High-fat diet promotes prostate cancer growth through histamine signaling
Makoto Matsushita
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorCorresponding Author
Kazutoshi Fujita
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Department of Urology, Kindai University, Faculty of Medicine, Osakasayama, Japan
Correspondence
Kazutoshi Fujita, Department of Urology, Kindai University Faculty of Medicine, 377-2 Oono-higashi, Osakasayama, Osaka 589-8511 Japan.
Email: [email protected]
Search for more papers by this authorKoji Hatano
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorTakuji Hayashi
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorHisako Kayama
Laboratory of Immune Regulation, Department of Microbiology and Immunology, Osaka University, Graduate School of Medicine, Suita, Japan
WPI Immunology Frontier Research Center, Osaka University, Suita, Japan
Institute for Advanced Co-Creation Studies, Osaka University, Suita, Japan
Search for more papers by this authorDaisuke Motooka
Department of Infection Metagenomics, Research Institute for Microbial Diseases, Osaka University, Suita, Japan
Search for more papers by this authorHiroaki Hase
Laboratory of Cell Biology and Physiology, Osaka University, Graduate School of Pharmaceutical Sciences, Suita, Japan
Search for more papers by this authorAkinaru Yamamoto
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorToshihiko Uemura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorGaku Yamamichi
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorEisuke Tomiyama
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorYoko Koh
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorTaigo Kato
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorAtsunari Kawashima
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorMotohide Uemura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorSatoshi Nojima
Department of Pathology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorRyoichi Imamura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorAysha Mubeen
Department of Pathology, UAB School of Medicine, Birmingham, Alabama, USA
Search for more papers by this authorGeorge J. Netto
Department of Pathology, UAB School of Medicine, Birmingham, Alabama, USA
Search for more papers by this authorKazutake Tsujikawa
Laboratory of Cell Biology and Physiology, Osaka University, Graduate School of Pharmaceutical Sciences, Suita, Japan
Search for more papers by this authorShota Nakamura
Department of Infection Metagenomics, Research Institute for Microbial Diseases, Osaka University, Suita, Japan
Search for more papers by this authorKiyoshi Takeda
Laboratory of Immune Regulation, Department of Microbiology and Immunology, Osaka University, Graduate School of Medicine, Suita, Japan
WPI Immunology Frontier Research Center, Osaka University, Suita, Japan
Search for more papers by this authorEiichi Morii
Department of Pathology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorNorio Nonomura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorMakoto Matsushita
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorCorresponding Author
Kazutoshi Fujita
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Department of Urology, Kindai University, Faculty of Medicine, Osakasayama, Japan
Correspondence
Kazutoshi Fujita, Department of Urology, Kindai University Faculty of Medicine, 377-2 Oono-higashi, Osakasayama, Osaka 589-8511 Japan.
Email: [email protected]
Search for more papers by this authorKoji Hatano
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorTakuji Hayashi
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorHisako Kayama
Laboratory of Immune Regulation, Department of Microbiology and Immunology, Osaka University, Graduate School of Medicine, Suita, Japan
WPI Immunology Frontier Research Center, Osaka University, Suita, Japan
Institute for Advanced Co-Creation Studies, Osaka University, Suita, Japan
Search for more papers by this authorDaisuke Motooka
Department of Infection Metagenomics, Research Institute for Microbial Diseases, Osaka University, Suita, Japan
Search for more papers by this authorHiroaki Hase
Laboratory of Cell Biology and Physiology, Osaka University, Graduate School of Pharmaceutical Sciences, Suita, Japan
Search for more papers by this authorAkinaru Yamamoto
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorToshihiko Uemura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorGaku Yamamichi
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorEisuke Tomiyama
