One-carbon metabolite ratios as functional B-vitamin markers and in relation to colorectal cancer risk
Abstract
One-carbon metabolism biomarkers are easily measured in plasma, but analyzing them one at a time in relation to disease does not take into account the interdependence of the many factors involved. The relative dynamics of major one-carbon metabolism branches can be assessed by relating the functional B-vitamin marker total homocysteine (tHcy) to transsulfuration (total cysteine) and methylation (creatinine) outputs. We validated the ratios of tHcy to total cysteine (Hcy:Cys), tHcy to creatinine (Hcy:Cre) and tHcy to cysteine to creatinine (Hcy:Cys:Cre) as functional markers of B-vitamin status. We also calculated the associations of these ratios to colorectal cancer (CRC) risk. Furthermore, the relative contribution of potential confounders to the variance of the ratio-based B-vitamin markers was calculated by linear regression in a nested case–control study of 613 CRC cases and 1,190 matched controls. Total B-vitamin status was represented by a summary score comprising Z-standardized plasma concentrations of folate, cobalamin, betaine, pyridoxal 5′-phosphate and riboflavin. Associations with CRC risk were estimated using conditional logistic regression. We found that the ratio-based B-vitamin markers all outperformed tHcy as markers of total B-vitamin status, in both CRC cases and controls. In addition, associations with CRC risk were similar for the ratio-based B-vitamin markers and total B-vitamin status (approximately 25% lower risk for high vs. low B-vitamin status). In conclusion, ratio-based B-vitamin markers were good predictors of total B-vitamin status and displayed similar associations as total B-vitamin status with CRC risk. Since tHcy and creatinine are routinely clinically analyzed, Hcy:Cre could be easily implemented in clinical practice.
Abstract
What's new?
While total homocysteine (tHcy) levels are an important biomarker of B-vitamin status and may be predictive for colorectal cancer (CRC) risk, they are influenced by a variety of factors, such as age, sex, and lifestyle. Here, tHcy was compared to ratio-based biomarkers of total B-vitamin status to assess functionality and relation to CRC risk. In CRC patients and controls, the ratio-based markers outperformed tHcy as indicators of total B-vitamin status. Their association with CRC risk was similar to that of total B-vitamin status. Ratio-based biomarkers could fill a valuable role in assessments of functional B-vitamin levels and disease risk.
Abbreviations
-
- AUC
-
- area under the curve
-
- CRC
-
- colorectal cancer
-
- KTR
-
- kynurenine-to-tryptophan-ratio
-
- MSP
-
- Mammary Screening Programme
-
- NSHDS
-
- Northern Sweden Health and Disease Study
-
- OR
-
- odds ratio
-
- PLP
-
- pyridoxal 5′-phosphate
-
- ROC
-
- receiver operating characteristic
-
- SAM
-
- S-adenosylmethionine
-
- SD
-
- standard deviation
-
- SDMA
-
- symmetric dimethylarginine
-
- tHcy
-
- total homocysteine
-
- VIP
-
- Västerbotten Intervention Programme
Introduction
One-carbon metabolism, a complex network of enzymatic reactions using B-vitamins as co-factors and co-substrates, is important for genome stability and gene translation, and disturbances in one-carbon metabolism are associated with anemia, cardiovascular disease, tumorigenesis and other diseases.1-3 Clinically, and in medical research, plasma total homocysteine (tHcy) concentrations are used as a functional marker of B-vitamin status, in particular folate, and also other components involved in one-carbon metabolism.4, 5 However, tHcy concentrations are influenced by several physiological, clinical and lifestyle factors, including age, sex, smoking, kidney function and blood pressure.6-9
tHcy is formed from S-adenosylhomocysteine, which is formed from the universal methyl group donor S-adenosylmethionine (SAM) in the course of transmethylation reactions.1 Elevated homocysteine may be harmful to the cell, and excess homocysteine is either metabolized through the transsulfuration pathway or remethylated into methionine.5 In the transsulfuration pathway, homocysteine provides sulfur functional groups in a series of vitamin B-6 dependent enzymatic reactions. The first step converts homocysteine into cystathionine with the help of the enzyme cystathionine β-synthase, cystathionine is then converted into cysteine by cystathionine γ-lyase. Remethylation of homocysteine by methionine synthase uses 5-methyltetrahydrofolate as a methyl donor and vitamin B-12 as a cofactor.1 Alternatively, homocysteine can be remethylated via betaine-homocysteine methyltransferase with betaine as methyl group donor.10 The supply of 5-methyltetrahydrofolate is dependent