Tumor eradication by immunotherapy with biodegradable PLGA microspheres—an alternative to incomplete Freund's adjuvant†
Conflict of interest: None
Abstract
In experimental tumor immunotherapy, incomplete Freund's adjuvant (IFA) has been considered as the “gold standard” for T-cell vaccination in mice and humans in spite of its considerable adverse effects. Recently, we succeeded in eliciting strong CTL responses in mice after vaccination with biodegradable poly(D,L-lactide-co-glycolide) (PLGA) microspheres (MS). In our study, we compared the immune response to IFA and PLGA-MS containing ovalbumin (OVA) and CpG-oligodeoxynucleotide (MS-OVA/CpG) or we used a mixture of MS-OVA/CpG and MS-polyI:C. A single vaccination with MS-OVA/CpG elicited long-lasting titers of IgG1 and IgG2a, but only low IgE titers, and also the T-cell response was biased toward Th1 differentiation. Antigen presentation to CD4+ and CD8+ cells and activation of a cytotoxic T-cell response in mice vaccinated with PLGA-MS and IFA lasted for over 3 weeks. Preconditioning of the injection site with TNF-α and heterologous prime-boost regimen further enhanced the cytotoxic response. PLGA-MS were as efficient or superior to IFA in eradication of preexisting tumors and suppression of lung metastases. Taken together, PLGA-MS are well-defined, biodegradable and clinically compatible antigen carrier systems that compare favorably with IFA in their efficacy of tumor immunotherapy in mouse models and hence deserve to be tested for their effectiveness against human malignant diseases.
The recent identifications of tumor antigens in numerous types of cancers and of ligands of pattern-recognition receptors are important prerequisites to elicit strong CTL responses against tumor cells by immunotherapy. Pattern molecules should be directed to their cognate receptors localized either on the cell surface, in the endosomal membrane or in the cytoplasm. Strong adjuvants like cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG) or polyriboinosinic:polyribocitidylic acid (polyI:C) need to enter the endosome to stimulate their cognate toll-like receptors (TLR) TLR9 and TLR3, respectively. It is also the endosome where antigens have to be delivered for cross presentation on MHC class I molecules and direct presentation on MHC class II molecules.1 To achieve an effective T-cell stimulation, it is beneficial if antigen and adjuvant reach the same endosome,2, 3 which makes their joint uptake highly desirable.
The progress in the development of antigen delivery systems that achieve this aim is lagging behind, thereby hampering a successful introduction of immunotherapy into clinical practice. For numerous clinical trials, antigens and pattern molecules are incorporated into a formulation, which has been introduced over 60 years ago, i.e., incomplete Freund's adjuvant (IFA).4 IFA is a water-in-oil emulsion, which can be mixed with antigens and pattern molecules. Although IFA/antigen emulsions elicit long-lasting IgG responses, they also stimulate the activation of CTL and T-helper cells.5, 6 In spite of such desired properties, IFA is not approved for routine immunotherapy in humans, but is merely used in investigational clinical trials. Recent studies described severe local skin reactions, sterile abscesses and cysts, inflammations and persistent painful granulomas at the injection site, when using IFA or a formulation similar to IFA (Montanide ISA-51).7 Moreover, oil-induced neoplasms have been observed.8 The need to develop potent and well-defined antigen delivery systems is therefore self-evident.
We and other groups have investigated microspheres (MS) consisting of poly(D,L-lactide-co-glycolide) (PLGA) as antigen delivery system targeting dendritic cells (DCs) and macrophages in vitro and in vivo.9-13 PLGA is approved for use in humans, e.g., for delivering drugs and as material for biodegradable surgical sutures.14 PLGA can be used to microencapsulate peptides, proteins, RNA or DNA by several procedures, such as spray drying, which produces MS of a size of 1–5 μm.15 Such size range is ideal for the phagocytic uptake by DC in vitro.16 PLGA hydrolyses in aqueous environment during several weeks thereby releasing slowly microencapsulated substances. Uptake of PLGA-MS by human monocyte-derived DC in vitro does not negatively affect their survival, migration, cytokine release or T-cell stimulation.16 After being ingested by DC, PLGA-MS release microencapsulated antigens intracellularly for processing and presentation on MHC class I and II molecules.17 Especially, cross presentation may be at least a 100-fold more sensitive in vitro if the antigen is taken up in association with PLGA-MS when compared to a soluble form.
On the basis of these in vitro properties, we and others have investigated the potency of PLGA-MS for T-cell stimulation in the mouse model.18-23 With a single injection of MS-OVA/CpG, OVA-specific CTL responses mounting up to 8% of CD8+ cells could be obtained; the CTL responses mediated lysis of target cells in vivo and in vitro, production of IFN-γ and protection of mice from infection with vaccinia virus.18 In our study, we tested the antitumor response achievable with PLGA-MS and compared it systematically with an IFA formulation. We found that PLGA-MS-based immunotherapy elicited long-lasting antibody titers, cytolytic and T-helper cell responses and protected mice from growth of melanoma and thymoma in a protective and therapeutic setting. The efficacy was equivalent or even better than that achieved with IFA, which underlines the potential of PLGA-MS for immunotherapy of cancer.
