The road to cervical cancer elimination in Malaysia: Evaluation of the impact and cost-effectiveness of human papillomavirus screening with self-collection and digital registry support
Abstract
The WHO has launched a global strategy to eliminate cervical cancer through the scale-up of human papillomavirus (HPV) vaccination, cervical screening, and cervical cancer treatment. Malaysia has achieved high-coverage HPV vaccination since 2010, but coverage of the existing cytology-based program remains low. Pilot studies found HPV self-sampling was acceptable and effective, with high follow-up rates when a digital registry was used, and recently the Malaysian Government announced plans for a national HPV-based screening program. We therefore evaluated the impact of primary HPV screening with self-collection in Malaysia in the context of Malaysia's existing vaccination program. We used the “Policy1-Cervix” modeling platform to assess health outcomes, cost-effectiveness, resource use and cervical cancer elimination timing (the year when cervical cancer rates reach four cases per 100 000 women) of implementing primary HPV testing with self-collection, assuming 70% routine-screening coverage could be achieved. Based on available data, we assumed that compliance with follow-up was 90% when a digital registry was used, but that compliance with follow-up would be 50-75% without the use of a digital registry. We found that the current vaccination program would prevent 27 000 to 32 200 cervical cancer cases and 11 700 to 14 000 deaths by 2070. HPV testing with a digital registry was cost-effective (CER = $US 6953-7549 < $US 11 373[<1×GDP per capita]) and could prevent an additional 15 900 to 17 800 cases and 9700 to 10 600 deaths by 2070, expediting national elimination by 11 to 20 years, to 2055 to 2059. If HPV screening were implemented without a digital registry, there would be 1800 to 4900 fewer deaths averted by 2070 and the program would be less cost-effective. These results underline the importance of HPV testing as a key elimination pillar in Malaysia.
Abbreviations
-
- APO
-
- adverse pregnancy outcomes
-
- ASCH
-
- atypical squamous cells, cannot exclude HSIL
-
- ASCUS
-
- atypical squamous cells of undetermined significance
-
- ASR
-
- age-standardized rate
-
- CER
-
- cost-effectiveness ratio
-
- CIN
-
- cervical intraepithelial neoplasia
-
- GDP
-
- gross domestic product
-
- HPV
-
- human papillomavirus
-
- HSIL
-
- high-grade squamous intraepithelial lesion
-
- LBC
-
- liquid-based cytology
-
- LEEP
-
- loop electrosurgical excision procedure
-
- LSIL
-
- low-grade squamous intraepithelial lesion
-
- LYS
-
- life-years saved
-
- PCR
-
- polymerase chain reaction
-
- VIA
-
- visual inspection with acetic acid
-
- WHO
-
- World Health Organization
What's new?
While Malaysia has achieved high-coverage human papillomavirus (HPV) vaccination, the coverage of the cytology-based screening program for cervical cancer remains low. This is the first modeling study to assess HPV self-sampling in Malaysia, and to model the impact of a digital registry in any setting. The scale-up of HPV self-sampling in addition to the existing vaccination program could save an additional 9,700-10,600 lives over the next 50 years, was cost effective, and could expedite cervical cancer elimination by 11-20 years. This supports the WHO's cervical cancer elimination strategy, and highlights the importance of HPV testing in achieving it.
1 INTRODUCTION
In Malaysia, cervical cancer is the third most common and the fourth most deadly cancer among women1; between 15 and 44 years of age, it is the second most common.2 Globocan 2018 estimates suggest that in that year, 1682 Malaysian women were diagnosed with cervical cancer and 944 died from it.1 From 2007 to 2011, it had an age-standardized rate (ASR) of incidence of 8.6 to 12.1 per 100 000.1, 2 The burden of disease is heterogenous, with higher incidence rates in East Malaysia vs Peninsular Malaysia.2
In 2020, the World Health Organization (WHO) launched its global initiative to eliminate cervical cancer as a public health problem through the “90/70/90” triple intervention strategy, which is (a) 90% of girls aged 15 fully vaccinated against human papillomavirus (HPV); (b) 70% of women screened with a high-sensitivity test by age 35 and again by age 45; and (c) 90% of women identified with cervical precancer or cancer receiving adequate treatment and care.3 Elimination is considered to have been achieved when the incidence rate for cervical cancer falls below four per 100 000 women. Recent comparative modeling showed that if these targets are reached across all low-and lower-middle income countries, elimination could be achieved in all countries analyzed.4
In 2010, Malaysia introduced a national vaccination program with a 3-dose schedule for all 13-year-old girls, switching to 2-dose in 2015,5 and having >80% coverage.6 Bivalent and quadrivalent vaccination have been alternately used since the vaccination program was introduced. Screening with 3-yearly Pap testing was introduced for women aged 20 to 65 in the 1960s but 3-yearly coverage has been <25%.7 This is partly because Malaysia has relied on opportunistic screening, with no registry for follow-up. Other factors include insufficient cytopathologists, lack of space and privacy in primary care facilities, lack of knowledge or time, and fear or embarrassment.8, 9 Any abnormalities detected in the current screening program would be referred to a hospital for follow-up. Cervical cancer treatments are available in Malaysia and depending on staging this typically involves chemoradiotherapy or hysterectomy.10 However, information on the level of access to such treatments is not fully available. Estimates of 5-year cervical cancer survival in Malaysia vary from 57% to 71%,11, 12 the upper value being similar to many high-income countries.
