Clinical and pathophysiological delineation of musculocontractural Ehlers—Danlos syndrome caused by dermatan sulfate epimerase deficiency (mcEDS-DSE): A detailed and comprehensive glycobiological and pathological investigation in a novel patient
Mari Minatogawa, Takuya Hirose, and Shuji Mizumoto contributed equally to this study.
Abstract
Musculocontractural Ehlers–Danlos syndrome caused by dermatan sulfate epimerase deficiency (mcEDS-DSE) is a rare connective tissue disorder. This is the first report describing the detailed and comprehensive clinical and pathophysiological features of mcEDS-DSE. The patient, with a novel homozygous nonsense variant (NM_013352.4:c.2601C>A:p.(Tyr867*)), exhibited mild skin hyperextensibility without fragility and small joint hypermobility, but developed recurrent large subcutaneous hematomas. Dermatan sulfate (DS) moieties on chondroitin sulfate/DS proteoglycans were significantly decreased, but remained present, in skin fibroblasts. Electron microscopy examination of skin specimens, including cupromeronic blue-staining to visualize glycosaminoglycan (GAG) chains, revealed coexistence of normally assembled collagen fibrils with attached curved GAG chains and dispersed collagen fibrils with linear GAG chains from attached collagen fibrils across interfibrillar spaces to adjacent fibrils. Residual activity of DS-epi1, encoded by DSE, and/or compensation by DS-epi2, a minor homolog of DS-epi1, may contribute to the mild skin involvement through this “mosaic” pattern of collagen fibril assembly.
Musculocontractural Ehlers–Danlos syndrome (mcEDS) is a subtype of EDS caused by defective dermatan sulfate (DS) biosynthesis, and has two forms attributable to biallelic loss-of-function variants in CHST14 (mcEDS-CHST14; MIM# 601776), encoding carbohydrate sulfotransferase 14/dermatan 4-O-sulfotransferase 1 (D4ST1), or dermatan sulfate epimerase (DSE) (mcEDS-DSE; MIM# 615539), encoding DSE (Brady et al., 2017; Malfait et al., 2017, 2020). Only 13 patients with mcEDS-DSE from seven families have been described to date (Lautrup et al., 2020; Müller et al., 2013; Schirwani et al., 2020; Syx et al., 2015; Ullah et al., 2021). In contrast, 66 patients with mcEDS-CHST14 from 48 families with mcEDS-CHST14 have been reported (Minatogawa et al., 2021). The major diagnostic criteria for mcEDS include congenital multiple contractures (adducted thumbs, talipes equinovarus), characteristic craniofacial features (evident at birth or early infancy), and characteristic cutaneous features (skin hyperextensibility, easy bruisability, fragility with atrophic scars, fine palmar creases) (Brady et al., 2017; Malfait et al., 2017). The general patterns of symptoms were reported to be similar between mcEDS-CHST14 and mcEDS-DSE (Lautrup et al., 2020; Minatogawa et al., 2021). Life-threatening complications were rare and no deaths occurred in patients with mcEDS-DSE (Lautrup et al., 2020), while critical episodes (infectious endocarditis, fulminant gastric ulcer, diverticular perforation) and 10 deaths were described in patients with mcEDS-CHST14 (Dündar et al., 2001, 2009; Janecke et al., 2001, 2016; Kono et al., 2016; Kosho, Miyake, et al., 2010; Minatogawa et al., 2021; Mochida et al., 2016; Syx et al., 2015). In addition, core skin features (hyperextensibility, bruisability, fragility, atrophic scars) as well as joint manifestations (hypermobility, recurrent joint dislocation), low-set ears, refractive errors, and constipation were significantly less common in patients with mcEDS-DSE than in patients with mcEDS-CHST14 (Minatogawa et al., 2021). Because of the limited clinical, glycobiological, and pathological information, the whole picture of the clinical features and courses as well as the pathophysiological mechanisms of mcEDS-DSE, especially the similarities and differences relative to mcEDS-CHST14, remain to be clarified.
In this report, we provide detailed and comprehensive descriptions of the clinical, glycobiological, and pathological features of a novel patient with mcEDS-DSE, thereby contributing to further delineation of the disorder.