Abbreviations
CpG: cytosine-phosphorothioate-guanine oligodeoxynucleotides; DC: dendritic cell; IFA: incomplete Freund's adjuvant; MS: microspheres; OVA: ovalbumin; PBS: phosphate-buffered saline; PLGA: poly(D,L-lactide-co-glycolide); polyI:C: polyriboinosinic:polyribocitidylic acid; TLR: toll-like receptors
Material and Methods
Preparation of microspheres
Microspheres (MS) were prepared from 14-kDa PLGA 50:50 (Resomer RG502H, Boehringer Ingelheim, Ingelheim, Germany). The antigens and TLR ligands were microencapsulated by spray drying as described elsewhere.15 Briefly, 50 mg ovalbumin (OVA, Grade V, Sigma) and 5 mg CpG oligodeoxynucleotides with a phosphothioate backbone (CpG-ODN 1826, Microsynth, Balgach, Switzerland) or 0.5 mg polyI:C (Calbiochem, VWR, Dietikon, Switzerland) (MS polyI:C) were dissolved in 0.5 ml 0.1 M NaHCO3 (aqueous phase) and mixed with 1 g of PLGA dissolved in 20 ml of dichloromethane (organic phase). The two phases were emulsified by ultrasonication (Hielscher, UP200 H, Ampl. 40%) for 10 sec on ice. The obtained w/o dispersion was immediately spray dried (Büchi, Mini Spray-Dryer 191, Büchi, Flawil, Switzerland) at a flow rate of 2 ml/min and inlet/outlet temperatures of 40°C/37°C. The obtained MS were within a size range of 0.5–5 μm for all batches; they were washed out of the spray-dryer's cyclone with 0.05% poloxamer 188 (Synperonic®F68, Serva Electrophoresis, Heidelberg, Germany), collected on a cellulose acetate membrane filter and dried under reduced pressure (20 mbar) for 18 hr at room temperature. The encapsulation efficacy of OVA in MS-OVA/CpG was determined to be 47% ± 2% according to a previously described protocol.18 The release of polyI:C from MS-polyI:C was determined to be 37.8% ± 6.6%. PLGA-MS were stored under desiccation at 4°C. Immediately before use, MS were dispersed in PBS by ultrasonication for 30 sec.
Mice and immunizations
OT-I mice were obtained from Dr. Ying Waeckerle-Men (University of Zurich, Switzerland). OT-II/Thy1.1 mice were obtained from Prof. Thomas Brocker (LMU Munich, Germany). C57BL/6 mice (H-2b) were purchased from Charles River Laboratories. All mice were kept in a specific pathogen-free facility and used at 6–10 weeks of age. Animal experiments were approved by Regierungspräsidium Freiburg. For Figures 4-6, mice were immunized s.c. at the base of the tail [which yielded superior CTL responses compared to i.p. immunization as previously determined (data not shown)] either with a mixture of 5 mg PLGA-MS loaded with OVA (250 μg) and CpG-ODN (25 μg) and 5 mg MS-polyI:C (2.5 μg) or the corresponding amounts of OVA, CpG-ODN and polyI:C in PBS:IFA (1:1) (IFA). This dose was previously shown to yield optimal CTL responses.18 For Figures 1-3, mice were only immunized with MS-OVA/CpG as outlined above. Control mice were either treated with empty MS (empty) or left untreated (naïve). All injections were performed at a single site with a total volume of 200 μl. The recombinant vaccinia virus expressing OVA24 was obtained from Dr. J. Yewdell (NIAID, Bethesda, MD) and propagated as previously described24; it was injected i.p. at a dose of 2 × 106 p.f.u.
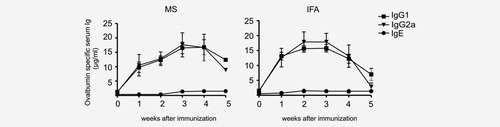
Serum levels of ovalbumin-specific Ig after a single injection of either MS-OVA/CpG or OVA and CpG in IFA. C57BL/6 mice (n = 6) were immunized with 5 mg of MS-OVA/CpG containing ovalbumin (250 μg) and CpG oligonucleotide (25 μg) or the same amounts of OVA and CpG in IFA. The sera were analyzed for ovalbumin-specific IgG1 (squares), IgG2a (triangles) or IgE (dots) at indicated time points. The values represent the means ± SEM of ovalbumin-specific Ig in μg/ml from two independent experiments.
