Evidence of X-Ray Magnetic Circular Dichroism and Low-Field Microwave Absorption in Room-Temperature Organic Ferromagnetic Semiconductor
ABSTRACT
Room-temperature organic ferromagnetic semiconductors represent a promising frontier in developing next-generation electronic and spintronic devices. However, the origin of magnetic moments in organic ferromagnets and the acquisition of critical evidence for magnetic ordering remain incompletely understood. This study presents compelling evidence for room-temperature ferromagnetism in N,N′-diamino perylene bisimide (2NH2-PBI) radical aggregates through a comprehensive analysis utilizing X-ray magnetic circular dichroism (XMCD), low-field microwave absorption (LFMA) techniques and magnetic characterization. The 2NH2-PBI samples, prepared via hydrothermal reduction, exhibit a significant saturation magnetization of 0.8 emu g−1 (336.3 emu mol−1) at 300 K, with a coercive field of 170 Oe. The XMCD measurements at the carbon K-edge exhibited a pronounced dichroic signal (~8.7%), confirming the origin of ferromagnetism in the π-conjugated electrons of the perylene core. Density functional theory calculations further support this finding by demonstrating that spin density is primarily delocalized on the π-conjugated skeleton, giving a microscopic explanation for the magnetic properties of 2NH2-PBI radicals. Furthermore, LFMA studies provide additional evidence of ferromagnetic ordering, showcasing hysteretic behavior consistent with domain wall dynamics. Our work indicates that imide-based radical molecules with extended π-conjugated structures constitute a class of effective magnetic functional units.
1 Introduction
Magnetic semiconductors, owing to their dual capabilities of semiconductor logic functions and magnetic material storage functions, are poised to address the challenges of the “post-Moore era” [1]. However, research on dilute magnetic semiconductors has not yet achieved magnetic ordering at ambient temperatures, necessitating a breakthrough in room-temperature applications [2-4]. Organic ferromagnets provide additional benefits over conventional magnets due to their flexibility, the ability for low-temperature synthesis, lightweight, and compatibility with biological systems [5-7]. Significant efforts have been devoted to discovering ferromagnetism in purely organic compounds based on radicals [8]. McConnell first suggested that π-radicals are promising candidates for pure organic magnets [9]. In 1989, Awaga et al. discovered ferromagnetism in the nitroxide radical para-nitrophenyl nitronyl nitroxide (p-NPNN) [10]. The quinoid resonance structure of the p-NPNN molecule leads to the delocalization of unpaired electrons and the exchange interaction of n-π electrons in the nitro group, significantly enhancing the spin polarization effect [10, 11]. The synergy of these two effects results in ferromagnetic interactions between molecules. Subsequently, Tamura et al. reported that the Curie temperature (Tc) of the orthorhombic β-phase of p-NPNN is only 0.6 K, with a saturation magnetization (Ms) of 10.1 emu g−1 [12]. Following this discovery, various design strategies have been employed to achieve ferromagnetic ordering in organic radicals at as high temperatures as possible [13-16]. The Tc in the nitroxide biradical diazaadamantane reaches 1.48 K, which is the highest value reported for nitroxide radicals to date [13]. Rawson et al. controlled molecular aggregation by substituting cyano and fluoro groups, increasing the Tc of β-phase crystals of dithiadiazole radicals to 36 K [14]. Its net magnetic moment arises from an equal number of canted antiferromagnetic arrangements that are not entirely antiparallel. The resulting Ms is 0.03 emu g−1. Baek et al. discovered room-temperature ferromagnetism in organic conjugated framework radical materials [15]. However, due to the inherent ordering limitations of cross-linked conjugated aggregates, this material exhibits a relatively weak Ms of only 10−3 emu g−1 and is insulating. Our group first reported room-temperature organic ferromagnetic/ferromagnet (OFM) semiconductors in the perylene bisimide (PBI) dyes [17]. The Ms reaches 1.2 emu g−1, two orders of magnitude higher than previously reported organic ferromagnetic materials, with a Hall mobility of 0.5 cm2 V−1 s−1. Subsequent density functional theory calculations, based on a systematic study of the π-dimer stacking conformations of NH4PBI, established a π-tetramer stacking model that exhibited strong ferromagnetic interactions (JAB = +381.2 cm−1) [18]. Zhou et al. replicated the hydrothermal PBI reaction with a strong magnetic field [19]. Due to the magnetic field's influence on the molecular arrangement and crystal growth during the reduction process, the prepared samples exhibited a Ms of only 0.063 emu g−1 at 10 K. While the observation of room-temperature OFM is a significant advancement, the origin of magnetism and the mechanism of magnetic interaction remain unanswered. To fully harness the potential of these materials, it is crucial to conduct comprehensive magnetic characterization studies.