Pilot trials of HPV-based testing have recently been conducted in Malaysia. One of these was Project ROSE, a collaboration between the University of Malaya, the Malaysian Ministry of Health and VCS Foundation conducted in five clinics in Selangor. It integrated self-sampling, primary HPV screening and a digital registry. Initial findings of the trial were that 1997 women were screened, of which 99% indicated they would repeat the test again; reasons given by patients included that it was simple, quick, and self-performed.9 The digital health platform allowed women to have an initial result sent to them quickly via SMS, with 91% of those positive attending follow-up.9 Another pilot study of HPV testing, conducted by the Ministry of Health in four states since 2019, was quoted by the Deputy Health Minister as showing encouraging results.13
The Malaysian government plans to introduce HPV-based testing nationally by 2023.13 However, the effectiveness and cost-effectiveness of such a program is yet to be evaluated. Furthermore, some rural parts of interior East Malaysia are remote, for example only being accessible by river.14 In such regions, a point-of-care approach using portable HPV tests and on-the-spot treatment may be appropriate.15, 16 We therefore aimed to evaluate the benefits, harms, timing of elimination and cost-effectiveness of primary HPV screening with self-collection in Malaysia with a point-of-care approach in rural East Malaysia. We accounted for the existing national HPV vaccination program and explicitly assessed the impact of the use of a digital registry to improve follow-up.
2 METHODS
2.1 Model platform and calibration
We used “Policy1-Cervix,” an extensively validated dynamic model of HPV transmission, HPV vaccination, cervical precancer and cancer, screening, diagnosis and treatment (Appendix, Section A). The platform has previously been used for policy evaluations of countries such as Australia,17, 18 England,19 New Zealand,20 USA,19 China21, 22 and Japan.23 It has also been used to evaluate the timeline to elimination globally24 and as part of a comparative modeling assessment of the health impact of the WHO intervention strategy on 78 low and lower-middle income countries.4, 25 Reporting follows the HPV-FRAME standards for modeling of HPV vaccination and cervical screening (Appendix, Section B).26
The model was calibrated separately to Peninsular and East Malaysia (Figure 1) by using data on age-specific cervical cancer incidence27, 28 and HPV type distributions,29 and national data on cervical cancer stage distributions.2 The data were also validated against age-specific HPV prevalence,29 survival rates11, 30 national cancer incidence1 and mortality1 (Appendix, Section C). WHO national all-cause mortality projections were used to evaluate other causes of mortality over time.31 The relative proportions of the populations in Peninsular and East Malaysia were based on state-specific statistics, with rural parts of East Malaysia determined by using rural non-Malay populations as a proxy.32
2.2 Vaccination assumptions
State-specific full-dose vaccination coverage in 13-year-old Malaysian citizens from 2012 to 2018 was used to calculate yearly coverage rates in Peninsular and East Malaysia,6 adjusted to account for the number of citizens in the total population.33 In the absence of state-specific data for 2010 to 2011, national level data was used to extrapolate these values.5 We assume coverage from 2019 onwards remains at observed 2018 levels, full-dose vaccination is 100% effective against HPV 16/18 and protection is lifelong. We assume the bivalent vaccine also provides 71.9% cross-protection against types 31/45/52 based on published Japanese data,34 with 20 years protection, consistent with at least 7 years of protection currently reported.34, 35
2.3 Screening assumptions
For Peninsular Malaysia and urban East Malaysia, we used primary HPV DNA self-sampling with referral to colposcopy for women with a positive result (Figure 2A). We also modeled an algorithm that incorporates triaging by HPV16/18 genotyping and liquid-based cytology (LBC) (Figure 2B), which reflects recent Ministry of Health Guidelines for HPV screening.16 Women who are positive for HPV16/18 are referred to colposcopy while those who are positive for other high-risk types are referred to cytology. Women with high-grade abnormal cytology (ASCH/HSIL) are then referred to colposcopy while those with normal or low-grade cytology (ASCUS/LSIL) are followed up in a year. For rural East Malaysia we assumed point-of-care HPV testing is used with visual assessment to determine eligibility for same-day thermoablation (Figure 2C); Ministry of Health Guidelines allow for point-of-care testing depending on geographical accessibility.16 This flowchart reflects WHO guidelines for screen-and-treat with thermoablation,15 and more specifically, that used in recent field trials in Papua New Guinea, which found the approach was highly acceptable, feasible to implement, time saving, and could treat >90% of women with underlying high-grade disease.36, 37 Follow-up after colposcopy (Figure 2D) was informed by local clinical expertise (Personal Communication, YL Woo).