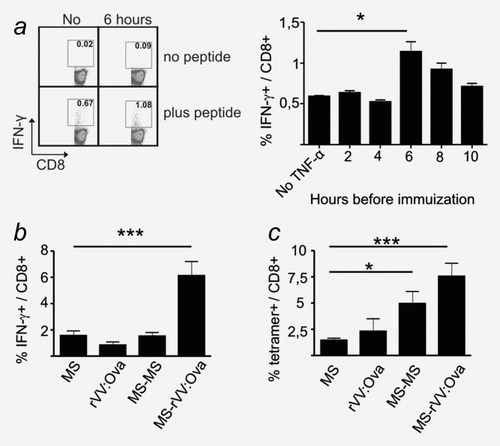
Effects of pretreatment of the injection site and prime-boost vaccination on CTL responses. (a) C57BL/6 mice (n = 3) were pretreated with 200 ng TNF-α at the site of injection at 2, 4, 6, 8 or 10 hr before immunization with 5 mg of MS-OVA/CpG containing ovalbumin (50 μg/mg MS) and CpG-ODN (5 μg/mg MS). Control animals were left untreated (No). Six days after immunization, splenocytes were analyzed by IFN-γ-ICS. Two representative flow cytometry dot plots (left panels) and mean values (right panel) from one out of three experiments with similar outcomes are shown. Values (mean ± SEM) are given in percent IFN-γ+ cells of CD8+ cells. Background signals (no peptide) were subtracted. The p value was calculated by a Student's t-test (p = 0.0317). (b, c) Effects of booster immunizations: C57BL/6 mice (n = 2) received single injections of MS-OVA/CpG (MS) or recombinant vaccinia virus expressing ovalbumin (rVV:OVA) or additional booster immunizations after 4 weeks (MS-MS or MS-rVV:OVA). Six days after the last immunization, splenocytes were analyzed by ICS (b) or SIINFEKL/H-2Kb-tetramer staining (c). Results are from one out of three experiments with similar outcomes. Values (mean ± SEM) for (b) are given in percent IFN-γ+ of CD8+ lymphocytes. Background signals (no peptide) were subtracted. The p value was calculated by a Student's t-test (p = 0.0001). Values (mean ± SEM) in (c) are given in percent SIINFEKL/H-2Kb-tetramer+ cells of CD8+ lymphocytes. The background (naïve mouse) was subtracted. The p values were calculated by a Student's t-test (*p = 0.0153; ***p = 0.0002).
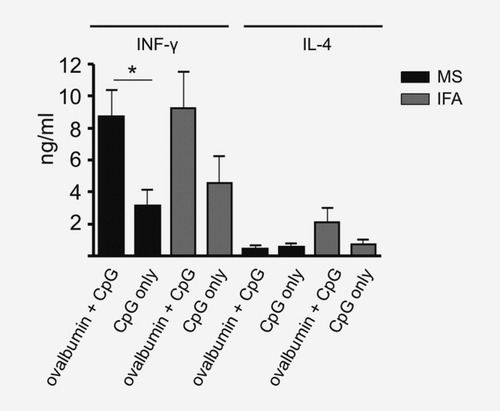
Production of IFN-γ and IL-4 by CD4+ cells after immunization with MS-OVA/CpG or OVA and CpG in IFA. C57BL/6 mice (n = 10) were immunized with 5 mg MS containing 250 μg ovalbumin and 25 μg CpG oligonucleotides (black bars) or the corresponding amounts of ovalbumin and CpG-ODN in IFA (gray bars). Control groups were immunized with corresponding amounts of encapsulated CpG alone or CpG alone in IFA. After 6 days, splenocytes were isolated and magnetically sorted for CD4+ cells. 5 × 105 CD4+ cells were restimulated by plate-bound anti-CD3 and anti-CD28 Ig for 18 hr. Supernatants were analyzed for IL-4 and IFN-γ by ELISA. Background levels of naive mice were subtracted. Values are given in ng/ml (mean ± SEM). Results are a summary of two independent experiments with similar outcomes.