on the activity of the methylenetetrahydrofolate reductase enzyme (MTHFR) in a reaction with 5,10-methylenetetrahydrofolate as substrate and vitamin B-2 as cofactor. Thus, mechanistically, methylation reactions in one-carbon metabolism depend on folate, cobalamin (vitamin B-12), betaine, riboflavin (vitamin B-2) and pyridoxal 5′-phosphate (PLP (vitamin B-6)). Accordingly, plasma concentrations of tHcy are inversely associated with dietary intake and/or plasma concentrations of these vitamins and nutrients.6, 11-13 Furthermore, a common variant homozygous polymorphism in MTHFR rs1801133 (MTHFR 677TT) is associated with higher plasma tHcy concentrations,13 and homocysteine and cysteine also increase with impaired renal function.14
Recently, three metabolite ratios all showed improved sensitivity and specificity over homocysteine alone for predicting plasma B-vitamin status in a cohort of patients with suspected stable angina pectoris.15 The ratios comprise tHcy and either cysteine (Hcy:Cys), creatinine (Hcy:Cre), or a combination of the two (Hcy:Cys:Cre), and are hereafter referred to as ratio-based B-vitamin markers. Biochemically, the ratios reflect the transsulfuration pathway and SAM-dependent methylation reactions. Cysteine is the output of the transsulfuration pathway,1 and creatinine is the catabolite of creatine, the synthesis of which consumes a significant portion of SAM and thus is methyl group demanding.16
Several B-vitamins and related nutrients and metabolites have been studied in relation to CRC risk. Higher plasma concentrations of riboflavin, PLP and betaine have each been associated with lower CRC risk.17-20 Plasma concentrations of cysteine have been observed to be associated with lower CRC risk in women,21 whereas cohorts including both men and women reported null associations.18, 22 For cobalamin, folate and tHcy, associations have been inconsistent or null.17, 21-26 However, in the Northern Sweden Health and Disease Study (NSHDS), and in a large US study with low folate status similar to the NSHDS, low plasma concentrations of folate were associated with decreased CRC.24, 27, 28
Studying one-carbon metabolites individually may give ambiguous results, due to the interdependence between the metabolites.29 We have previously used a machine learning-based Bayesian network approach to simultaneously study >30 factors involved in one-carbon metabolism in relation to CRC risk.18 The study confirmed the importance of folate, riboflavin and PLP relative to other metabolites involved in one-carbon metabolism. Another approach is to use more general markers reflecting the dynamics of the one-carbon metabolism network. tHcy is one such marker,5 but its usefulness is hampered by the influence of factors such as kidney function and inflammation.15 The novel, ratio-based B-vitamin markers Hcy:Cys, Hcy:Cre and/or Hcy:Cys:Cre may provide a better estimation of overall B-vitamin status with limited additional biochemical analyses and have previously not been evaluated in relation to cancer risk.
In this population-based, prospective study of 613 colorectal cancer (CRC) cases and 1,190 healthy matched controls, we assessed three ratio-based B-vitamin markers, namely tHcy:Cys, tHcy:Cre and tHcy:Cys:Cre, as markers of total B-vitamin status and in relation to CRC risk.
Materials and Methods
Study participants
This was a case–control study nested within the Northern Sweden Health and Disease Study (NSHDS). Participants from two population-based subcohorts were included, the Västerbotten Intervention Programme (VIP, 78% of the study participants) and the Mammography Screening Project in Västerbotten (MSP, 22% of the study participants).30 In the VIP, established in 1985, residents of Västerbotten County are invited to participate in a health survey upon turning 30 (years 1990–1996), 40, 50 and 60 years of age. The health survey consists of laboratory tests and a medical examination, donation of a fasting blood sample and completion of an extensive lifestyle questionnaire. The VIP included 115,147 blood samples from 85,877 individuals as of March 31, 2009, the final date for case identification for our study (Supporting Information Fig. S1). Selection bias has been found to be low,31 and comparisons of cancer incidence in the cohort with expected incidence (based on data from the Swedish Cancer Registry) support the population-based structure of the VIP cohort.32 The MSP was established in 1995 and concluded in 2006, and invited women residing in Västerbotten County and about 50–70 years of age, to complete a lifestyle questionnaire and donate a blood sample while attending mammography screening. The MSP includes a total of 54,401 blood samples from 28,802 women.