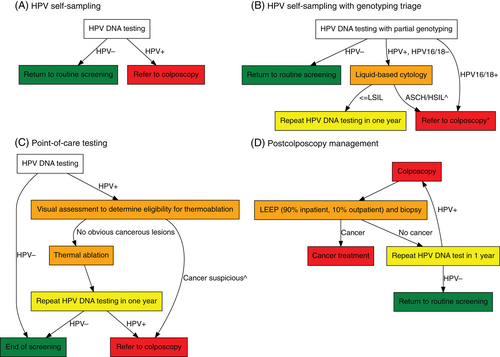
As modeled previously,4, 25 we assumed screening twice in a lifetime at ages 35 and 45 years and that scale-up would reach 45% by the year 2023, 70% by 2030 and 90% by 2045.4, 25 For rural East Malaysia, women are assumed to be screened only once at ages 35, as multiple screens would be physically challenging for women to attend in these remote regions. We assumed a digital registry is implemented for the baseline scenarios, allowing 90% compliance to follow-up to be achieved, based on findings of Project ROSE9; it also reflects the compliance assumptions for modeling performed to support the 2021 WHO guidelines for cervical screening.38 The sensitivity and specificity of screening tests, and the success rates of precancer treatments, were based on systematic reviews and pooled analyses (see Appendix, Section D for a table of values and references).
2.4 Costs
We assume a government payer perspective and therefore use direct medical and direct nonmedical costs. Costs were obtained from local fee schedules when available; this included colposcopies, loop electrosurgical excision procedures (LEEPs), and cancer treatment.39 Costs for LBC and HPV DNA were informed by local trials and expert opinion. Vaccination costs were based on the label price for Cervarix with additional administrative and wastage costs based on an international review of rotavirus vaccine costs, which has a similar overhead to HPV vaccines.40 All costs were converted to 2019 $US41, 42 (Appendix, Section E). We assume the digital registry would cost $US 8.45 per woman (Personal Communication, VCS Foundation).
2.5 Scenarios
We considered four scenarios, namely (1) a counter-factual in which neither screening nor vaccination were introduced (“No vax or screening”); (2) the status quo in which only the existing HPV vaccination program is in place (“Vax only”); (3) the existing HPV vaccination program and primary HPV screening with self-sampling (“Vax + HPV self-sampling”); and (4) the existing HPV vaccination program and primary HPV screening with self-sampling with HPV16/18 genotyping and LBC triage (“Vax + HPV self-sampling + triage”). Note that in each of the screening scenarios (2–4) point-of-care testing is used for rural East Malaysia (Figure 2C) rather than primary HPV screening with follow-ups (Figure 2A) or including triage (Figure 2B).
2.6 Outcomes
We assessed two effectiveness outcomes: (a) ASRs of cervical cancer incidence and mortality over time, using the 2015 Female World Standard Population for ages 0 to 9931; and (b) the number of cumulative cervical cancer cases and deaths averted. We assessed yearly resource use over 2020 to 2069 of precancer treatments and colposcopies (the numbers of these needed to avert a cervical cancer case or death are given in Appendix, Section F; for simplicity these ratios are represented for an unvaccinated cohort). The number of precancer treatments are also a direct surrogate measure of the obstetric harms associated with intervention, given excisional treatments can result in adverse pregnancy outcomes (APOs).43 Although we did not calculate the absolute number of APOs, the relative harms of different strategies can be compared using the treatment information. In an exercise such as this we could not directly assess psychosocial harms, but they are known to be closely linked to women with an abnormal test result, many of which receive precancer treatments and colposcopies.44 We performed a cost-effectiveness analysis over 2023 to 2100, assuming a 3% discounting rate for costs and life-years. A strategy was considered cost effective if it has a cost-effectiveness ratio [CER] <1×gross domestic product [GDP] per capita (pc) of Malaysia ($US 11 373).45, 46 For each outcome, we provided a range, based on whether bivalent or quadrivalent vaccination was used.
2.7 Supplementary evaluations and sensitivity analysis
As a supplementary approach, we evaluated the same national outcomes for screening scenarios without a digital registry (Appendix, Section G). To do this we did not apply the $US 8.45 registry cost per women initially screened and we also assumed a lower compliance to follow-up of 50% to 75%. The upper bound of 75% compliance reflected average follow-up observed in several Central American countries.47 It also matched alternative assumptions of 75% explored in sensitivity analysis in modeling performed to support the 2021 WHO cervical cancer screening guidelines.38 Although 75% compliance was considered as a lower bound of compliance in the context of appropriate and recommended data/registry support for screening,3 we also used it to inform our assumption for the most optimistic compliance that could be achieved without registry support. The lower bound of 50% for compliance in the absence of registry support reflects that observed without the use of one in Project ROSE (Personal Communication, YL Woo) as well the lowest compliance observed in Central American countries.46
Recent Malaysian screening guidelines recommend primary HPV screening every 5 years starting at age 30.16 Therefore, we also evaluated the same national outcomes for the screening programs but at ages 30 to 65 every 5 years, all else being equal (Appendix, Section H). Given the remoteness of rural East Malaysia, we modeled 3× lifetime screening at ages 30, 40, 50 with all else being equal.