Soft X-ray magnetic circular dichroism (XMCD) is a powerful tool for defining the contribution of different elements to ferromagnetic behavior, including carbon materials [20]. After undergoing treatments such as ion irradiation, high-purity graphite crystals can exhibit ferromagnetic properties despite the absence of traditional magnetic elements [21]. Ohldag et al. investigated this phenomenon using XMCD technology and discovered that the π-bonds of carbon atoms in graphite play a pivotal role in its ferromagnetic behavior [22]. Recently, a study has utilized XMCD to delve into the K-edge properties of carbon and nitrogen in pure organic radical films [23]. The experiment was performed at 1.1 K and 7 T, revealing differences in XMCD characteristics of the films based on preparation methods. A low-field nonresonant microwave absorption has recently been observed in a variety of magnetically ordered materials at low DC fields (−1000 Oe ≤ HDC ≤ 1000 Oe), which is known as low-field microwave absorption (LFMA) [24]. The LFMA technique is commonly detected using commercial electron paramagnetic resonance (EPR) spectrometers. The LFMA, regarded as an indicator of ferromagnetic properties in a wide range of materials, relates to the onset of ordered phases in ferrites and magnets [25, 26]. LFMA signal has been reported previously in other soft magnetic materials and interpreted as due to low-field spin magnetization processes [27]. Miller et al. observed broad near-zero-field resonance and narrow anti-resonance in the EPR spectra of molecular-based magnets V(TCNE)x·y(solvent), composed of different solvents [6, 28]. This low-field signal is similar to the signals observed in domain wall resonance. A distinct zero-field signal was present in each composition below the Tc. Previous studies have reported similar observations on spin glasses, soft and hard magnetic layers [29], and magnetic metal nanowires [30].
Such investigations will shed light on the mechanisms of magnetic interactions arising from unpaired electrons in s and p molecular orbitals and elucidate the origin of magnetic moments in these organic systems. Currently, the utilization of XMCD and LFMA in room-temperature OFM semiconductors is still an unexplored area. Here, we have confirmed the existence of room-temperature ferromagnetism in organic semiconductors through magnetic measurements, XMCD, and LFMA. XMCD tests have evidenced that the ferromagnetism originates from the π-electrons of reduced 2NH2-PBI (r-2NH2-PBI) microcrystals. The LFMA studies provide insights into domain dynamics and shape anisotropy in r-2NH2-PBI.
2 Results and Discussions
2.1 Fabrication and Structural Characterizations
The reduction and dissolution treatment of 2NH2-PBI was performed via the hydrothermal method with an excess hydrazine hydrate at 120°C for 12 h. After cooling, a purple-red suspension was obtained. Subsequently, the solvent was heated and evaporated in a glove box (H2O < 1 ppm and O2 < 4 ppm) to prepare r-2NH2-PBI powder (Figure 1A). The powder exhibited a closely packed morphology of nanorods (Figure 1B). The prepared film exhibited peaks at 810 and 965 nm, facilitating the identification of the generation of r-2NH2-PBI radical anions (Figure 1C) [31]. EPR spectroscopy was utilized to investigate the formation of radical anions in the r-2NH2-PBI powder. A strong resonance signal was observed near g = 2.003, originating from unpaired electrons, indicating a sharp increase in radical anions (Figure 1D). The X-ray diffraction (XRD) pattern of the prepared r-2NH2-PBI powder is presented in Figure 1E. The peak at 27.7° corresponds to π–π stacking with a calculated interplanar distance of 3.22 Å (shorter than van der Waals contact), while the 11.6° peak aligns with edge-to-face stacking [32, 33]. Compared to the pristine sample, the slightly increased π–π interlayer spacing after reduction results from the insertion of cations and electrostatic repulsion arising from electronic interactions between functional groups and ions. Given the repulsive forces between anions, the ability to maintain a closely packed structure in the ionic state indicates the presence of strong π–π interactions between molecules. The intense interaction between polarized π-electrons favors magnetic coupling. After reduction treatment, the relative intensity ratio of these peaks (I27.7°/I11.6°) increases from 0.3 to 1.0, accompanied by a reduction in full width at half maximum (FWHM) for the 27.7° peak. These observations collectively suggest that the reduction process significantly enhances the π–π stacking, establishing it as the dominant structural motif with improved long-range ordering.