The patient is currently a 37-year-old Japanese man, the second child of consanguineous parents who were second cousins. His older brother is healthy. He was born at full-term by normal delivery after an uneventful pregnancy. His birth weight was 2,800 g. At birth, he had bilateral adducted thumbs but did not exhibit other major congenital abnormalities including talipes equinovarus. An episode of cyanosis of unknown cause occurred at several hours after birth. He was transferred from an obstetric clinic to a neonatal intensive care unit and admitted for approximately 1 month. Craniofacial features in infancy included a large skull, short and downslanting palpebral fissures, strabismus, short nose with hypoplastic columella, low-set ears, micrognathia, and thin upper lip vermilion (Figure 1a,b). His growth and psychomotor development were normal: he walked unsupported and spoke single meaningful words at approximately 12 months of age. Joint stiffness and muscle weakness were noted in childhood. Closure of the anterior fontanel was delayed to 3 years of age. From childhood to adolescence, his face became slender with a protruding jaw (Figure 1c,d) and a slender build became evident (Figure 1s,t).
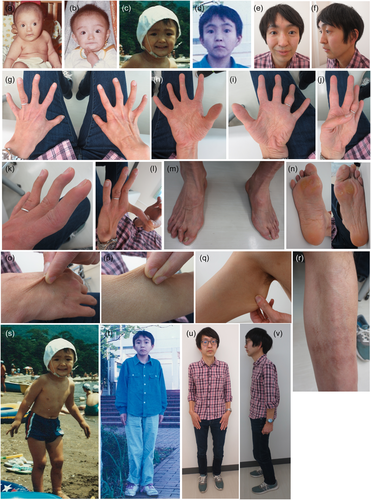
He often tumbled and incurred severe subcutaneous hemorrhages with minor injuries during childhood. Consequently, he tended to wear elastic bandages around his knees to prevent injuries while attending elementary school. At 10 years of age, he hit his right knee on the ground while practicing gymnastics in a physical education class and developed a rapidly progressive large subcutaneous hematoma. During hospitalization for 1 month, he underwent surgical drainage and detailed evaluations for his bleeding tendency. The plasma activity of von Willebrand factor (VWF) was low, and he was suspected to have von Willebrand disease; however, the VWF activity gradually increased to reach the lower limit of the normal range. He developed recurrent large subcutaneous hematomas on his feet or buttocks after minor injuries and required hospitalization. At approximately 12 years of age, intravenous administration of 1-desamino-8-d-arginine vasopressin was introduced on an as-needed basis; however, it appeared to be ineffective and always induced hyponatremia. He heard a sound of “blood vessel rupture” just before subcutaneous hematoma formation, and felt that the subcutaneous tissue was tearing, with severe pain, during hematoma expansion. Small hematomas (several centimeters in the major or minor axis) developed about once every 2 months, while large hematomas (approximately 10 cm in the major axis and several centimeters in the minor axis) developed once every 1 or 2 years and required hospitalization. He compressed the affected area to prevent hematoma formation or expansion and rested for half a day when he suffered an injury likely to cause a large subcutaneous hematoma.
He had bilateral cryptorchidism and underwent an orchiopexy for the left testis and orchiectomy for the right testis in his early childhood. Ophthalmological examinations showed moderate myopia and strabismus at 4 years of age, high myopia and astigmatism at 8 years of age that were corrected with contact lenses and eyeglasses, and bilateral glaucoma accompanied by optic disc cupping at 15 years of age that was treated with eye drops containing timolol maleate. He had significant dental crowding due to micrognathia and started orthodontic treatment at 15 years of age. Kyphoscoliosis was noted at 12 years of age and gradually progressed to the level for surgical indication; however, corrective surgery was withheld considering the difficulties and risks of surgical intervention. He was suspected to have vascular EDS when he was at high school, but a skin biopsy-based biochemical investigation showed no abnormal production of type III procollagen. A cardiac ultrasonography examination in his twenties showed mitral valve prolapse (myxomatous changes with elongated anterior leaflet and hypoplastic posterior leaflet) and regurgitation, but they did not progress through serial cardiology evaluations. He got married at 26 years of age and had three daughters after natural conception at 27, 29, and 31 years of age. At 34 years of age, he presented with a massive hemorrhage from the diverticula of the ascending colon. He was admitted to an emergency hospital and received conservative treatment, including administration of carbazochrome sodium sulfonate hydrate and tranexamic acid. He was also found to have renal calculi.