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorYoko Koh
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorTaigo Kato
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorAtsunari Kawashima
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorMotohide Uemura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorSatoshi Nojima
Department of Pathology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorRyoichi Imamura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorAysha Mubeen
Department of Pathology, UAB School of Medicine, Birmingham, Alabama, USA
Search for more papers by this authorGeorge J. Netto
Department of Pathology, UAB School of Medicine, Birmingham, Alabama, USA
Search for more papers by this authorKazutake Tsujikawa
Laboratory of Cell Biology and Physiology, Osaka University, Graduate School of Pharmaceutical Sciences, Suita, Japan
Search for more papers by this authorShota Nakamura
Department of Infection Metagenomics, Research Institute for Microbial Diseases, Osaka University, Suita, Japan
Search for more papers by this authorKiyoshi Takeda
Laboratory of Immune Regulation, Department of Microbiology and Immunology, Osaka University, Graduate School of Medicine, Suita, Japan
WPI Immunology Frontier Research Center, Osaka University, Suita, Japan
Search for more papers by this authorEiichi Morii
Department of Pathology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorNorio Nonomura
Department of Urology, Osaka University, Graduate School of Medicine, Suita, Japan
Search for more papers by this authorFunding information: Japan Society for the Promotion of Science, Grant/Award Number: JP21K09421; Japanese Urological Association; Yakult Bio-Science Foundation
Abstract
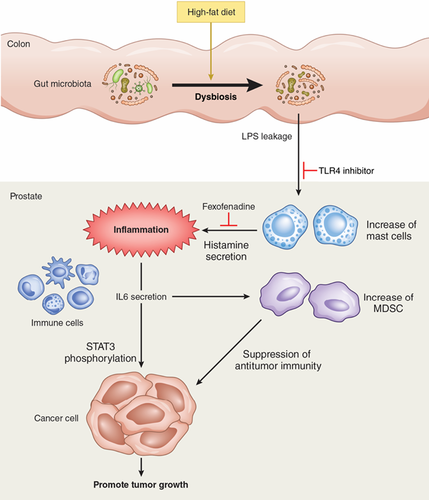
Western high-fat diets (HFD) are regarded as a major risk factor for prostate cancer (PCa). Using prostate-specific Pten-knockout mice as a PCa model, we previously reported that HFD promoted inflammatory PCa growth. The composition of the gut microbiota changes under the influence of diet exert various effects on the host through immunological mechanisms. Herein, we investigated the etiology of HFD-induced inflammatory cancer growth and the involvement of the gut microbiome. The expression of Hdc, the gene responsible for histamine biosynthesis, and histamine levels were upregulated in large prostate tumors of HFD-fed mice, and the number of mast cells increased around the tumor foci. Administration of fexofenadine, a histamine H1 receptor antagonist, suppressed tumor growth in HFD-fed mice by reducing the number of myeloid-derived suppressor cells and suppressing IL6/STAT3 signaling. HFD intake induced gut dysbiosis, resulting in the elevation of serum lipopolysaccharide (LPS) levels. Intraperitoneal injection of LPS increased Hdc expression in PCa. Inhibition of LPS/Toll-like receptor 4 signaling suppressed HFD-induced tumor growth. The number of mast cells increased around the cancer foci in total prostatectomy specimens of severely obese patients. In conclusion, HFD promotes PCa growth through histamine signaling via mast cells. Dietary high-fat induced gut dysbiosis might be involved in the inflammatory cancer growth.
Graphical Abstract
What's new?
High-fat diets consisting of large amounts of animal fat are linked to an increased risk of prostate cancer, an association attributed in part to the ability of excess fat to induce inflammation in prostate immune cells. While the mechanism behind this phenomenon is unknown, the present study shows that high animal fat intake promotes prostate cancer growth via histamine signaling by mast cells. In addition, in mice, gut dysbiosis induced by a high-fat diet resulted in elevated lipopolysaccharide (LPS) levels, whereas inhibition of LPS signaling suppressed tumor growth. The findings reveal novel links between fat intake and inflammation in cancer.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The reported cDNA microarray data are available at GEO under the accession number GSE184381. Other data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| ijc34028-sup-0001-Supinfo.pdfPDF document, 19.8 MB | Appendix S1 Supporting Information. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394-424.
- 2Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020; 77: 38-52.
- 3Lloyd T, Hounsome L, Mehay A, Mee S, Verne J, Cooper A. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008-2010. BMC Med. 2015; 13: 171.
- 4Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci. 2020; 21: 1447.