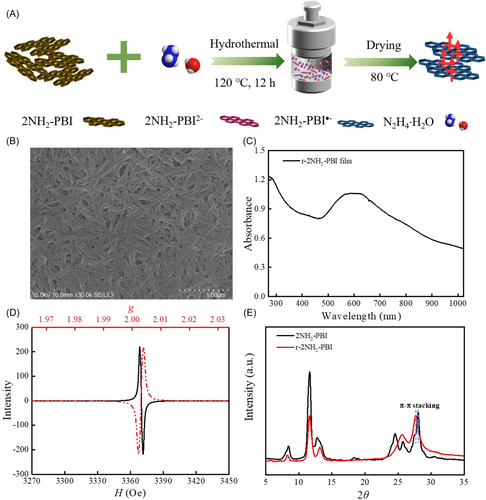
2.2 Magnetic and Semiconducting Properties
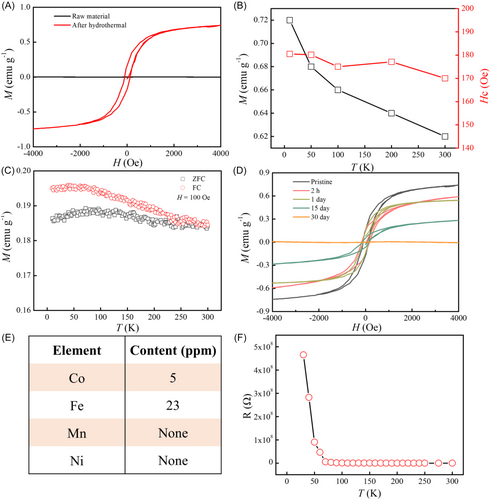
We investigated the semiconductor properties and magnetic behaviors of r-2NH2-PBI using the physical property measurement system. The magnetization-magnetic field (M-H) curve at 300 K exhibits a representative ferromagnetic hysteresis loop (Figure 2A). The magnetization reaches saturation at 2 kOe with a coercive field of 170 Oe. The extracted Ms at 300 K is 0.8 emu g−1 (336.3 emu mol−1), which is notably large among organic magnets, significantly exceeding that of weak ferromagnets in porous organic radical frameworks linked by 1,3,5-triazine [34], and comparable to previously reported PBI ferromagnetism [17]. In contrast, the linear M-H curve of pristine 2NH2-PBI shows diamagnetic characteristics, strongly suggesting that the ferromagnetic signal arises from the reduction process. Furthermore, hysteresis loops were measured within a temperature range of 10–300 K (Supporting Information S1: Figure S1). The temperature dependences of Ms and the coercivity field indicate ferromagnetic behavior above room temperature (Figure 2B). The temperature dependences of magnetization under zero-field-cooled (ZFC) and field-cooled (FC) conditions at a magnetic field of 100 Oe are shown in Figure 2C. The ZFC magnetization curve exhibits a maximum near 100 K. Furthermore, the splitting of ZFC and FC curves below about 300 K, indicating a Tc value above 300 K for the ferromagnetic r-2NH2-PBI powder. We further observed that after approximately 1 month of continuous exposure to air (Figure 2D), the ferromagnetic property of r-2NH2-PBI gradually transformed into diamagnetism. Due to the natural chemical activity of free radicals, the previously reported organic ferromagnetic crystals are generally unstable. For instance, tetrakis(dimethylamino)ethylene-C60 will ultimately lose its magnetic properties after exposure to air for just a few seconds [35]. Compared with such organic ferromagnets with low Tc, our ferromagnetic powder exhibits more excellent stability in the air. The total content of magnetic metallic impurities is 28 ppm (Figure 2E), and the magnetization intensity calculated based on this content would not exceed 6 × 10−5 emu g−1 (Fe = 2.2 μB, 218 emu g−1; Co = 1.7 μB, 161 emu g−1). This value is four orders of magnitude smaller than the measured saturation magnetization, conclusively excluding extrinsic ferromagnetism from impurities. Figure 2F depicts the temperature-dependent resistance curve exhibiting typical semiconductor characteristics. With the increased air exposure time, the electrical conductivity (σ) increased first and then decreased. The maximum is 0.26 S cm−1 (Supporting Information S1: Figure S2).