CRC case participants diagnosed between October 17, 1986, and March 31, 2009, were identified by linkage with the essentially complete Cancer Registry of Northern Sweden (ICD-10 C18.0 and C18.2–C18.9 for colon, C19.9 and C20.9 for rectum). A single pathologist specialized in gastrointestinal pathology verified all cases and tumor data. Patient records were used to verify tumor site. Exclusion criteria were previous cancer diagnosis other than non-melanoma skin cancer (68 cases), insufficient volume of stored plasma samples (46 cases, nine controls), location of the primary tumor outside of the colorectum (18 cases), samples prioritized to other studies (eight cases, 18 controls), serious infectious disease (one case, excluded for the safety of the laboratory staff) or no matching control available (three cases). Two control participants were randomly selected for each case, matched by age at and year of blood sampling and data collection, cohort, sex and fasting status. The same exclusion criteria were applied to the control participants as the case participants. Furthermore, controls were all alive and had no diagnosed cancer other than non-melanoma skin cancer at the time of diagnosis of their index cases. After exclusions (81 cases and 41 controls), 613 cases and 1,190 controls were in included for data analyses (Supporting Information Fig. S1).
The data handling procedures and study protocol were approved by the Research Ethics Committee of Umeå University, Umeå, Sweden (Dnr 03-186). All participants gave a written informed consent at the time of recruitment to the cohorts. All data and samples were de-identified.
Blood sampling and analysis
The blood samples for our study were collected in EDTA sample tubes, separated into plasma, erythrocyte fractions and buffy coat and cryopreserved at −80°C at a central location. Samples were frozen within 1 hr of collection, either at −80°C or at −20°C for up to 1 week before transfer to a −80°C freezer. In the VIP samples are collected in the morning, and only 2% had fasted <4 hr, and 21% <8 hr. In the MSP, blood samples were collected throughout the day, and 96% had fasted for <4 hr.
All biochemical analyses were performed at Bevital A/S (http://www.bevital.no), Bergen, Norway, in 2011. Plasma concentrations of betaine, riboflavin, creatinine, neopterin, symmetric dimethylarginine (SDMA) and PLP were measured with liquid chromatography–mass spectrometry methods (between-day coefficient of variation (CV): 3–13%).33, 34 Plasma concentrations of tHcy, tCys, kynurenine and tryptophan were measured using an isotope dilution gas chromatography–mass spectrometry method (between-day CV: 2–9%).35 Folate and cobalamin concentrations were determined by a microbiological method using Lactobacillus casei and Lactobacillus leichmannii, respectively, adapted to a microtiter plate format and carried out by a robotic workstation (between-day CV: 5%),36, 37 MTHFR rs1801133 genotype was determined using MALDI-TOF mass spectrometry (estimated average error rate of ≤0.1% in duplicated samples).38 The investigators and laboratory staff were blinded to case and control status.
Statistical analyses
All computations were conducted in R v.3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and p-values < 0.05 were considered statistically significant. Mann–Whitney U-test or χ2 tests were used to test for univariate differences in variable distributions between groups.