The patient was examined by us at 35 years of age. At that time, his height was 170 cm (−0.1 SD), his weight was 53.6 kg (−1.2 SD), and his occipitofrontal circumference was 58 cm (+0.2 SD), and his arm span was 175 cm. He had craniofacial features including a mildly slender facial shape with a protruding jaw, hypertelorism, short palpebral fissures, blue sclerae, and large ears (Figure 1e,f). Facial asymmetry, downslanting palpebral fissures, short nose with hypoplastic columella, low-set ears, long philtrum, thin upper lip vermilion, and high palate were not apparent. His skeletal features included tapering fingers, hypermobility in several interphalangeal joints, flat chest, pes planus, hallux valgus, and deformed and overlapping toes (Figure 1g–m). The fingers, especially the thumbs, could not be moved smoothly (Supporting Information: Video S1). The Beighton score was 0/9. His cutaneous features included mild hyperextensibility, fine palmar creases, and sole clavuses (Figure 1n−r). Atrophic scars or translucencies were not apparent. He had a Marfanoid habitus (Figure 1u,v). Radiological examination revealed straight alignment of the neck, degenerative spondylosis of the neck and lumbar spine, and severe thoracolumbar kyphoscoliosis with a Cobb angle of 45°. Dual-energy X-ray absorptiometry showed normal bone mineral density at L1−L4 (0.992 g/cm2; Z-score −0.8), but reduced bone mineral density at the right hip (0.585 g/cm2; Z-score −3.3) and left hip (0.488 g/cm2; Z-score −4.0).
We conducted an extensive molecular, glycobiological, and pathological study using specimens from the patient. Peripheral blood samples, urine samples, and skin specimens from his left upper arm were collected after obtaining written informed consent. The study was approved by the Ethics Committees at Shinshu University School of Medicine (Matsumoto, Japan) (#610) and Meijo University (Nagoya, Japan) (2015-30-3). Detailed descriptions of the methods are provided in Supporting Information.
Genomic DNA was extracted from the peripheral blood lymphocytes. A next-generation sequencing-based custom panel analysis including 52 genes for heritable connective tissue disorders was performed on an Ion PGM System (Thermo Fisher Scientific Inc.) (Koitabashi et al., 2018). A novel nonsense variant in exon 6 of DSE (NM_013352.4:c.2601C>A:p.(Tyr867*)) was identified homozygously. The variant was confirmed by Sanger sequencing.
The disaccharide compositions of the chondroitin sulfate (CS) and DS chains in cultured skin fibroblasts (conditioned medium and cell fraction) and urine samples from the patient and an age- and sex-matched healthy control subject were analyzed by anion-exchange high-performance liquid chromatography profiles after digestion with chondroitinase AC or B (Mizumoto et al., 2017; Müller et al., 2013). DS moieties were markedly reduced, but remained present, in the conditioned medium and cell fraction from cultured fibroblasts of the patient, and were not detected in urine from the patient, compared with the findings in the control subject (Supporting Information: Table S1, Figure S1). The DSE activities in cultured skin fibroblasts from the patient and the control subject as well as in COS-7 cells transfected with p.Tyr867*-mutant or wild-type DSE were evaluated by measuring the total amount of glucuronic acid (GlcUA) converted from iduronic acid (IdoUA) in DS. The DSE activities were significantly reduced in the fibroblasts of the patient, compared with those of the control subject (Figure 2a, Supporting Information: Figure S2a–c), and were also reduced in the COS-7 cells transfected with p.Tyr867*-mutant DSE compared with those transfected with wild-type DSE (Figure 2b, Supporting Information: Figure S2d–f).
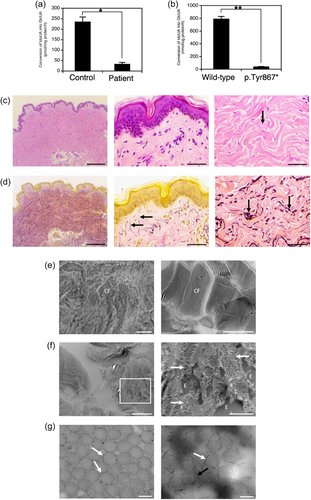
The pathological investigations included light microscopy (LM) with hematoxylin-eosin (H&E) or elastica van Gieson (EVG) staining and transmission electron microscopy (TEM) with or without cupromeronic blue (CB)-staining to visualize glycosaminoglycan (GAG) chains linked to adjacent collagen fibrils (Watanabe e al., 2016). LM with H&E staining showed fine collagen fibers in the papillary dermis and partially disorganized collagen bundles with intrabundle spaces (Figure 2c). LM with EVG staining showed a normal structure (oxytalan fibers) in the papillary dermis and increased elastic fibers in the reticular dermis (Figure 2d). TEM findings in the papillary dermis included loss of the lamellar structure of collagen fibers and less dense or dispersed collagen fibrils (Figure 2e, left). TEM findings in the reticular dermis included coexistence of normally assembled collagen fibrils (Figure 2e, right) and dispersed collagen fibrils with elastin deposits in the interfibrillar spaces (Figure 2f). TEM with CB-staining in the reticular dermis demonstrated GAG chains that curved in close contact along the contour of attached collagen fibrils (Figure 2g, left) in some views as well as linear GAG chains stretching from the attached collagen fibrils across interfibrillar spaces to adjacent fibrils in other views (Figure 2g, right).