2.3 Origin of Magnetism
To elucidate the elemental contributions to the observed ferromagnetism, we employed the XMCD technique. This powerful technique, illustrated schematically in Figure 3A, involves a two-step process. First, circularly polarized X-rays with their helicity vector aligned parallel (or antiparallel) to the orbital moment excite electrons with a preferred spin orientation. Subsequently, these excited electrons occupy available states in the unoccupied valence band, resulting in a net positive or negative peak in the XMCD spectrum, depending on the relative abundance of spin-up and spin-down holes [20, 36]. Figure 3B depicts the room-temperature X-ray absorption spectroscopy (XAS) and XMCD spectra at the C K-edge, with the X-ray incident on the film at a 90° angle and an applied magnetic field strength of 3 kOe. The resonance peak located in the 280–290 eV region corresponds to the π* resonance of the perylene core, while the resonance peak in the 290–300 eV region is primarily attributed to the σ* resonance [37, 38]. The first peak at 284 eV is associated with transitions from the C 1s level of carbon atoms in the perylene core to the lowest unoccupied molecular orbital (LUMO, L0). The peak at 285.4 eV belongs to transitions from core levels to higher excited energy orbitals, L1–L3. The peaks near 287.2 and 288 eV can be assigned to transitions from carbon atoms on the imide moiety to L0 and L1–L3, respectively. The XMCD signals were acquired by the difference in the normalized X-ray absorption spectra recorded with right- and left-circularly polarized beams. A nonnegligible C XMCD signal was observed. The strength of XMCD reached ~8.7% by the division between amplitudes of XMCD (0.075) and XAS (0.86). The signal intensity is comparable to the Co L-edge XMCD signal (5%) observed in Co(Cp)2/SnS2 [39], underscoring the significance of our findings. Crucially, XMCD signals are exclusively observed in the π* region (280–290 eV) with no detectable magnetic dichroism above 290 eV (σ* region), confirming that the magnetism originates from the polarized π-conjugated electrons within the r-2NH2-PBI perylene core.
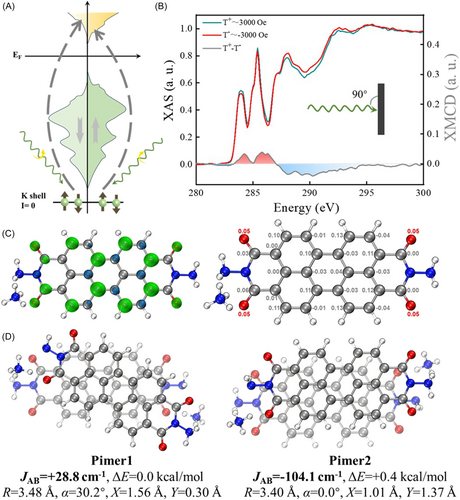
To visualize the spin density of the monomer that constructs this OFM semiconductor, a NH4-doped r-2NH2-PBI radical model (r-2NH2-PBI-NH4) is calculated at M05-2X/def2-TZVP theoretical level. The spin density distributions and Mulliken spin population of r-2NH2-PBI-NH4 are displayed in Figure 3C. It can be seen from the spin density distributions that the oxygen atoms carry an excess of α-electron density, and the total magnetic moment on these atoms is 0.20 μB. The spin density distributions on the perylene core follow the spin alternation rule, which means that the spins on neighboring atoms are preferred to be successively antiparallel due to spin polarization. It can be concluded that the unpaired electron of r-2NH2-PBI-NH4 is distributed over the π-orbital according to the shape of the isosurface. It is delocalized over the whole radical, and the most significant magnetic moment of the carbon atoms is 0.13 μB. Moreover, the total magnetic moment on carbon atoms is 0.83 μB, indicating that the spin density contains a significant contribution from the atomic orbitals of carbon atoms compared to that of oxygen atoms. These computational results are in excellent agreement with our XMCD experimental findings, providing a theoretical foundation for understanding the origin of ferromagnetism in r-2NH2-PBI. A π-dimer (pimer) model consisting of two r-2NH2-PBI-NH4 radicals was established to theoretically investigate the origin of ferromagnetism observed in ferromagnetic powders without anionic radical crystal structure characterization. Various π-stacking patterns of r-2NH2-PBI-NH4 pimers were generated and optimized, yielding six distinct pimers. The definitions of the structural indicators for r-2NH2-PBI-NH4 radical pimers are illustrated in Supporting Information S1: Figure S3. The most representative pimers are summarized in Figure 3D, with the other structures shown in Supporting Information S1: Figure S4. By comparing the relative total energies of the pimers, it is found that Pimer1 has an energy 0.4 kcal mol−1 lower than Pimer2, which may be attributed to the charged hydrogen bonds between the ammonium and the oxygen of the carbonyl group in Pimer1. The relative total energies of the other pimers are