We calculated total B-vitamin status score by summing log-transformed and standardized (z = x-mean/standard deviation (SD)) plasma concentrations of folate, cobalamin, betaine, PLP and riboflavin. The influence of the B-vitamin score and other variables on tHcy and B-vitamin markers was then evaluated in multiple linear regression models. The relative contribution to total explained variance (R2) for tHcy and B-vitamin markers were assessed using the lmg algorithm in the relaimbo R package. The lmg algorithm decomposes total R2 into a contribution for each predictor in a regression model by taking the average R2 contribution of each predictor over all possible model sizes and predictor combinations.39 Four linear regression models were fitted, with log-transformed standardized tHcy, Hcy:Cys, Hcy:Cre and Hcy:Cys:Cre as response variables, and sex, age, cohort (VIP or MSP), fasting status (<4 hr, 4–8 hr, ≥8 hr), sample year, smoking status (non-, current-, former-smoker), BMI, kynurenine-to-tryptophan-ratio (KTR), neopterin, SDMA and B-vitamin score as predictor variables. We also evaluated the contribution of individual B-vitamins by estimating the same models but with log-transformed standardized B-vitamins included as separate variables. To compare associations between B-vitamin score and ratio-based B-vitamin markers, we evaluated regression-based sensitivity (defined as B-vitamin marker variance explained by B-vitamin score), regression-based specificity (defined as B-vitamin marker variance explained by B-vitamin score divided by total variance explained) and performance (defined as sensitivity multiplied by specificity). In addition, we also calculated more clinically applied metrics, i.e., diagnostic sensitivity, specificity and area under the receiver operating characteristic (ROC) curve (AUC) for B-vitamin deficiency defined as a B-vitamin score below the lowest 5th percentile in control participants.
To further evaluate the specificity of the ratio-based B-vitamin markers in relation to total B-vitamin status and not as markers of kidney dysfunction and inflammation, we calculated a combined measure of kidney dysfunction and inflammation by summing log-transformed standardized plasma concentrations of the sensitive kidney function marker SDMA,40 and the inflammatory markers KTR and neopterin.41 The score was then used in ROC analysis by defining a dichotomous kidney dysfunction and inflammation variable using the highest 95 percentile of the score in controls as the cut-off.
Odds ratios (ORs) for CRC risk by biomarker levels were calculated using conditional logistic regression, taking the matched control design into account. The markers were stratified by tertiles, with cut-off values based on the distribution of the controls, or as standardized log-transformed continuous variables. ORs were obtained from crude models adjusted only for matching (Model 1), as well as from models adjusted for variables with a plausible link to both exposure and CRC risk. Model 2 was adjusted for smoking status (current-, former-, non-smoker), body mass index (BMI) (<25, 25–30, ≥30 kg/m2), alcohol intake (zero intake, above/below the sex-specific median of self-reported grams of alcohol/day), dietary fiber intake (above/below sex and age-specific (10-year groups)) median of self-reported intake in grams/2,000 kcal), recreational- and occupational physical activity (self-reported on a scale from 1 to 5, ranging between 1 for sedentary and 5 for highly active),18 and kidney dysfunction/inflammation score. Model 3 was additionally adjusted for MTHFR rs1801133 genotype.
For plasma biomarkers, participants with missing data for a variable were excluded from analysis (betaine: n = 8; cobalamin: n = 27; creatinine: n = 8; PLP: n = 9; riboflavin: n = 9; SDMA: n = 8; B-vitamin score: n = 38 and kidney dysfunction/inflammation score: n = 13). Missing values for potential confounders were assigned to a separate category in the multivariable analyses.
The interaction between MTHFR rs1801133 genotype and biomarkers were tested using a likelihood ratio test comparing the interaction model to a model with a main effects model without interaction term. Heterogeneity of the risk associations by tumor subgroups was tested with likelihood ratio tests, comparing a model in which the risk association could vary across subgroups to a model in which all associations were held constant.42
Results
Baseline characteristics
The study cohort consisted of 41% males with a median age of approximately 60 years, most of whom were from the VIP cohort (78%) (Table 1). About 20% of both cases and controls were current smokers, whereas the frequency of former smokers was slightly higher in cases (p = 0.01). Baseline data for BMI, physical activity (occupational and recreational), alcohol intake and dietary fibre intake were comparable for cases and controls. Of the B-vitamins, plasma concentrations of cobalamin, betaine and riboflavin were significantly lower in cases than in controls, and, consequently, the total B-vitamin score was also significantly lower in cases than in controls. Plasma concentrations of the inflammatory marker neopterin were significantly higher in CRC cases than in controls. Of the ratio-based B-vitamin markers, Hcy:Cys was significantly higher in CRC cases than in controls. The median age at diagnosis for CRC cases was 65.2 years (5th–95th percentile: 50.3–76.2 years), and the median follow-up time was 8.2 years from sampling to diagnosis. The CRC cases were approximately equally distributed with respect to tumor location in the right colon (30%), left colon (35%) and rectum (35%), as well as tumor stage (53% stages I–II, 47% stages III–IV).