We used one-way sensitivity to assess the uncertainty in the CER and the number of deaths averted for both screening algorithms (Appendix, Section I). The parameters we varied included costs, compliance and alternative screening modes.
3 RESULTS
3.1 Health impact
By the year 2070 the current vaccination program alone would prevent 27 000 to 32 200 cervical cancer cases and 11 700 to 14 000 deaths. Scaling-up primary HPV testing with self-collection and a digital registry could avert 44 800 to 48 000 cases of cervical cancer and 22 400 to 23 700 deaths (Table 1). The addition of screening therefore represents a 49% to 65% increase in cases averted and 69% to 91% increase in deaths averted. Additional compliance assumed to be achievable by use of a digital registry accounts for 3000 to 8400 of these cases averted and 1800 to 4900 of the deaths averted (Appendix, Section G). If 5-yearly screening were employed, a further 34 500 to 36 300 cases and 17 700 to 18 600 deaths would be averted (Appendix, Section H). By the year 2100, HPV self-sampling with vaccination could avert 112 300 to 121 900 cervical cancer cases and 57 300 to 61 500 deaths.
| Cumulative number | Reduction vs no vaccination or screening | Reduction vs current vaccination | ||||
|---|---|---|---|---|---|---|
| Scenarios | Year 2070 | |||||
| Cases | Deaths | Cases | Deaths | Cases | Deaths | |
| No vax or screening | 124 900 | 60 100 | ||||
| Vax only | 92 700-97 800 | 46 100-48 400 | 27 000-32 200 | 11 700-14 000 | ||
| Vax + HPV self-sampling | 76 800-80 000 | 36 500-37 800 | 44 800-48 000 | 22 400-23 700 | 15 900-17 800 | 9700-10 600 |
| Vax + HPV self-sampling + triage | 77 900-81 500 | 37 000-38 400 | 43 400-47 000 | 21 800-23 100 | 14 800-16 300 | 9100-10 000 |
| Cumulative number | Reduction vs no vaccination or screening | Reduction vs current vaccination | ||||
|---|---|---|---|---|---|---|
| Scenarios | Year 2100 | |||||
| Cases | Deaths | Cases | Deaths | Cases | Deaths | |
| No vax or screening | 218 800 | 106 800 | ||||
| Vax only | 121 800-136 300 | 60 600-67 700 | 82 500-97 000 | 39 000-46 200 | ||
| Vax + HPV self-sampling | 96 900-106 600 | 45 300-49 500 | 112 300-121 900 | 57 300-61 500 | 24 900-29 700 | 15 300-18 200 |
| Vax + HPV self-sampling + triage | 98 900-109 400 | 46 400-50 900 | 109 400-119 900 | 55 900-60 400 | 22 900-26 800 | 14 200-16 900 |
- Note: Vax + HPV self-sampling and Vax + HPV self-sampling + triage uses twice lifetime screening at ages 35 and 45 in Peninsular Malaysia and in urban East Malaysia. However, for rural East Malaysia, once-lifetime at age 35 point-of-care testing is used. See Section 2 for details.
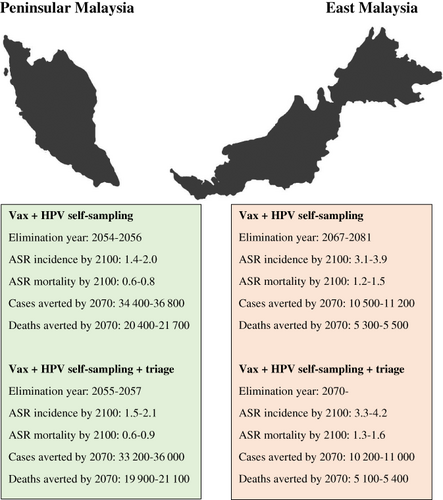
Without any vaccination or screening programs, the national ASR of cervical cancer incidence would remain at 9.9 per 100 000. The ASR for Peninsular Malaysia would be 9.0 per 100 000 and for East Malaysia it would be 14.0 per 100 000. The existing vaccination program could eliminate cervical cancer at a national average level by the year 2066 to 2079 (Figure 3), leveling out at an ASR of 2.7 to 3.7 per 100 000 in the longer term. However, in East Malaysia, elimination would not be possible, with rates leveling out at 5.4 to 6.5 per 100 000. This is due to the lower vaccination coverage and higher burden of disease in this region. The combination of vaccination and HPV self-sampling could bring forward the timing of national elimination by >10 years to 2055 to 2059, leveling out at an ASR of 1.7 to 2.4 per 100 000. It would also allow for elimination in East Malaysia by 2067 to 2081, albeit at a later time than in Peninsular Malaysia, where elimination could occur by 2054 to 2056. A similar pattern occurs for mortality rates (Figure 3), with rates falling from 4.6 at status quo to 0.7 to 0.9 per 100 000 with HPV-based screening. If lower compliance in the context of a digital registry not being used was observed, the timeline to elimination would be delayed by 3 to 7 years (Appendix, Section G). On the other hand, if 5-yearly screening were employed, the national timeline to elimination could be further expedited to 2038 (Appendix, Section H).