at least 3 kcal mol−1 higher than these two, leading to low Boltzmann populations at room temperature. Pimer3 and Pimer6 exhibit similar stacking structures but have significant energy differences due to different cationic positions. Pimer4 and Pimer5 possess distinct twist angles corresponding to different spin states. The superposition of slipping and twisting enhances the transition of magnetic coupling interaction from antiferromagnetic to ferromagnetic. The calculation results indicate that the relative positions between r-2NH2-PBI-NH4 radicals significantly influence the magnetic coupling constant, with the interaction between radicals in Pimer1 being ferromagnetic. This could be due to the slipping and twisting that reduce the Pauli repulsion between electrons, making the ferromagnetic state's electron energy (+28.8 cm−1) lower than that of the antiferromagnetic state. However, in Pimer2, the parallel stacking of r-2NH2-PBI-NH4 radicals increases the orbital overlap area, leading to enhanced singly occupied molecular orbital (SOMO)-SOMO interactions and resulting in antiferromagnetic coupling interactions between radicals (−104.1 cm−1). It should be noted that due to the simplification of interactions between adjacent radicals and pimer, some quantitative discrepancies may exist compared to experimental results regarding the obtained magnetic coupling interaction.
2.4 LFMA
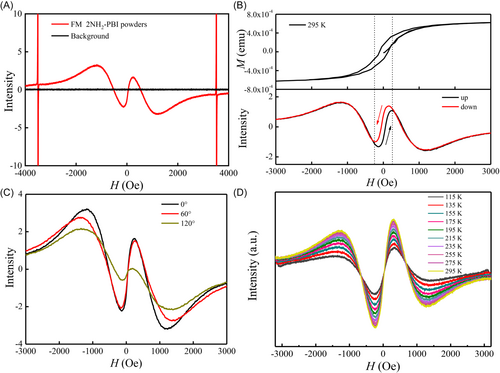
A typical EPR spectrum of r-2NH2-PBI powder at room temperature is presented in Figure 4A. Compared to the quiescent background, three distinct resonance signals are observed for the ferromagnetic r-2NH2-PBI powder: (1) the primary resonance with g = 2.003, (2) a broad near-zero-field resonance, and (3) a narrow zero-field non-resonance. The appearance of LFMA is particularly significant, as it is associated with the onset of magnetic ordering and serves as a sensitive indicator of such ordering [25, 26]. Figure 4B illustrates an apparent hysteresis of this signal on cycling the field. By comparing the M-H hysteresis loop, we observe that the field range exhibiting absorption hysteresis corresponds directly to that associated with the magnetization reversal process. The hysteretic nature of the LFMA signal, typically encountered in domain structures subjected to DC fields below the saturation field, implies a strong connection to the spin responses of various irreversible domain configurations as the DC field is cycled [40]. The hysteresis effect of the LFMA signal appears to be due to nonuniform surface magnetization processes. A ferromagnetic conducting system's absorption of electromagnetic radiation is influenced by domain structure, anisotropy, radiation orientation, conductivity, frequency, and amplitude. This absorption can be adjusted using HDC, which modifies magnetic properties, domain structures, and spin dynamics, resulting in hysteresis effects observed in domain configurations under sub-saturation DC fields [27]. Interestingly, we observed that rotating the sample results in a diminution of the LFMA signal (Figure 4C). This angular dependence implies the presence of shape anisotropy in r-2NH2-PBI samples, a characteristic that could be harnessed in future device designs. Figure 4D illustrates the EPR spectra of ferromagnetic r-2NH2-PBI powder as a function of temperature within the range of 295–115 K. It can be observed that the peak intensity gradually decreases as the temperature decreases. In contrast to the angle-dependent EPR spectra, no significant changes in the shape and position of the EPR spectra are evident across the entire temperature range. This is markedly different from the broadening of linewidth and the shift of resonance field observed in inorganic ferromagnetic materials such as zinc-cobalt oxide [26] and AgMnxSby [29]. As shown in Supporting Information S1: Figure S5, the LFMA intensity of r-2NH2-PBI exhibits a non-monotonic temperature dependence (295–455 K): the initial increase (295–335 K) may arise from thermally enhanced spin dynamics, while the subsequent decrease (335–455 K) could reflect progressive domain wall relaxation, potentially linked to reduced exchange coupling at higher temperatures [41]. These trends suggest a competition between thermal activation and magnetic ordering decay, which aligns with prior observations of magnon-driven dynamics in systems with Dzyaloshinskii–Moriya interactions [42].