| Baseline characteristics | Cases (n = 613) | Controls (n = 1,190) | pb |
|---|---|---|---|
| VIP cohort, n (%) | 479 (78) | 931 (78) | – |
| Male sex, n (%) | 253 (41) | 487 (41) | – |
| Age, years | 59.8 (50.1–60.1) | 59.7 (50.1–60.1) | – |
| Fasting ≥8 hr, n (%) | 365 (60) | 716 (60) | – |
| BMI, kg/m2 | 25.7 (23.5–28.2) | 25.6 (23.2–28.1) | 0.43 |
| Smoking status, n (%) | |||
| Current smoker | 114 (19) | 239 (20) | 0.01 |
| Former smoker | 136 (22) | 197 (17) | |
| No recreational physical activity, n (%)c | 211 (45) | 354 (40) | 0.39 |
| Sedentary or standing work, n (%)c | 90 (22) | 163 (20) | 0.21 |
| Alcohol intake, g/dayc, d | 2.4 (0.2–5.8) | 2.3 (0.3–5.7) | 0.94 |
| Dietary fiber intake, g/2,000 kcalc, d | 22.4 (18.5–26.7) | 22.7 (18.5–26.3) | 0.93 |
| eGFR, mL/min/1.73m2 | 61.9 (52.3–73.0) | 61.7 (50.9–72.3) | 0.48 |
| SDMA, μmol/L | 0.65 (0.55–0.75) | 0.64 (0.56–0.75) | 0.73 |
| Creatinine, μmol/L | 65.1 (57.2–73.5) | 64.9 (57.4–72.9) | 0.78 |
| tHcy, μmol/L | 10.1 (8.4–11.9) | 9.9 (8.2–11.7) | 0.15 |
| Cysteine, μmol/L | 275 (253–299) | 276 (255–298) | 0.74 |
| B-vitamins | |||
| Folate, nmol/L | 7.25 (4.89–10.37) | 7.15 (4.60–10.24) | 0.49 |
| Cobalamin, pmol/L | 413 (337–498) | 426 (353–510) | 0.02 |
| Betaine, μmol/L | 29.9 (25.7–34.4) | 30.8 (26.1–35.5) | 0.05 |
| Riboflavin, nmol/L | 10.8 (7.4–16.0) | 11.8 (7.9–17.9) | 0.002 |
| PLP, nmol/L | 35.9 (25.9–51.5) | 38.2 (28.0–51.5) | 0.08 |
| B-vitamin score | −0.48 (−1.95 to 1.54) | −0.00 (−1.73 to 1.69) | 0.01 |
| Ratio-based B-vitamin markers | |||
| Hcy:Cys (×100) | 3.65 (3.15–4.23) | 3.57 (3.05–4.17) | 0.05 |
| Hcy:Cre (×100) | 15.3 (12.9–18.4) | 15.1 (12.6–18.6) | 0.29 |
| Hcy:Cys:Cre (×10,000) | 5.59 (4.75–6.64) | 5.47 (4.66–6.61) | 0.25 |
| Inflammatory markers | |||
| KTR (×1,000) | 22.5 (19.4–26.0) | 22.1 (19.3–25.7) | 0.23 |
| Neopterin, nmol/L | 9.69 (8.21–11.90) | 9.36 (7.95–11.36) | 0.01 |
| MTHFR rs1801133 genotype, n (%) | |||
| CC | 326 (53) | 580 (49) | 0.23 |
| CT | 232 (38) | 489 (41) | |
| TT | 53 (9) | 112 (9) | |
| Case characteristics | |||
| Age at diagnosis, years | 65.2 (59.3–70.2) | – | |
| Follow-up time, years | 8.2 (4.7–11.9) | – | |
| Tumor site, n (%) | |||
| Right colon | 183 (30) | – | |
| Left colon | 215 (35) | ||
| Rectum | 214 (35) | ||
| Tumor stage, n (%) | |||
| I–II | 308 (53) | – | |
| III–IV | 276 (47) |
- Abbreviations: VIP: Västerbotten intervention programme; BMI: body mass index; eGFR: estimated glomerular filtration rate; SDMA: symmetric dimethylarginine; tHcy: total homocysteine; PLP: pyridoxal 5′-phosphate; KTR: kynurenine-to-tryptophan ratio (given as kynurenine (nmol/L) divided by tryptophan (μmol/L)); MTHFR: methylenetetrahydrofolate reductase.