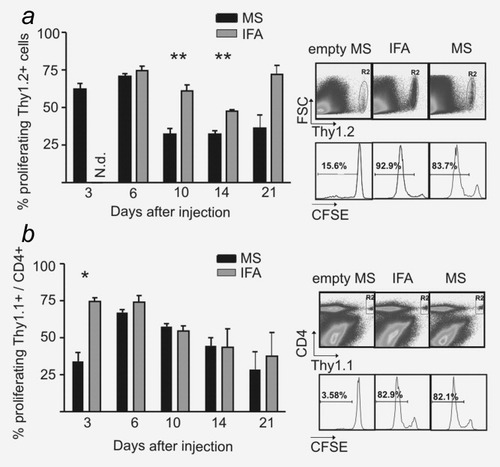
Time course of antigen presentation after immunization with MS and IFA in vivo. (a) Thy1.1 mice (n = 3) were immunized with a mixture of MS-OVA/CpG and MS-polyI:C (black bars) or the corresponding amounts of ovalbumin (250 μg), CpG-ODN (25 μg) and polyI:C (2.5 μg) in IFA (gray bars). Three days before mice were sacrificed, 1 × 107 sorted CD8+ and CFSE-labeled splenocytes from OT-I/Thy1.2 mice were injected i.v. into Thy1.1 mice. Then, Thy1.2+ splenocytes were analyzed for CFSE fluorescence. Representative results from one out of two experiments with similar outcomes are shown (left panel). Values (mean ± SEM) are given in percent proliferating Thy1.2+ cells. Background values (PBS-treated mice) were subtracted. The p values were calculated by a Student's t-test (Day 10: p = 0.008; Day 14: 0.009). The right panels show representative flow cytometry dot plots for gating of Thy1.2+ cells and histograms for CFSE staining of gated cells from three mice. Panels in (b) show evidence for the potential to stimulate proliferation of CD4+ cells. C57BL/6 mice (n = 2) were immunized as outlined above. Three days before mice were sacrificed, 2 × 106 CFSE-labeled splenocytes from OT-II/Thy1.1 mice were injected i.v. Then, Thy1.1+CD4+ splenocytes were analyzed for CFSE fluorescence. Representative results from one out of two experiments with similar outcomes are shown (left panel). Values (mean ± SEM) are given in percent proliferating Thy1.1+CD4+ cells. The p value was calculated by a Student's t-test (p = 0.019). The gating of Thy1.1+CD4+ cells in dot plots and representative histograms for CFSE staining of gated cells is shown on the right.
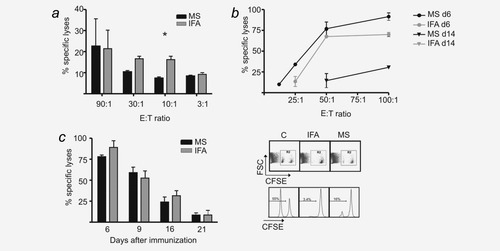
CTL-mediated cytotoxicity elicited by vaccination with PLGA-MS and IFA. (a) Cytotoxicity as determined by 51-chromium release assay. C57BL/6 mice (n = 2) were immunized with a mixture of MS-OVA/CpG and MS-polyI:C (black bars) or the corresponding amounts of ovalbumin (250 μg), CpG-ODN (25 μg) and polyI:C (2.5 μg) in IFA (gray bars). On Day 6, splenocytes were coincubated for 4 hr with 51-chromium-labeled EL-4 or Eg-7 cells expressing ovalbumin. Values (mean ± SEM) are given in percent-specific lysis. Data from one out of two experiments with similar outcomes are shown. The p value for the E:T ratio of 10:1 was calculated by a Student's t-test (p = 0.039). (b) Cytotoxicity as determined by BaTDA-cytotoxicity assay described in the Material and Methods section. C57BL/6 mice (n = 2) were immunized with a MS mixture (black lines) or IFA (gray lines), as outlined above. Spleens were removed after 6 days (circles) or 14 days (triangles) and coincubated with BaTDA-labeled TrampC2 cells that express ovalbumin (Vf10 cells). Values (mean ± SEM) are given in percent-specific lysis with indicated effector-to-target ratios. Data from one out of three experiments with similar outcomes are shown. No lysis was found 14 days after vaccination with IFA. (c) In vivo cytotoxicity assay. C57BL/6 mice (n = 3) were immunized as described under (a). In vivo cytotoxicity was analyzed on indicated days, and representative dot plots and histograms of gated CFSE+ cells are shown (right). Representative data (mean ± SEM) from one out of three experiments with similar outcomes are shown (left).