From the perspective of the fundamental principles of magnetism in physics, the prerequisites for designing organic molecular materials with bulk magnetic properties are (i) persistence of spin units. Perylene diimide derivatives possess an extensive planar π-conjugated system, which favors the stabilization of radicals and promotes the delocalization of spin density [18]. Utilizing large π-conjugated structures to stabilize radicals provides a stable source for magnetism. (ii) Establishment of magnetic coupling pathways between adjacent spin units. π-conjugated radicals with face-to-face geometry and short π–π distances for atomic-atomic interactions can form strong SOMO-SOMO interactions comparable to covalent bonds [43-45]. The r-2NH2-PBI radicals form nanorod aggregates with a π–π distance of just 3.22 Å, shorter than typical van der Waals distances. The synergistic effect of strong intermolecular π–π stacking and hydrogen bonding enhances the spin electron exchange between SOMOs, achieving interaction strengths comparable to those of chemical bonds, thereby significantly enhancing the intermolecular ferromagnetic coupling. (iii) Effective transmission of magnetic interactions within the material is crucial. Magnetic domains arise when intrinsic magnetic moments are confined within minute crystalline domains and undergo spontaneous magnetization through charge exchange between spin electrons. The interactions between these magnetic domains determine the overall magnetic properties of the material. The r-2NH2-PBI radical aggregates exhibit long-range π–π interactions, enabling the maintenance of magnetic interactions along the material, forming a long-range magnetically ordered structure. Through π–π stacking, the coupled radicals established long-range magnetic order via the spin of unpaired electrons. This discovery deepens our understanding of the mechanism of intermolecular magnetic interaction and the design of magnetic organic semiconductor materials.
3 Conclusions
In summary, we provide compelling evidence supporting the existence of room-temperature ferromagnetism in organic semiconductors based on perylene bisimide derivatives. By employing a combination of magnetic measurements, XMCD, and LFMA techniques, we have successfully demonstrated that the observed ferromagnetism originates from the π-electrons of the r-2NH2-PBI microcrystals. The XMCD measurements conducted at the carbon K-edge reveal a significant dichroic signal, directly linking the ferromagnetic behavior to the carbon atoms in the perylene core. Density functional theory calculations provide additional support for this experimental finding by illustrating that the unpaired electron is primarily distributed over the π-orbitals of carbon atoms and showcasing a range of stacked geometries for 2NH2-PBI-NH4 pimers on low spin-high spin state. The LFMA studies confirm the ferromagnetic ordering, revealing hysteretic behavior consistent with domain wall dynamics. Moreover, the materials exhibit promising semiconductor properties with tunable electrical conductivity.
Author Contributions
Jiaji Yang, Hanlin Gan, Jiang Zhang, Yuguang Ma, and Qinglin Jiang conceived the concept. Jiaji Yang and Qinglin Jiang designed the experiments. Jiaji Yang, Yanuo Zhu, Shaohua Tong, and Wei Cui performed the materials and characterizations. Hanlin Gan, Xiandong He, and Jiang Zhang assist with the data analysis. The manuscript was drafted by Jiaji Yang and Hanlin Gan, and revised together with Jiang Zhang, Qinglin Jiang. All authors contributed to the discussion of the manuscript.
Acknowledgments
We gratefully appreciate the financial support from Natural Science Foundation of China (92463310 and 52203221), the open research fund of Songshan Lake Materials Laboratory (2023SLABFK05), National Key R&D Program of China (2020YFA0714604), Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (2023B1212060003), Guangdong Provincial Quantum Science Strategic Initiative (GDZX2301002). Funding by Science and Technology Projects in Guangzhou (2024A04J2529), Young Talent Support Project of Guangzhou Association for Science and Technology (QT2024-001), the Fundamental Research Funds of State Key Laboratory of Luminescent Materials and Devices (No. Skllmd-2023-03 and Skllmd-2024-23).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