This is the 14th published case of mcEDS-DSE (Lautrup et al., 2020; Müller et al., 2013; Schirwani et al., 2020; Syx et al., 2015; Ullah et al., 2021), but the first report to provide detailed and comprehensive pathophysiological findings including extensive glycobiological analyses and TEM observations with CB-staining as well as detailed and longitudinal clinical features. The variant (NM_013352.4:c.2601C>A:p.(Tyr867*)) detected homozygously in DSE was concluded to be pathogenic (PVS1, PS3, and PM2) according to the 2015 guideline from the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (Richards et al., 2015).
The clinical findings of the 14 patients with mcEDS-DSE are summarized and compared with those of the 66 reported patients with mcEDS-CHST14 in Supporting Information: Table S2. To determine the significance of differences in the prevalences of the findings in the patients with the two forms, the nonparametric Mann–Whitney U-test was conducted using EZR statistical software (V.1.38) (Kanda, 2013). Although the clinical picture of mcEDS-DSE was essentially similar to that of mcEDS-CHST14, joint manifestations (recurrent/chronic joint dislocations, joint hypermobility), skin features (hyperextensibility, bruisability, fragility, atrophic scars), constipation, hypotonia, and motor developmental delay were significantly less common in mcEDS-DSE than in mcEDS-CHST14 (p<0.01). These findings were similar to those in our previous report (Minatogawa et al., 2021). However, refractive errors were excluded, and hypotonia and motor developmental delay were included by adding five patients reported by Ullah et al. (2021) and the current patient. Life-threatening complications and complications affecting activities of daily living occurred in patients with mcEDS-DSE as well as in patients with mcEDS-CHST14. Large subcutaneous hematomas with minor injuries were described in two sisters (Syx et al., 2015), a boy (forehead, elbow, arm, buttock, knee), a man (calf) (Lautrup et al., 2020), and the present patient (buttock, knee, foot). A woman described by Syx et al. (2015) had a myxomatous mitral valve with severe prolapse and regurgitation as well as chordal rupture and required valve replacement. The present patient exhibited a massive diverticular hemorrhage from the ascending colon. Therefore, multisystem surveillance and preparation for emergency complications would be indispensable for patients with mcEDS-DSE, similar to the case for patients with mcEDS-CHST14 (Brady et al., 2017; Minatogawa et al., 2021).
The pathophysiology of the skin involvement in mcEDS-CHST14 was clarified using skin specimens. The findings revealed that complete loss of activity of dermatan 4-O-sulfotransferase 1 (D4ST1), encoded by CHST14, results in production of a negligible amount of DS and an excessive amount of CS in affected cultured skin fibroblasts (Dündar et al., 2009; Kosho, Mizumoto, et al., 2019; Miyake et al., 2010) as well as in affected urine (Mizumoto et al., 2017). A subsequent compositional change (complete loss of DS, replaced by CS) in the GAG chains of decorin (Miyake et al., 2010), a major DS-proteoglycan in the skin with a critical role in collagen fibril assembly, results in structural alteration of the GAG chains visualized by TEM with CB-staining (curved and in close contact with attached collagen fibrils in normal skin; linear and stretching from the outer surface of collagen fibrils to adjacent fibrils in affected skin) (Hirose et al., 2019; Kosho, Mizumoto, et al., 2019). The structural alteration in the GAG chains on decorin leads to connective tissue fragility mediated by spatial disorganization of collagen networks (Hirose et al., 2019; Kosho, Mizumoto, et al., 2019).