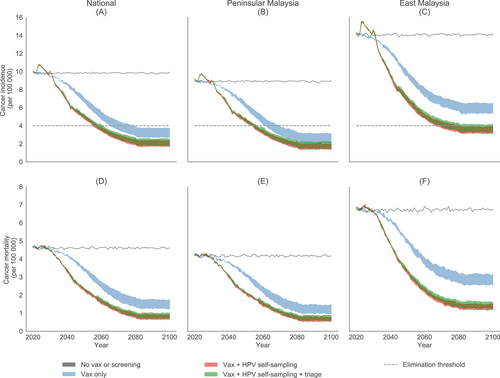
If we instead scale-up using primary HPV testing with a triage for HPV positive women, there would be 1000 to 1400 more cervical cancer cases and 600 to 600 more deaths (Table 1) by 2070 than the strategy in which all HPV positive women are referred to colposcopy. The ASR of incidence and mortality would also level out at slightly higher values (Figure 3), with national elimination occurring in 2056 to 2062. Strictly speaking, the 4/100 000 elimination threshold is not achieved in East Malaysia without assuming there is cross-protection as has been shown for bivalent vaccination. Nevertheless, similar rates are achieved with quadrivalent vaccination (4.2/100 000). With lower compliance in the context of no digital registry, there would be delays in elimination of 4 to 9 years and 2200 to 5700 fewer deaths averted by 2070 (Appendix, Section G). On the other hand, if 5-yearly screening were employed, the national timeline to elimination could be expedited to 2038 and 17 400 to 18 200 more deaths averted by 2070 (Appendix, Section H).
Despite differences in ASRs and timing to elimination between the regions being modeled, Peninsular Malaysia accounts for 76% to 80% of cervical cancer cases and deaths prevented by 2070 (Box 1), due to it representing 79% of the national population.31
BOX 1. Summary of the health impact in Peninsular Malaysia and East Malaysia [Color figure can be viewed at wileyonlinelibrary.com]
Note that Vax + HPV self-sampling and Vax + HPV self-sampling + triage uses twice lifetime screening at ages 35 and 45 in Peninsular Malaysia and in urban East Malaysia. However, for rural East Malaysia, once-lifetime at age 35 point-of-care testing is used. See Section 2 for details.
3.2 Budget impact and cost-effectiveness
Table 2 shows that the implementation of HPV self-sampling is cost-effective over 2023 to 2100, with a CER of $US/LYS 6953 to 7549 (LYS stands for life-years saved), which is <1×GDP pc of Malaysia, that is, $US 11 373 ($RM 45 948).41, 47 HPV self-sampling with triage for HPV positive women was similarly cost-effective, being within the same range as for HPV self-sampling in which all HPV positive women are referred to colposcopy (ie, neither dominates the other). The average budget impact of vaccination and HPV self-sampling with triage would be $US 44.3 to 44.4 million per year, slightly less than 50% more than that of current vaccination only. Not using triage would further increase this by about $US 1 million per year. With lower compliance in the context of no digital registry, both screening strategies would be less cost-effective, with triaging of HPV positive women potentially not being cost-effective (Appendix, Section G). Five-yearly screening was not cost-effective when assumed to be delivered to all women over the next century regardless of vaccination status (Appendix, Section H) although it was cost-effective for unvaccinated cohorts (Appendix, Section F). These cost-effectiveness results were robust to sensitivity analysis: varying costs, having a steadier initial scale-up of 10% per year from 2024 to 2030, using higher compliance and using the alternative postcolposcopy management in which women without a visible transformation zone receive cytology, was still cost-effective, as well as having comparable health impact (Appendix, Section I).
| Scenarios | Discounted costs per woman (USD) | Discounted life-years per woman | Cost-effectiveness ratio (USD/LYS) | Annual budget impact (USD millions) over 2020-2029 | Annual cost difference versus “Vax only” (USD millions) |
|---|---|---|---|---|---|
| Vax only | $74.57-77.38 | 30.5490-30.5504 | – | $30.8-30.9 | – |
| Vax + HPV self-sampling | $111.77-115.38 | 30.5545-30.5553 | $6953.32-7549.10 | $45.2-45.4 | $14.4-14.5 (+46.9-46.9%) |
| Vax + HPV self-sampling + triage | $109.69-113.06 | 30.5542-30.5550 | $6935.36-7607.96 | $44.3-44.4 | $13.5-13.5 (+43.8-44.0%) |
- Note: All values are a yearly average. Vax + HPV self-sampling and Vax + HPV self-sampling + triage uses twice lifetime screening at ages 35 and 45 in Peninsular Malaysia and in urban East Malaysia. However, for rural East Malaysia, once-lifetime at age 35 point-of-care testing is used. See Section 2 for details.
