (Figure 4E). Cyclosporin A, which is an inhibitor of the transcription factor NFAT (NFATi), was included as a positive control (Figure 4F). We found that IL-15-differentiated memory-like T cells maintained high-level cytokine production in a glucose-free medium. Interestingly, glutamine deprivation enhanced cytokine release, which is consistent with findings indicating that glutaminolysis plays a role in suppressing effector cytokine production.27 CD8 mAb treatment results in increased production of the effector cytokines IFNγ and TNFα only in CD8+ T cells that were cultured with both glucose and glutamine. Nutrient depletion, especially glucose deprivation, almost completely abrogated the CD8 mAb-induced production of effector cytokines by CD8+ T cells.
Because CD8+ T cell density and CD8+ T cell cluster area positively correlate with IL-2 production,28 and also because CD8 mAb treatment increased the formation of cell clusters (Figure 2E), we tested whether IL-2 was required for CD8 mAb to induce effector cytokine production. The addition of increasing concentrations of an anti-IL-2 neutralizing antibody had no impact on enhanced cytokine release in CD8-ligated cells (Figure S2), suggesting that autocrine IL-2 was not required for CD8 mAb to induce effector cytokine production by CD8+ T cells.
3.5 CD8 mAb treatment enhances antitumor CD8+ T cell responses
To determine if the enhanced effector function of CD8-ligated T cells would translate into improved antitumor activity in vivo, we used the subcutaneous B16-GP33 melanoma model.11 First, we asked whether the enhanced antigen-induced proliferation we observed in CD8-ligated cells in vitro was maintained in vivo. Memory-like CD8+ T cells were differentiated with IL-15 in the presence or absence of CD8 mAb and labeled with Cell Trace Violet (CTV) before adoptive transfer into B16-GP33 tumor-bearing mice (Figure 5A). We observed a significant decrease in the number of CD8-ligated donor cells both in the tumor and the spleen 2 days after the adoptive transfer (Figure 5B). This may have been due to the clearance of CD8 mAb-bound cells by antibody-dependent cellular cytotoxicity (ADCC). However, we did detect an increase in the fraction of proliferating donor cells, as well as the percentages of donor cells undergoing four or more divisions (red lines and numbers in the figure) in both tumors and spleens of mice receiving CD8 mAb-ligated cells (Figure 5C). Despite their rapid proliferation, CD8-ligated cells were not more prone to exhaustion as suggested by similar expression levels of the exhaustion markers PD1, Lag3 and TIGIT in both CD8 mAb-treated and -untreated cells (Figure 5D).
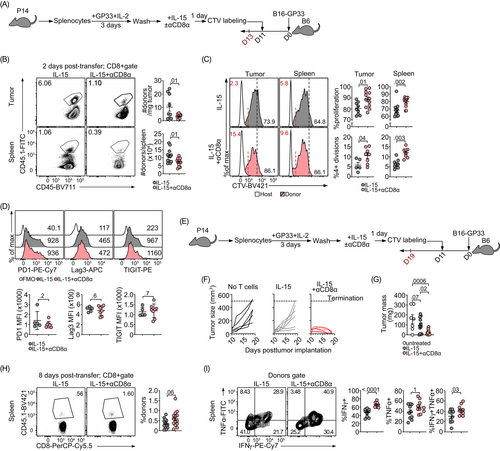
We then asked whether the rapid proliferation of CD8-ligated cells might compensate for their depletion. We followed B16-GP33 tumor growth over an extended time interval (Figure 5E). Compared to B16-GP33 tumor-bearing mice that received no donor T cells, those receiving IL-15-differentiated P14 CD8+ donors had smaller tumors, although the difference did not reach statistical significance (Figure 5F,G). By contrast, P14 CD8+ donor T cells pretreated with both IL-15 and CD8 mAb achieved dramatically superior tumor control. Tumor sizes were reduced on average by around 80% compared to tumors in mice that received untreated donor T cells (Figure 5G). Because the tumors were very small, we were unable to recover tumor-infiltrating lymphocytes (TILs) in numbers sufficient for phenotypic characterization. Therefore, we characterized donor T cells in the spleen (Figure 5H), where CD8 mAb-treated cells tended to persist better despite their initial disadvantage. Furthermore, CD8 mAb-treated cells isolated from mouse spleens produced more IFNγ (Figure 5I). These findings suggest that CD8 mAb-induced cell proliferation can compensate for the initial partial depletion of ligated cells. Further, descendants of CD8-ligated cells are able to efficiently control tumor growth. Together, our results demonstrate the utility of agonistic CD8 antibodies to enhance adoptive T cell cancer therapies.
4 DISCUSSION
The findings presented in our study build on the traditional view of CD8 as a TCR co-receptor that facilitates its interaction with peptide-MHC I complexes. We discovered that engagement of cell surface CD8 alone triggers TCR signal transduction and functionally activates memory CD8+ T cells both in vitro and in vivo. Therefore, our study has revealed a unique functional role of CD8 protein in memory CD8+ T cells.
We systematically analyzed changes in CD8 expression within the course of an active immune response. Our findings revealed a dynamic association between surface CD8 levels and the activation history of the cell. This result is consistent with previous observations that CD8 is involved in the intricate regulation of T cell differentiation. For example, over expression of CD8 reduces cellular differentiation and locks T cells in a metabolically-quiescent, naive-like state.29 By contrast, failure to upregulate CD8 limits the long-term persistence of memory T cells.30 Interestingly, this had a particular impact on KLRG1+IL-7Rα− memory T cells, which, as we show here, have comparatively lower levels of CD8. It is possible that these effector memory cells express minimal levels of CD8 necessary for survival and that any further downregulation compromises their maintenance.