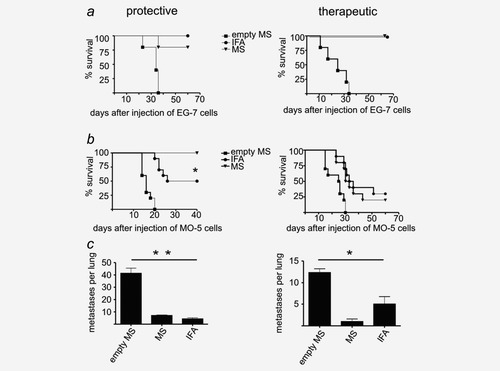
Antitumor responses elicited with PLGA-MS and IFA against EG-7 thymoma and MO-5 melanoma. (a, b) Left panels show the results from a protective setting. C57BL/6 mice (n = 5) were immunized with a mixture of MS-OVA/CpG and MS-polyI:C (triangles) or IFA containing ovalbumin, CpG oligo and polyI:C in equivalent amounts (dots) or empty PLGA-MS (squares). After 6 days, mice were challenged s.c. with 5 × 105 EG-7 or MO-5 cells. Tumor sizes were measured daily until they reached a mean size of 15 mm. (a, b) Right panels show the results from a therapeutic setting. C57BL/6 mice (n = 10) were challenged s.c. with 5 × 105 EG-7 or MO-5 cells. When palpable tumors were established, mice were immunized with a mixture of MS (triangles), IFA (dots) or empty PLGA-MS (squares), as outlined above. The p value was calculated by a survival curve analysis (p = 0.0496). (c) Left panel: metastatic tumor challenge in the protective setting. C57BL/6 mice (n = 3) were immunized as outlined above. After 6 days, mice were challenged i.v. with 5 × 104 melanin-expressing MO-5 cells. After 2 weeks, mice were sacrificed and lungs removed. Visible metastases were counted and are given as means ± SEM. (c) Right panel: metastatic tumor challenge in the therapeutic setting. C57BL/6 mice (n = 3) were challenged i.v. with 2 × 104 MO-5 cells. After 1 week, mice were immunized as described above. After 14 days, mice were sacrificed and lungs removed. Metastases numbers are given as means ± SEM. All graphs show one representative experiment out of three with similar outcomes. The p values were calculated by a one-way analysis of variance for the protective setting (p = 0.0017) and for the therapeutic setting (p = 0.03).
Statistical analyses
For statistical analyses, groups from similar experiments were pooled and analyzed for significant differences as indicated in the graph. The p values for experiment composites are given in the figure legends.
Tumor cell lines and inoculation of mice
EL-4 thymoma cells25 and the OVA-expressing transfectant EG-726 were kindly provided by Dr. Wolfram Osen (DKFZ Heidelberg) and kept in RPMI medium. The OVA transfectant of B16F10 melanoma, MO-5, was kindly provided by Dr. Antje Heit19 (LMU Munich) and kept in DMEM medium. All media contained GlutaMAX, 10% fetal calf serum (FCS) and 100 U/ml of penicillin/streptomycin (P/S). To produce the clone Vf10, TrampC2 cells27 were transfected with a plasmid encoding for full-length, cytosolic OVA. Vf10 cells were kept in DMEM (-pyruvate) and complemented with 5% FCS, 5% Nu-serum, 5 μg/ml of insulin, 10−8 M dihydrotestosterone and 1% P/S (5 ml). To maintain the expression of OVA in EG-7, MO-5 and Vf10 transfectants, the media were supplemented with G418 (0.2 mg/ml). For the inoculation of mice, a number of 5 × 105 EG-7 or MO-5 cells, which we titrated to yield optimal tumor growth, were injected subcutaneously into the right flank. Mice were either treated 6 days earlier (protective setting) or as soon as palpable tumor occurred (therapeutic setting). For lung metastasis in the protective setting, 5 × 104 melanin-expressing MO-5 cells were injected into the tail vein at Day 6 after treatment. After 14 days, mice were sacrificed, lungs were removed and visible metastases were counted. Therapeutic treatment of lung metastases started 1 week after inoculation with 2 × 104 melanin-expressing MO-5 cells via the tail vein, and mice were sacrificed after 2 additional weeks.
Intracellular cytokine staining and MHC tetramer staining
Splenocytes were isolated and incubated with or without 10 μM SIINFEKL peptide (Eurogentec, Cologne) in the presence of brefeldin A (10 μg/ml, Sigma-Aldrich) for 5 hr at 37°C. After washing, cells were stained with PE-Cy5-conjugated anti-mouse CD8α antibody (BD Biosciences Pharmingen, Clone 53-6.7) for 20 min at 4°C. The cells were washed and fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. After washing the cells twice with PBS, cells were labeled intracellularly with FITC-conjugated rat anti-IFN-γ antibody (clone XGM1.2, diluted in PBS/0.1% saponin) at 4°C overnight. The next day, cells were washed and resuspended in PBS for flow cytometry. Background levels of each sample (without peptide) were subtracted. The H-2Kb/SIINFEKL tetramers used in our study were kindly provided by Prof. Dirk Busch (TU Munich). For MHC tetramer staining, splenocytes were stained with PE-labeled SIINFEKL/H-2Kb-tetramer for 20 min at 37°C and subsequently with PE-Cy5-conjugated rat anti-CD8 IgG for 20 min at 4°C. Cells were washed twice and measured by flow cytometry.
In vivo cytotoxicity assay
CTL activity in vivo was assessed exactly as described elsewhere.28 The percentage of specific cytolysis was calculated as follows: 100 − [(% peptide pulsed cells in vaccinated mice/% unpulsed cells in vaccinated mice)/(% peptide pulsed cells in control mice/% unpulsed cells in control mice)] × 100.