- a Plasma biomarker levels are described in medians (25th–75th percentile).
- b From test for difference in variable distribution between cases and controls. Mann–Whitney U-tests for continuous variables, χ2 tests for categorical variables. Not conducted for case–control matching variables.
- c Variables only available in the VIP cohort.
- d Estimated from self-administered, semi-quantitative food frequency questionnaires (FFQs) designed to measure intakes from the previous year in mass/day.
Associations between B-vitamin markers and B-vitamin score
The predictors (sex, age, cohort, fasting status, smoking, BMI, KTR, neopterin, SDMA and total B-vitamin score) explained about one-third of the variance of tHcy and the ratio-based B-vitamin markers in both cases and controls (Fig. 1). Although contributions to explained variance were slightly higher in cases for some variables (total B-vitamin score and neopterin), there were overall no large differences in between cases and controls. As expected, total B-vitamin score had the strongest association with tHcy and the ratio-based B-vitamin markers (inverse association). Regression-based sensitivity (defined as variance explained by total B-vitamin score) and specificity (defined as variance explained by B-vitamin score divided by total variance explained by model) were 16.6 and 55.9% for tHcy, 23.5 and 72.7% for Hcy:Cys, 21.5 and 73.7% for Hcy:Cre and 27.0 and 83.3% for Hcy:Cys:Cre, respectively, in controls and similar in cases (Table 2). Thus, overall performance (sensitivity multiplied by specificity) was better for the ratio-based B-vitamin markers compared to tHcy (performances: 9.3, 17.1, 15.8 and 22.5 for tHcy, Hcy:Cys, Hcy:Cre and Hcy:Cys:Cre, respectively, in controls). In models with separate B-vitamins as predictors, folate was the strongest contributor for all ratio-based B-vitamin markers (explaining 10–15% of the variance for tHcy and the ratio-based B-vitamin markers), followed by cobalamin, betaine, PLP and riboflavin (each explaining 1–10% of variance) in controls. No other predictors explained >5% of the total variation for tHcy or the ratio-based B-vitamin markers, including markers of kidney function or inflammation.
| tHcy | Hcy:Cys | Hcy:Cre | Hcy:Cys:Cre | |||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| Sensitivity, %a | 16.7 | 16.6 | 26 | 23.5 | 23.1 | 21.5 | 31.6 | 27.0 |
| Specificity, %b | 55.6 | 55.9 | 75.1 | 72.7 | 76.1 | 73.7 | 89.4 | 83.3 |
| Performancec | 9.3 | 9.3 | 19.5 | 17.1 | 17.6 | 15.8 | 28.2 | 22.5 |
| Performance ratio | 1 (ref) | 1 (ref) | 2.1 | 1.8 | 1.9 | 1.7 | 3.0 | 2.4 |
- a Defined as variance explained by B-vitamin score.
- b Defined as variance explained by B-vitamin score divided by total variance explained by the model.
- c Sensitivity multiplied by specificity divided by 100.
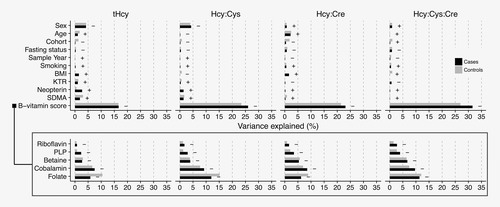
Regression-based sensitivity, specificity and performance were higher for both tHcy and the ratio-based B-vitamin markers in participants with the homozygous variant MTHFR rs1801133 (TT) genotype (Supporting Information Fig. S2, Table S1). However, differences by genotype were smaller for the ratios, in particular, ratios with Hcy:Cys and Hcy:Cys:Cre, for which performance was above 15 for all genotypes.