- 5Hatano K, Fujita K, Nonomura N. Application of anti-inflammatory agents in prostate cancer. J Clin Med. 2020; 9: 2680.
- 6Hayashi T, Fujita K, Matsushita M, Hayashi Y, Uemura M, Nonomura N. Metformin inhibits prostate cancer growth induced by a high-fat diet in Pten-deficient model mice. Int J Urol. 2019; 26: 307-309.
- 7Hayashi T, Fujita K, Nojima S, et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin Cancer Res. 2018; 24: 4309-4318.
- 8Matsushita M, Fujita K, Hayashi T, et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021; 81: 4014-4026.
- 9Dzutsev A, Badger JH, Perez-Chanona E, et al. Microbes and cancer. Annu Rev Immunol. 2017; 35: 199-228.
- 10Fox EM, Morris CP, Hübner MP, Mitre E. Histamine 1 receptor blockade enhances eosinophil-mediated clearance of adult filarial worms. PLoS Negl Trop Dis. 2015; 9:e0003932.
- 11Suo H, Feng X, Zhu K, Wang C, Zhao X, Kan J. Shuidouchi (fermented soybean) fermented in different vessels attenuates HCl/ethanol-induced gastric mucosal injury. Molecules. 2015; 20: 19748-19763.
- 12Fleming K, Donnelly RF. Physical compatibility and chemical stability of injectable and oral ranitidine solutions. Hosp Pharm. 2019; 54: 32-36.
- 13Maher HM, Sultan MA, Olah IV. Development of validated stability-indicating chromatographic method for the determination of fexofenadine hydrochloride and its related impurities in pharmaceutical tablets. Chem Cent J. 2011; 5: 76.
- 14Ono Y, Maejima Y, Saito M, et al. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci Rep. 2020; 10: 694.
- 15Ittmann M, Huang J, Radaelli E, et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the mouse models of human cancers consortium prostate pathology committee. Cancer Res. 2013; 73: 2718-2736.
- 16Fujita K, Hayashi T, Matsushita M, Uemura M, Nonomura N. Obesity, inflammation, and prostate cancer. J Clin Med. 2019; 8: 201.
- 17Hayashi T, Fujita K, Matsushita M, Nonomura N. Main inflammatory cells and potentials of anti-inflammatory agents in prostate cancer. Cancer. 2019; 11: 1153.
- 18Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007; 67: 1378-1384.
- 19Nonomura N, Takayama H, Nishimura K, et al. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br J Cancer. 2007; 97: 952-956.
- 20Moriguchi T, Takai J. Histamine and histidine decarboxylase: immunomodulatory functions and regulatory mechanisms. Genes Cells. 2020; 25: 443-449.
- 21Davis SC, Clark S, Hayes JR, Green TL, Gruetter CA. Up-regulation of histidine decarboxylase expression and histamine content in B16F10 murine melanoma cells. Inflamm Res. 2011; 60: 55-61.
- 22Liu J, Zhang Y, Zhao J, et al. Mast cell: insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011; 30: 177-184.
- 23Nguyen PL, Cho J. Pathophysiological roles of histamine receptors in cancer progression: implications and perspectives as potential molecular targets. Biomolecules. 2021; 11: 1232.
- 24Shi Z, Fultz RS, Engevik MA, et al. Distinct roles of histamine H1- and H2-receptor signaling pathways in inflammation-associated colonic tumorigenesis. Am J Physiol Gastrointest Liver Physiol. 2019; 316: 205-216.
- 25Habel LA, Levin TR, Friedman GD. Cimetidine use and risk of breast, prostate, and other cancers. Pharmacoepidemiol Drug Saf. 2000; 9: 149-155.
10.1002/(SICI)1099-1557(200003/04)9:2<149::AID-PDS481>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 26Fritz I, Wagner P, Olsson H. Improved survival in several cancers with use of H1-antihistamines desloratadine and loratadine. Transl Oncol. 2021; 14:101029.
- 27Wang WT, Chen YH, Hsu JL, et al. Terfenadine induces anti-proliferative and apoptotic activities in human hormone-refractory prostate cancer through histamine receptor-independent Mcl-1 cleavage and Bak up-regulation. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387: 33-45.