3.3 Impact of triaging HPV positive women
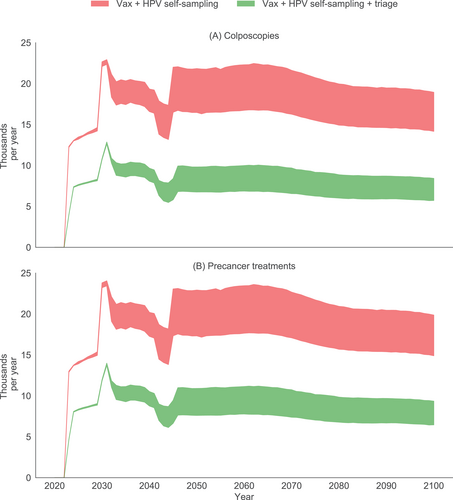
If HPV self-sampling were implemented in addition to current vaccination, the number of precancer treatments (Figure 4) would increase from 12 900 to 13 000 in the year 2023 (the first screening program year) to a peak of 23 400 to 24 000 in 2031 (after screening coverage is scaled up in 2030), and decrease to 14 900 to 19 900 by 2100. The number of colposcopies follow a similar pattern. If vaccination and HPV self-sampling with triage for HPV positive women were instead implemented, the number of precancer treatments is greatly reduced, with the peak yearly number of precancer treatments and colposcopies half those observed without triage. In unvaccinated cohorts, the number-needed-to-treat to avert a cancer death decreased from 36 with no triage to 22 with triage (Appendix, Section F). Thus, precancer treatments and colposcopies are halved, with small impacts on health outcomes, resulting in a more efficient program.
4 DISCUSSION
We evaluated the benefits, harms, impact on elimination timing and cost-effectiveness of implementing primary HPV testing using self-collection with a digital registry. We found that the current vaccination program alone would prevent 27 000 to 32 200 cervical cancer cases and 11 700 to 14 000 deaths by 2070, and that additionally scaling-up HPV testing to 70% coverage by 2030 could prevent another 15 900 to 17 800 cervical cancer cases and 9700 to 10 600 deaths. This would bring forward the timing to elimination of cervical cancer by 11 to 20 years to 2055 to 2059 and be cost-effective (CER < $RM 45,947.86 [<1×GDP pc]). If a digital registry were not implemented, 1800 to 4900 fewer deaths would be averted by 2070, elimination would be delayed, and the program would be less cost-effective (Appendix, Section G).
To our knowledge, this is the first comprehensive evaluation of the combination of primary HPV screening with self-collection and a digital registry for any setting. Whereas clinician-based testing may be a barrier for some women, almost 90% of women surveyed in the Project ROSE trial approved of the fact that the HPV test could be self-sampled.9 Provided that the polymerase chain reaction (PCR) is used, self-sampling is comparable to the sensitivity of clinician-collected samples,48 as we have modeled here, and higher than the sensitivity of the current Pap tests.9 However, the preference for self-collection or clinician-collection will vary from setting to setting and an option for both would be ideal. Project ROSE adapted the canSCREEN49 digital health platform developed by the VCS Foundation for digital registration and rapid follow-up via SMS.9 This software is designed to give a comprehensive multilevel approach for population health screening, managing and integrating data on participation and effectiveness.49 Of the HPV-positive women in that study, 91% attended follow-up, similar to our modeled compliance of 90%. Without this facility for recall, much lower compliance rates to follow-up would be observed.46 Supplementary analysis in our evaluation showed that digital registration has a substantial impact on both the effectiveness and cost-effectiveness of an HPV-based screening program (Appendix, Section G). This is because, even with only 1 or 2 routine lifetime screens, HPV-positive women may need many follow-ups in the scenarios explored for any given routine screen (a minimum of 2 but often 4 or more depending on follow-up outcomes), woman may be lost to each of these follow-ups and without a digital registry the increased losses are magnified. Although the modeled registration costs were based on canSCREEN, there are other monitoring approaches that result in a lower cost per woman. For example, District Health Information Software 2 (DHIS2) is an open-source model which has been utilized in various public health programs in Africa including cancer control.50 A system could also be developed in-house by the Malaysian government, which already has other health information systems to draw upon. Further reductions in costs could make the use of an appropriately functional registry even more cost-effective, as demonstrated in our sensitivity analysis (Appendix, Section I).