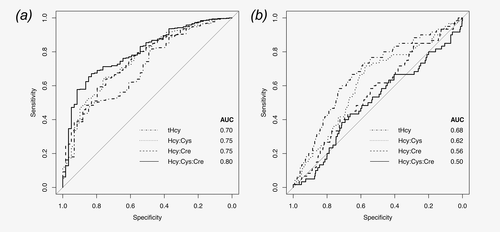
In the ROC analysis, results consistent with the regression-based analyses were observed (Fig. 2). The ratio-based B-vitamin markers, of which Hcy:Cys:Cre performed best, were better than tHcy at predicting low total B-vitamin score (AUC 0.75–0.80 for B-vitamin markers, 0.70 for tHcy in controls). AUCs were slightly higher in cases, as in the linear regression analyses (data not shown). The ratio-based B-vitamin markers were weaker predictors of kidney dysfunction/inflammation (AUCs 0.50–0.62) than tHcy (AUC 0.68), again confirming the specificity of the ratios as markers of total B-vitamin status observed in the regression-based analyses.
Associations between B-vitamin markers and CRC risk
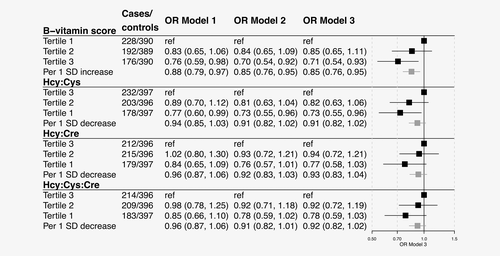
ORs for CRC risk by tertiles (categorical) and per standard deviation (SD, continuous) increase in B-vitamin score, and decrease in Hcy:Cys, Hcy:Cre and Hcy:Cys:Cre, are shown in Figure 3. Higher B-vitamin status was associated with a reduced risk of CRC, independently of the observed potential confounders. The negative association for total B-vitamin score was not weakened by the removal of folate from the B-vitamin score calculation (Supporting Information Fig. S3). Associations for ratio-based B-vitamin markers were similar to those for the B-vitamin score. None of the ratio-based marker ORs differed significantly from each other in z-tests based on the estimated standardized regression coefficients and covariance matrices (all p > 0.25). tHcy, cysteine or creatinine included as separate variables were not associated with CRC risk, regardless of whether the variables were mutually adjusted for each other or not (ORs: 0.85–0.90, all p > 0.30, data not shown). We observed no large differences with respect to follow-up time between sampling and diagnosis (pheterogeneity = 0.15–0.76, data not shown).
Next, we evaluated interactions between MTHFR rs1801133 genotype and the total B-vitamin score and the ratio-based B-vitamin markers. Homozygous or heterozygous variant genotypes are weakly associated with a reduced CRC risk in this cohort, as previously reported.19 The group with common homozygous rs1801133 genotype in combination with low B-vitamin status as estimated by total B-vitamin score or the ratio-based markers, had the highest CRC risk in all interaction models. However, there was no multiplicative increase in CRC risk in participants with both homozygous or heterozygous variant rs1801133 genotypes and high B-vitamin status (pinteraction = 0.16 to 0.76, Supporting Information Fig. S4).
Discussion
We evaluated ratios of metabolites (Hcy:Cys, Hcy:Cre and Hcy:Cys:Cre) as functional ratio-based markers of total B-vitamin status in a nested case–control study of 613 CRC cases and 1,190 matched control participants from the population-based cohorts of the NSHDS. Compared to tHcy, the three ratio-based B-vitamin markers explained a greater portion of the total B-vitamin score in both case and control participants. Furthermore, similar to the total B-vitamin status, B-vitamin status as estimated by the ratio-based B-vitamin markers was also inversely associated with CRC risk.