Ex vivo cytotoxicity assays
For the classical chromium release assay (Fig. 5a), C57BL/6 mice were immunized either by a mixture of PLGA-MS or the corresponding components in IFA. Six days later, splenocytes were used as effectors in a primary chromium release assay as previously described.29 EL-4 (H-2b) (negative control) or EG-7 cells served as targets. For measurement of ex vivo cytolytic activity of splenocytes from vaccinated C57BL/6 mice in Figure 5b, a time-resolved fluorometric assay based on the DELFIA® EuTDA reagent (PerkinElmer) was used.30 Briefly, 5 × 104 Vf10 cells served as target cells and were labeled with BaTDA (PerkinElmer). After 4 hr of coincubation, supernatants were mixed with europium solution for 1 hr and measured at ex/em wavelengths of 340/615 nm and a lag time of 200 nsec. Specific cytolysis was calculated for both assays as follows: [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100%.
ELISA
For detection of OVA-specific immunoglobulin (Ig) in plasma, blood samples were collected at indicated time points after immunization using a heparin-coated microvette (Sarstedt, Nümbrecht, Germany) and spun down for 5 min at 1,000g. Ninety-six-well ELISA plates were coated with 0.1 mg/ml OVA in PBS overnight at 4°C. The next day, plates were washed three times with PBS-Tween 20 (0.05%), and serum samples were applied for 2 hr at room temperature. For detection of mouse Ig isotypes, the mouse-Ig-isotyping-kit (BD Bioscience) was used. For detection of IL-4 and IFN-γ, the respective kit (mouse IFN-γ/IL-4 ELISA set, BD Bioscience) was used, following the manufacturer's instruction.
Proliferation assay
Proliferation of lymphocytes was measured via CFSE dilution. Immunized mice received 1 × 107 CFSE (10 μM)-labeled splenocytes derived from OT-I or OT-II/Thy1.1 mice. For transfer of CD8+ lymphocytes, OT-I splenocytes were treated with erythrocyte-lysis buffer (155 mM NH4CL and 0.01 M Tris) for 5 min at room temperature. CD8+ cells were magnetically sorted, labeled with CFSE and injected i.v. into Thy1.1 recipient mice. Three days later, spleens of Thy1.1 recipient mice were stained for Thy1.2+ cells [PE-Cy5-conjugated anti-mouse CD90.2 (Thy1.2), eBioscience, Clone 53-2.1] for 20 min at 4°C, washed with PBS and analyzed by flow cytometry. For transfer of OT-II/Thy1.1 splenocytes, spleens of OT-II/Thy1.1 mice were homogenized and treated with erythrocyte-lysis buffer for 5 min at room temperature. Cells were labeled with CFSE and injected i.v. into C57BL/6 recipient mice. Three days later, spleens of recipient mice were harvested, homogenized and stained for Thy1.1 [PE-Cy5-conjugated anti-mouse CD90.1 (Thy1.1) Ig, eBioscience, Clone HIS51] and for CD4 (PE-conjugated anti-mouse CD4 Ig, eBioscience, Clone RMA4-5) for 20 min at 4°C. CD4/Thy1.1 double positive cells were analyzed for CFSE fluorescence.
Results
Humoral response to single injection of PLGA-MS
To determine the humoral immune response to PLGA-MS, mice received a single injection of MS containing OVA and CpG oligonucleotides (MS-OVA-CpG) or the same amount of antigen and TLR ligand in IFA. The OVA-specific Ig isotype concentrations indicate a robust IgG1 and IgG2a response but only low levels of IgE (Fig. 1). Empty MS showed no significant increase of Ig isotypes within 4 weeks (data not shown). The maximum concentration of about 18 μg/ml of OVA-specific IgG1 and IgG2a was reached after 3 weeks and stayed at that level until the end of the experiment after 5 weeks post-MS-based vaccination, whereas we observed a marked decline of the titers after the 5th week when IFA-based immunization was performed. The relative levels of OVA-specific IgG1 to IgG2a suggested a balanced Th1/Th2 response.
Enhancement of CTL responses by TNF-α pretreatment and prime-boost vaccination
We tested two options to enhance the CTL response to PLGA-MS: (i) by pretreatment of the injection site with TNF-α31 and (ii) by administering a booster injection. The pretreatment of the injection site with 200 ng TNF-α at least 6 hr before immunization increased the CTL response markedly, and this effect was still visible when the pretreatment was performed up to 8 hr before immunization (Fig. 2a). Homologous priming-boosting with MS-OVA/CpG within an interval of 4 weeks failed to increase OVA-specific CTL responses, as determined by intracellular IFN-γ staining (ICS), whereas a heterologous boosting using recombinant vaccinia virus expressing OVA improved the CTL response (Fig. 2b). Flow cytometric analysis with SIINFEKL/H-2Kb tetramers confirmed the enhanced response after heterologous as opposed to homologous boosting with MS-OVA/CpG, but this read-out evidenced also an effect of a homologous boosting (MS-MS) (Fig. 2c). It was noteworthy that not all tetramer+CD8+ cells obtained after homologous boosting (MS-MS) also showed IFN-γ production in the ICS.