This is also the first modeled evaluation of the effectiveness and cost-effectiveness of an HPV-based screening program in Malaysia. A previous analysis considered the cost-effectiveness of cytology-based screening, quadrivalent vaccination or both. It found that vaccination is more cost-effective due to the infeasibility of long-term screening adherence,51 but it did not explore HPV-based self-sampling. In an earlier study published by our group, we estimated the timeline to elimination in Malaysia as part of a wider global analysis.24 In our current study, we found that Malaysia could eliminate cervical cancer by 2065 to 2070. This is later than what we reported in the earlier analysis (2055 to 2059). However, the global study was based on Globocan 2012 disease burden estimates, whereas the current study uses Globocan 2018 estimates (Appendix, Section J). Furthermore, our current analysis builds on this earlier work by considering primary HPV testing and triage management options based on local clinical experience obtained from Project ROSE. This updated study also performed a detailed implementation of different burden-of-disease rates between Peninsular and Easy Malaysia and also captured detailed vaccination rates for Peninsular and East Malaysia.
We predicted that the scale-up of primary HPV testing without triage in Malaysia would lead to a yearly peak in precancer treatments of 23 400 to 24 000. However, using genotyping and LBC as a triage could halve the peak in precancer treatments and reduce the number-needed-to-treat, a key factor when considering potential adverse obstetric outcomes resulting from LEEP.43 Because both algorithms are equally cost-effective and using triage would confer only a slight reduction in health impact, an HPV-based screening program that uses such triaging may be preferred.
We modeled a point-of-care approach for rural East Malaysia, and found that HPV-based screening was crucial for achieving elimination in East Malaysia. We assumed that women offered point-of-care HPV testing would receive results within about an hour, enabling same-day treatment of screen positive women. This approach has been trialed in various low- and middle-income settings37, 52 and protocols developed for scale-up.36, 53 Those suitable for treatment undergo thermocoagulation using a portable instrument. Although cryotherapy has traditionally been used in this context, it is increasingly falling out of favor due to logistical difficulties related to the requirement to transport CO2. A recent trial in Papua New Guinea, upon which our modeled algorithm was based, also showed that point-of-care testing with HPV had high sensitivity >90% for high-grade disease, and that visual inspection with acetic acid (VIA) is useful in determining which treatment to apply but not for triage.36, 37 However, women who are not found to be suitable for ablative treatment would need to attend a district hospital for further investigation.15
We found that a high-coverage 5-yearly screening program, as recommended in recent Malaysian guidelines, would expedite elimination compared to HPV vaccination alone, to 2038 with ≥75% more deaths averted over 2020 to 2069 (Appendix, Section H). This was not found to be as cost-effective, but our estimation of HPV screening costs is based on local trial data, whereas unit costs are likely to decrease significantly with a national scale-up. Furthermore, our cost-effectiveness analysis was performed over from 2023 to 2100 during which time all cohorts would become vaccinated, reducing the additional benefits of more frequent screening. Indeed, for unvaccinated cohorts, 5-yearly screening was cost-effective (Appendix, Section F). An area of future investigation may be to apply different screening frequencies depending on if a cohort is vaccinated, as has been modeled in other settings such as China.54
An alternative paradigm for cervical modeling has previously been proposed, which uses broad categories of natural history, namely reproductive HPV infection, and precancer (transitional HPV infection).55 In the Policy1-Cervix platform, the natural history states are defined on the basis of histopathological CIN (ie, different grades of CIN), because it is specifically CIN2+ that is treated, and because this allows us to directly relate model outputs to the data. This has allowed the model to be validated across a wide range of settings (Appendix, Section A). However, recently we and colleagues have demonstrated that structural differences may not generate different outcomes so long as model platforms are extensively calibrated and validated. A recent comparative modeling exercise for the US compared several models including Policy1-Cervix and the Harvard model,56 which does employ the alternative paradigm. This found similar outcomes in terms of average time from infection to cancer (25.7 years for Policy1-Cervix and 26.0 years for Harvard); in both models 50% of women acquired their carcinogenic infection by ages 19 to 23 years. In contrast, another model in the same comparative evaluation had different results, with average time from infection to cancer being 17.5 years and 50% of women only obtaining carcinogenic infection by 34 years, suggesting that other model differences can be more important in outcomes. We have also demonstrated very similar results between Policy1-Cervix and Harvard models across a range of policy evaluations. This detailed comparative modeling exploration shows that Policy1-Cervix and Harvard models, both well calibrated models that have been validated in many different settings, have very similar outcomes.
Our study has some limitations. It did not directly account for the alternating use of quadrivalent and bivalent vaccination in Malaysia between 2010 and 2016. However, we covered both these possibilities by exploring an upper and lower bound in all of our outputs (ongoing quadrivalent and bivalent vaccination, respectively). The sexual behavior was generalized rather than specific to the case of Malaysia but in our previous global analysis we found that substantially varying herd effects had minimal impact on cases averted.24 Although we have used the best population projections available, they span a period in which substantial demographic changes and technological development could occur. The relative proportion of women in Peninsular and East Malaysia was assumed to remain fixed but this is based on Malaysian population projections, which show the proportion of women remain stable.57 We did not explicitly model differential management of women with no visible transformation zones at colposcopy in the baseline because screening was modeled for women at ages 35 and 45 who would be premenopausal and thus likely to have a clear transformation zone.58 However, we did consider a scenario in sensitivity analysis (Appendix, Section I) in which women who did not have a visible transformation zone returned for a cytology test, and found that this did not affect our conclusions. Finally, we took a government payer perspective when applying costs. However, while Malaysia's healthcare system has a public sector that is almost entirely funded by budget allocations, it also has a fee-for-service private sector that offers many parallel services at primary care level and increasingly, secondary and tertiary hospitals.59 Nonetheless, Malaysia's HPV vaccination program is largely public59 and an HPV screening program roll-out might reasonably be expected to also fall under the purview of the government.