Our investigation validates the performance of the ratio-based B-vitamin markers previously observed in two Norwegian cohorts of patients with suspected stable angina pectoris.15 Now, for the first time, we demonstrate the utility of these ratio-based B-vitamin markers in a general population-based sample. Nutritionally, the main difference in metabolite concentrations between the Norwegian cohorts and the NSHDS is the lower median folate concentrations in the NSHDS. Folate and cobalamin are both important determinants of tHcy concentrations, especially at lower total concentrations of B-vitamins.8, 43
Ratio-based markers minimize influences from shared determinants (e.g., kidney dysfunction being associated with both plasma concentrations of tHcy and creatinine), and the ratio-based B-vitamin markers were not influenced by common confounders such as kidney function, smoking and inflammation. Moreover, the ratio-based B-vitamin markers performed equally well in healthy controls and in participants who later developed CRC. Hcy:Cys:Cre had the highest sensitivity and specificity as a functional marker of total B-vitamin status. However, both Hcy:Cys and Hcy:Cre performed significantly better than tHcy alone, and both plasma concentrations of tHcy and creatinine are simple and cost-efficient markers already routinely analyzed in clinical practice. The metabolite ratio of urine albumin:creatinine is recommended in clinical and laboratory guidelines for the definition of chronic kidney disease, and has been observed in population studies to accurately predict cardiovascular risks.44 Similarly, Hcy:Cre would be easy to implement in clinical practice as a routine output in clinical chemistry reports to aid interpretation of test results, and could provide additional information in investigations of one-carbon metabolism in disease risk. Hcy:Cys and Hcy:Cys:Cre also show promise as biomarkers of total B-vitamin status, but the necessary component cysteine is not commonly analyzed in clinical practice.
In our study, the functional ratio-based markers give new important insights into the relationship between one-carbon metabolites and CRC risk. Low circulating folate levels are associated with lower CRC risk in our cohort,24, 28 as well as in a previous study of a population with similar low folate status.27 In our study, folate is the main factor explaining variation in the ratio-based B-vitamin markers, yet higher B-vitamin status estimated with the ratio-based B-vitamin markers or the total B-vitamin score (with or without folate) demonstrated linear inverse associations with CRC risk. This indicates that the higher risk associated with higher folate in a population with low folate status could be attenuated by an adequate supply of other one-carbon metabolites. Cobalamin appears to be almost as important as folate in explaining variations in the ratio-based B-vitamin markers, and it has been suggested that vitamin B-12 should accompany folic acid in mandatory fortification programs.45
Strengths of our study include high-quality prediagnostic blood samples with respect to the fasting status and sample collection, handling and storage (low-preanalytical variability). The NSHDS population is characterized by a general low folate status,46-48 giving us the opportunity to study one-carbon metabolism in a population with a high portion of total folate intake derived from naturally occurring folates. However, results based on the NSHDS, may not be generalized to other cohorts and populations subject to folic acid fortification. We used a total B-vitamin score comprising of standardized concentrations of betaine, riboflavin, PLP, folate and cobalamin, whereas a B-vitamin score without betaine has previously been used to estimate general B-vitamin status.48 Betaine was included in the score in the present work because it is an important methyl donor associated with tHcy concentrations, particularly in individuals with the variant MTHFR rs1801133 genotype and/or poor folate status.49 A weakness in the ROC analysis was the definition of low total B-vitamin status as the lowest fifth percentile of the total B-vitamin score distribution. Participants in this group may not have a clinically significant B-vitamin deficiency, potentially leading to an underestimation of the AUC in our study.50
In conclusion, the ratio-based B-vitamin markers, Hcy:Cys, Hcy:Cre and Hcy:Cys:Cre, all outperform tHcy as markers of total B-vitamin status. tHcy and creatinine are simple and cost-efficient markers routinely analyzed in clinical practice. Hcy:Cre could therefore easily be implemented in routine clinical chemistry output. B-vitamin status, estimated with either total B-vitamin status or the ratio-based B-vitamin markers, was inversely linearly associated with CRC risk. Ratio-based B-vitamin markers may thus be an efficient means of assessing overall B-vitamin status in investigations of disease risk.
Acknowledgements
We thank the participants of the NSHDS as well as the staff at the Department of Biobank Research.