Persistent antigen exposure leads to the desensitization of the TCR signaling pathway.31 Recently, it was reported that T cells with high or low antigen affinity are equally unable to inhibit tumor growth due to either dysfunction or inadequate engagement of the effector machinery, respectively.32 Genetic ablation of Cd8a in cells with high affinity TCRs decreases their avidity, protects them from rapid exhaustion and increases their antitumor function. We observed a slight downregulation of CD8β after treatment with CD8 mAb, enhanced proximal TCR signaling and effector cytokine production and better tumor control. Therefore, using CD8 mAb enhances the TCR signal strength without subjecting the cells to the threat of rapid exhaustion.
An understanding of strategies that might be used to enhance stem cell-like features is critical for the success of adoptive T cell therapies used for many types of cancers.33, 34 While effector cells are efficient at producing cytokine in vitro, they do not persist after adoptive transfer and ultimately fail to control the tumor.35 Strong response to therapy correlates with the abundance of stem cells in the transferred cell pool.36 IL-15, in particular, has been shown to maintain T cell stemness after prolonged in vitro culture. IL-15-differentiated cells upregulate stem cell markers, persist longer after adoptive transfer independent of antigen recognition and exhibit superior tumor control.35, 37, 38 Our results reveal that CD8 ligation induces effector T cell features without significantly compromising stemness, allowing them to efficiently control tumor growth. In this regard, the use of an agonistic CD8 mAb is similar to a partial IL-2 agonist, which combines the IL-2-induced effector phenotype with long-term persistence of stem cell-like cells.39
We found that IL-15-differentiated CD8+ T cells cultured in glucose-free or glutamine-free medium secreted effector cytokines at similar, if not higher, levels in comparison to cells cultured in complete medium. This observation might appear at odds with previous reports showing that inhibition of glycolysis suppressed IFNγ production.40, 41 We want to emphasize that the previously reported results were obtained using T cells cultured under conditions that promoted effector T cell differentiation, as opposed to the memory-like T cells that were used in our study. Collectively, these results suggest that different T cell subsets adapt their nutrient requirements to control T cell cytokine production, in line with the report that inhibition of glutaminolysis has opposite roles in regulating cytokine secretion in different T cell subsets.42
Studies using anti-CD8 antibodies frequently report contradictory findings depending on the specific clone of the antibody used. Some anti-CD8 clones led to enhanced effector functions, proliferation and/or cytotoxicity while others either had no effect or even diminished CD8+ T cell responses.20, 21 Different conformational changes may be induced in CD8 after antibody binding at different epitopes. The antibody clone we used (clone 53-6.7) was previously shown to support positive co-operativity of TCR-peptide-MHC-I complex formation using adhesion frequency assays.43 However, and despite its agonistic effects in vitro, this antibody is of the IgG2a isotype, which efficiently binds and activates Fcγ receptors (FcγR) expressed by professional phagocytes.44, 45 Thus, injecting mice with this antibody leads to CD8+ T cell depletion by ADCC.46
Therefore, the direct administration of anti-CD8 to tumor-bearing mice remains hindered by the lack of a nondepleting antibody. In the current study, we overcame this technical limitation by pretreating the donor T cells with this agonistic anti-CD8 antibody prior to adoptive transfer to tumor-bearing mice. The CD8 mAb increased T cell proliferation in vivo, and the progeny cells derived from the donors were not coated with anti-CD8 antibodies. Therefore, the progeny CD8+ T cells were not cleared by ADCC. Overall, our findings suggest that the enhanced CD8+ T cell proliferation compensated for initial ADCC-mediated reductions in donor CD8+ T cell numbers. We also observed an enhanced abundance of CD8-ligated donor cells in the spleen of B16-tumor bearing hosts 8 days after T cell transfer. In combination with the enhanced expression of the stemness and survival markers Tcf1 and Bcl2, as well as the increased maximal respiration and SRC of CD8-ligated cells, our results show that the durability and long term survival of CD8-ligated T cells are improved.
Another way to avoid the depletion of CD8-ligated cells is to engineer a nondepleting antibody by changing the fragment crystallizable (Fc) domain to another isotype that either binds to inhibitory FcγR or does not bind to FcγR at all. Alternatively, the antibody could be selectively digested to retain the antigen-binding fragment (Fab) region. These modified antibodies could be employed during the ex vivo expansion and activation of T cells prior to adoptive cell therapies, potentially enhancing both the activity and the stemness/durability of the transferred cells.
In conclusion, our results highlight the critical, and tightly regulated, role of the CD8 co-receptor within the course of an immune response. We propose that targeting CD8 will be a useful, intrinsically specific and importantly, patient-agnostic approach to antitumor immunotherapy.
AUTHOR CONTRIBUTIONS
Alaa Madi and Guoliang Cui designed the experiments. Alaa Madi, Nina Weisshaar, Michael Buettner, Gernot Poschet, Sicong Ma, Jingxia Wu, Alessa Mieg Marvin Hering, Yanan Ming, Kerstin Mohr and Nora ten Bosch performed the experiments. Alaa Madi analyzed the results. Alaa Madi and Guoliang Cui wrote the article. The work reported in the article has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENTS
We thank our DKFZ FACS core facility colleagues for their assistance. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
Guoliang Cui receives research funding from Bayer AG and Boehringer Ingelheim, but the funding is not relevant to our study. The rest of the authors declare no competing interests.
ETHICS STATEMENT
All experiments were approved by the German regional council at the Regierungspräsidium Karlsruhe and performed in accordance with internal DKFZ regulations.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.