IFN-γ but no IL-4 production by CD4+ splenocytes from PLGA-MS-vaccinated mice
The propensity for Th1 as opposed to Th2 differentiation after MS-based immunization was further characterized by the IL-4 and IFN-γ concentrations in the supernatant of splenocytes harvested from mice, either immunized with MS-OVA/CpG or a formulation of the same substances in IFA. As a control, we used MS or IFA containing CpG oligonucleotides alone. Subsequent to stimulation with anti-CD3 and anti-CD28 antibodies in vitro, the supernatants of both splenocyte preparations contained predominantly IFN-γ and barely detectable levels of IL-4, arguing for a Th1 bias conferred by both vaccination modes (Fig. 3).
Persistence of CTL and T-helper cell stimulation after PLGA-MS- and IFA-based vaccination
As PLGA-MS and IFA are expected to create a depot of antigen at the site of injection, we investigated the potential of PLGA-MS- and IFA-based vaccination to induce in vivo proliferation of OVA-specific CD8+ and CD4+ T cells derived from OT-1 and OT-2 mice at several time points after vaccination. To this aim, we vaccinated the mice with either a mixture of MS-OVA/CpG and MS-polyI:C, which we determined to be the most potent vaccination scheme (M. Mueller, unpublished data), or the same amounts of OVA, CpG oligos and polyI:C in IFA. Following CFSE dilution assays, both PLGA-MS- and IFA-based vaccination induced the proliferation of transferred CD4+ and CD8+ T cells in vivo even when the T-cell transfer occurred up to 21 days after inoculation (Figs. 4a and 4b).
In vivo and ex vivo cytotoxicity elicited by PLGA-MS- and IFA-based vaccination
The cytotoxic effect of the generated CTLs was assessed by in vivo and ex vivo assays. At 6 days after immunization with either a mixture of MS-OVA/CpG and MS-polyI:C or the same amounts of OVA, CpG oligos and polyI:C in IFA, the IFA formulation elicited a slightly superior ex vivo cytotoxicity in the 51-chromium cytotoxicity assay at effector to target ratios of 30:1 and 10:1 than the PLGA-MS formulation (Fig. 5a). However, in the nonradioactive EuBATDA cytotoxicity assay, the PLGA-MS- and IFA-based vaccinations elicited similar cytolytic responses, yielding a detectable cytolytic response ex vivo even 14 days after immunization (Fig. 5b). The in vivo cytotoxicity assay showed robust responses at 6 days after PLGA-MS- and IFA-based vaccinations, which remained detectable when target cells were injected 9, 16 or even 21 days after vaccination, thus indicating that both vaccine formulations yielded long-lasting cytotoxic responses (Fig. 5c).
Tumor eradication by PLGA-MS- and IFA-based immunotherapy
For comparing the potential of PLGA-MS- and IFA-based vaccination for tumor immunotherapy, we investigated the antitumor responses to EL-4 thymoma (Fig. 6a) as well as to the very aggressive and fast growing B16F10 melanoma (Fig. 6b). The corresponding OVA transfectants EG-7 and MO-5 were used in either a protective setting (Fig. 6, left-hand side panels), where therapy was started 6 days before challenge, or in a therapeutic setting (Fig. 6, right-hand side panels), where therapy was started not before palpable tumors had been established. Eighty percent of mice receiving treatment with a mixture of MS-OVA/CpG and MS-polyI:C in a protective setting survived tumor free using EG-7 model, and all MS-treated mice survived in the protective MO-5 model. For comparison, all mice being treated with IFA containing the same ingredients remained tumor free in the EG-7 model, but only 60% survived inoculation with MO-5 tumor cells. The therapeutic settings showed that both delivery systems had the potency to cure all mice from EG-7 tumors. For MO-5, however, only 20% of the PLGA-MS group and 30% of the IFA group survived tumor free. In a further assay, metastases in the lung were established by i.v. injection of melanin-producing MO-5 cells. Here, both IFA and PLGA-MS formulations markedly reduced the number of metastases in the protective setting (Fig. 6c). The therapeutic setting showed a clear benefit for the group being treated with PLGA-MS when compared to the IFA group. Taken together, we could show that vaccination with PLGA-MS stimulates a robust immune response that has the potency to eradicate solid tumors and to prevent metastasis formation at an extent equivalent or superior to that of IFA.
Discussion
The pivotal function of DCs for T-cell priming has spurred tremendous efforts to target tumor antigens for uptake and presentation by DC. In the last decade, great efforts have been invested to cultivate autologous human DC in vitro to pulse them with antigen and inject them into patients.32-36 This approach, besides yielding largely disappointing clinical results, is very labor and cost intensive. An interesting alternative is the in vivo delivery of antigens and immunostimulants to DC for which IFA formulations have been used most frequently.