The scale-up of screening coverage which we modeled (45% by 2023, 70% by 2030 and 90% by 2045) matches that used in recent comparative modeling to inform the WHO global initiative for cervical cancer elimination.4, 25 Our study acknowledged that such a target can be considered aspirational: many obstacles would need to be negotiated including screening test supply and delivery challenges along with scale-up of treatment and colposcopy services for referral, not to mention disruptions related to COVID-19. As part of Malaysia's “movement control order” for COVID-19, schooling of girls was disrupted, with likely repercussions on vaccine uptake. Women wishing to attend screening or with symptoms of cervical cancer may have also delayed presenting to clinicians due to the restrictions on travel. The burden-of-disease for COVID-19 has recently been increasing in Malaysia,60 which may limit diagnosis, precancer and cancer treatment access as resources and staff are impacted by the epidemic.61 However, we did not model screening scale-up until 2023, and it is likely that COVID-19 vaccination would be rolled out by then and therefore screening scale-up would be unaffected. Our sensitivity analysis has shown that a more gradual scale-up toward the 2030 target of 10% coverage per year would still be cost-effective and could save a similar number of lives by 2070. If scale-up is achieved more slowly, then reductions in incidence and mortality would correspondingly be delayed. Cost-effectiveness and efficiencies are likely to be similar as costs and resource use are similarly scaled downwards.
A major strength of our study is that it uses a multicompartmental model that has been used to evaluate screening and vaccination across a range of settings.4, 20-25 It has also been validated in many contexts, including as part of a recent comparative modeling exercise of 78 low and lower-middle income countries coordinated by the WHO.4, 25 We adhered to the HPV-FRAME guidelines for reporting of cervical cancer modeling.26 The calibration targets were based on Malaysian data, accounted for regional differences, and validated against national Globocan estimates. This multitarget exercise was performed against metrics including age-specific cancer incidence and mortality, and age- and type-specific HPV prevalence. The screening strategies were informed by local data, trials and guidelines, and devised in consultation with local experts, and are therefore highly salient for policymakers. Important distinctions in region-specific population structures and vaccine coverage were accounted for over time, and costs were sourced from local expert opinion and data. A variety of parameters were also found to be robust to sensitivity analysis, including higher compliance, and costs of HPV tests, registration and vaccine costs.
Our modeling shows that the scale-up of HPV-based screening in Malaysia could accelerate the timeline to elimination and nearly the double the number of lives saved in the next 50 years. Screening is especially critical in the short-to-medium term given a vaccination program was only introduced recently and many women will be unvaccinated. It also accounts for other high-risk types not fully protected by the bivalent vaccine, and which globally make up 30% of cervical cancer cases. Our evaluation is unique in modeling the combination of HPV DNA testing, self-collection and a digital registry. This combination is cost-effective for screening scale-up in Malaysia, maximizing compliance, and is likely to be an important consideration in other settings.
ACKNOWLEDGMENTS
Adam Keane and Kate Simms receive salary support from Cancer Institute NSW (CDF1004). Karen Canfell receives salary support from the National Health and Medical Research Council (NHMRC; APP1194679). This study was in part funded by Cancer Institute NSW (CDF1004).
CONFLICT OF INTEREST
Marion Saville is Executive Director at VCS Foundation which has developed the canSCREEN digital health platform utilized in Project ROSE, which is a partnership between the University of Malaya and VCS Foundation. VCS Foundation is a not-for-profit organization that offers services to implement, support, monitor and manage population health programs. Karen Canfell and MS are co-PIs of an investigator-initiated trial of cytology and primary HPV screening (Compass; ACTRN12613001207707 and NCT02328872), which is conducted and funded by the VCS Foundation (VCS), a government-funded health promotion charity. Karen Canfell is also an investigator of Compass New Zealand (ACTRN12614000714684), which was conducted and funded by Diagnostic Medlab (DML), now Auckland District Health Board. The VCS Foundation received equipment and a funding contribution from Roche Molecular Systems and Ventana USA and DML received equipment and a funding contribution for Compass from Roche Molecular Systems. However, neither Karen Canfell nor her institution on her behalf (Daffodil Centre) receives direct funding from industry for this trial or any other project. Adam Keane, Chiu Wan Ng, Kate T. Simms, Diep Nguyen and Yin Ling Woo declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The model input parameters and year-and-cohort level simulated outputs of the model that support the findings of this study are available from the corresponding author upon reasonable request.