- 28Marone G, Granata F, Spadaro G, Genovese A, Triggiani M. The histamine-cytokine network in allergic inflammation. J Allergy Clin Immunol. 2003; 112: 83-88.
- 29Martin RK, Saleem SJ, Folgosa L, et al. Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J Leukoc Biol. 2014; 96: 151-159.
- 30Triggiani M, Gentile M, Secondo A, et al. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J Immunol. 2001; 166: 4083-4091.
- 31de la Sarasola MP, Táquez Delgado MA, Nicoud MB, Medina VA. Histamine in cancer immunology and immunotherapy. Current status and new perspectives. Pharmacol Res Perspect. 2021; 9:e00778.
- 32Kim JH, Kang YJ, Kim DS, et al. The relationship between mast cell density and tumour grade in transitional cell carcinoma of the bladder. J Int Med Res. 2011; 39: 1675-1681.
- 33Rao Q, Chen Y, Yeh CR, et al. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget. 2016; 7: 7842-7855.
- 34Glajcar A, Szpor J, Pacek A, et al. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch. 2017; 470: 505-515.
- 35Carpenco E, Ceauşu RA, Cimpean AM, et al. Mast cells as an indicator and prognostic marker in molecular subtypes of breast cancer. In Vivo. 2019; 33: 743-748.
- 36Chng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006; 19: 149-159.
- 37Kim HY, Jung H, Kim HM, Jeong HJ. Surfactin exerts an anti-cancer effect through inducing allergic reactions in melanoma skin cancer. Int Immunopharmacol. 2021; 99:107934.
- 38Takezawa K, Fujita K, Matsushita M, et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. Prostate. 2021; 81: 1287-1293.
- 39Matsushita M, Fujita K, Motooka D, et al. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci. 2021; 112: 3125-3135.
- 40Liss MA, White JR, Goros M, et al. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur Urol. 2018; 74: 575-582.
- 41Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015; 33: 496-503.
- 42Maharshak N, Packey CD, Ellermann M, et al. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes. 2013; 4: 316-324.
- 43Sae-Khow K, Charoensappakit A, Visitchanakun P, et al. Pathogen-associated molecules from gut translocation enhance severity of cecal ligation and puncture sepsis in iron-overload β-thalassemia mice. J Inflamm Res. 2020; 13: 719-735.
- 44Ondee T, Pongpirul K, Visitchanakun P, et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci Rep. 2021; 11: 6367.
- 45Endo Y, Kikuchi T, Takeda Y, Nitta Y, Rikiishi H, Kumagai K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol Lett. 1992; 33: 9-13.
- 46Deng X, Yu Z, Funayama H, et al. Mutual augmentation of the induction of the histamine-forming enzyme, histidine decarboxylase, between alendronate and immuno-stimulants (IL-1, TNF, and LPS), and its prevention by clodronate. Toxicol Appl Pharmacol. 2006; 213: 64-73.
- 47Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001; 167: 2250-2256.
- 48Lei J, Wang Y, Paul J, Thompson S, Jha R, Gupta K. Pharmacological inhibition of TLR4 reduces mast cell activation, neuroinflammation and hyperalgesia in sickle mice. Blood. 2015; 126: 278.
- 49Żelechowska P, Brzezińska-Błaszczyk E, Różalska S, Agier J, Kozłowska E. Mannan activates tissue native and IgE-sensitized mast cells to proinflammatory response and chemotaxis in TLR4-dependent manner. J Leukoc Biol. 2021; 109: 931-942.
- 50Massari NA, Nicoud MB, Medina VA. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol. 2020; 177: 516-538.
- 51Chen J, Hu XY. Inhibition of histamine receptor H3R suppresses prostate cancer growth, invasion and increases apoptosis via the AR pathway. Oncol Lett. 2018; 16: 4921-4928.
- 52Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013; 339: 1084-1088.
- 53Poutahidis T, Springer A, Levkovich T, et al. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014; 9:e84877.
- 54Pernigoni N, Zagato E, Calcinotto A, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science. 2021; 374: 216-224.