Admittedly, IFA formulations have been improved in terms of safety and tolerance by the patients. MF-59, e.g., is an emulsified squalene in combination with Tween and Span85, a fatty acid ester of polyhydric alcohol, which stabilizes the water in oil emulsion. Besides alum, MF-59 is the only carrier system approved for clinical use in humans.37, 38 Nevertheless, both alum and MF-59 are known to favor Th2 responses and show poor efficacy in regard to antigen or adjuvant depot formation.39, 40 In combination with Th1-polarizing adjuvants like monophosphoryl lipid A (MPL),41 a detoxified LPS derivate binding TLR442 or QS-21,43 a subfraction of Quil-A, both have been successfully used in malaria vaccines, in HPV as well as in HIV studies.40, 44-46
In our study, we investigated the potency of PLGA-MS based vaccination in immunotherapy of mice against two model tumors. We showed that PLGA-MS were equal or superior to IFA-based vaccination currently used in clinical trials.5, 47, 48 A major rationale for developing PLGA-MS as antigen delivery systems resided on their capacity to elicit strong antibody responses after a single administration without the need of booster immunizations.49 We could also show that high titers of OVA-specific IgG1 and IgG2a are obtained by a single vaccination and that these titers are maintained for several weeks (Fig. 1). Systemic IgE levels remained low arguing against an enhanced risk of allergic reactions. We hypothesized that the slow biodegradation of PLGA-MS over several weeks would allow the maintenance of an antigen depot, which might sustain CTL responses over an extended period of time. Indeed, the T-cell transfer experiments demonstrated that both IFA- and PLGA-MS-based vaccinations sustain antigen presentation for over 21 days in vivo (Fig. 4). More importantly, the induced CTLs were capable to lyse antigen-charged cells for up to 21 days after immunization. This result came as a surprise, because we had previously shown by IFN-γ ICS that PLGA-MS-based vaccination led to a peak of IFN-γ-producing CD8+ cells on Day 6 after vaccination and declined rapidly thereafter.18 Hence, the capacity to lyse target cells is sustained longer than that of producing IFN-γ. We were concerned that the persistence of antigen in PLGA-MS would induce regulatory T cells, but no accumulation of CD4+CD25+FOXP3+ T cells was observed over time following PLGA-MS-based vaccination (M. Mueller, unpublished data). Consistently, IFN-γ production and the switch to IgG2a was much more prominent after IFA- or PLGA-MS-based vaccination than IL-4 production or the switch to IgE. It therefore appears that both formulations induce a Th1 differentiation, which is probably due to CpG oligo and polyI:C coadministered with the antigen.
In our efforts to optimize PLGA-MS-based vaccination, two parameters turned out to be helpful for enhancing the Th1 responses to PLGA-based vaccination: (i) the preconditioning of the injection site with TNF-α31 and (ii) the use of two different pattern molecules, CpG and polyI:C. Also, the capacity to maintain IFN-γ production by antigen-specific CD8+ cells by a heterologous boost with recombinant vaccinia virus is an important finding of our study, which could be exploited in clinical trials. Both the ability of CTLs to directly lyse tumor cells as well as the production of IFN-γ by CTL and Th1 cells will most likely contribute to the observed antitumor responses (Fig. 6).
Finally, it is interesting to compare the efficacy of the antitumor response of PLGA-MS- and IFA-based vaccination. Although both vaccination regimes mediated the complete eradication of well-palpable EG-7 thymomas (Fig. 6a), the protection against this tumor and the aggressive MO-5 melanoma was enhanced when PLGA-MS-based vaccination preceded tumor inoculation. Also, the suppression of metastasis formation in the lung after MO-5 inoculation was clearly stronger when PLGA-MS based vaccination was used in a therapeutic setting (Fig. 6c). Our tumor protection and immunotherapy data are consistent with other tumor vaccination studies using PLGA nanoparticles or microparticles containing protein antigens and TLR ligands, which showed that the coencapsulation of antigen and pattern molecules markedly improves the antitumor response21 and even allowed the eradication of preexisting B16F10 melanoma.19 Taken together, PLGA-MS-based vaccination combines excellent biocompatibility with high and long-lasting antitumor responses resulting in powerful tumor eradication in mice. Therefore, the investigation of PLGA-MS-based vaccination in a phase 1 clinical trial of immunotherapy against cancer is clearly warranted.
Acknowledgements
The authors thank Dirk Busch for the contribution of MHC tetramers, Wolfram Osen and Antje Heit for cell lines, Jonathan Yewdell for recombinant vaccinia virus and Thomas Brocker and Ying Waeckerle-Men for transgenic mice. This study was supported by a grant from Deutsche Krebshilfe to M.G.