For mcEDS-DSE, pathophysiological studies were limited. Müller et al. (2013) reported reduced activity of DSE in cultured skin fibroblasts from a patient with a homozygous missense variant (c.308C>T:p.Ser268Leu), resulting in a marked reduction of DS disaccharides, compared with healthy control subjects. The total amount of CS in the cell fraction from the patient's fibroblasts was increased by approximately 1.5-fold, suggesting increased synthesis and/or reduced conversion of CS chains (Müller et al., 2013). To date, two DSE enzymes have been identified: the first and major one is encoded by DSE and named DS-epi1, and the second and minor one is encoded by dermatan sulfate epimerae-like and named DS-epi2 (Pacheco et al., 2009). A minor fraction of DS from decorin was found in affected fibroblasts, suggesting residual activity of DS-epi1 or compensation by DS-epi2 (Syx et al., 2015). Considering the typically milder phenotype in a patient with a possible “null” variant (c.1150_1157del:p.Pro384Trpfs*9) (Schirwani et al., 2020), compensation by DS-epi2 may be a predominant factor. Residual activity of DS-epi1 may also have contributed to the milder skin phenotype in the present patient, whose DSE protein lacks approximately 100 amino acids in the C-terminal portion, which encompasses a transmembrane domain (Pacheco et al., 2009) and is considered to interact with D4ST1, rather than bind to the Golgi membrane (Tykesson et al., 2018).
TEM performed on two adult sisters showed no apparent abnormalities in the collagen fibril architecture, although the observed layers in the skin specimens were not mentioned (Syx et al., 2015). DS was not detected in the urine of a patient reported by Lautrup et al. (2020). In the present patient, the DS moieties of CS/DS chains were significantly decreased, but still present, in the conditioned medium and cell fraction from affected cultured skin fibroblasts, consistent with the patient reported by Müller et al. (2013) and the patients reported by Syx et al. (2015). DS was not detected in urine from the present patient, similar to the report by Lautrup et al. (2020), possibly reflecting a low level of DS in the urine. The detailed pathological investigations including TEM with CB-staining, which have never been conducted in patients with mcEDS-DSE, demonstrated coexistence of normally assembled collagen fibrils and abnormally dispersed collagen fibrils, probably depending on the structure of the GAG side chains on decorin (curved GAG chains contribute to normally assembled collagen fibrils, while linear GAG chains contribute to abnormally dispersed collagen fibrils). Furthermore, considering the locations of the reported variants in DSE with six exons, three missense variants were located in exons 4 and 6 and four nonsense or frameshift variants were located in exons 5 and 6 (Supporting Information: Figure S3), suggesting that mRNA decay is unlikely to occur. Taken together, these findings suggest that the “mosaic” pattern of collagen fibril assembly may be related to the milder skin involvement in mcEDS-DSE (typically mild skin hyperextensibility with no fragility) than in mcEDS-CHST14 (typically progressive severe skin hyperextensibility with marked fragility), and the possible underlying pathophysiology could involve residual activity of DS-epi1 and/or compensation by DS-epi2 (Lautrup et al., 2020; Syx et al., 2015).
In conclusion, the 14th case with mcEDS-DSE, described herein, underwent detailed and comprehensive clinical and pathophysiological investigations that suggested a glycobiological base for the disorder and a biological role for DSE in the biosynthesis of DS.
AUTHOR CONTRIBUTIONS
Tomoki Kosho designed the study. Mari Minatogawa evaluated clinical data, reviewed reported cases, and wrote the draft with Tomoki Kosho, Takuya Hirose, and Takafumi Watanabe performed pathological experiments and interpreted the data. Shuji Mizumoto and Shuhei Yamada performed glycobiological experiments and interpreted the data. Tomomi Yamaguchi performed the molecular study. Chiai Nagae and Masashi Taki provided clinical data. The final draft was reviewed and approved by all authors.
ACKNOWLEDGMENTS
We are grateful to the patient for participating in the study, Prof. Kazushige Takehana for his support in the pathological study, and the late Prof. Kazuyuki Sugahara for his long-standing mentorship and support with kindness and encouragement for this collaborative study team. We also thank Dr. Muhammad Ilyas, MD (Center for Omic Sciences, Islamia College, University Peshawar, Pakistan) and Dr. Keng W. Teik (Genetic Department, Hospital Kuala Lumpur, Malaysia) for sharing images of their reported cases. Finally, we thank Alison Sherwin, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. The study was supported by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#19H03616) (S. M., T. Y., S. Y., T. W., and T. K.); a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (#19K07054) (S. M.); the Research Program on Policy of Measures for Intractable/Rare Diseases (20FC1046) (2020−202) (T. K.) from the Ministry of Health, Labour and Welfare, Japan; the Initiative on Rare and Undiagnosed Diseases (IRUD) (21445007) (2021−2023) (T. K.), Japan Agency for Medical Research and Development (AMED); and a Grant-in-Aid for Research Center for Pathogenesis of Intractable Diseases from the Research Institute of Meijo University (S. M. & S. Y.).
CONFLICTS OF INTEREST
T. Y. and T. K. are members of an endowed chair named “Division of Clinical Sequencing, Shinshu University School of Medicine” sponsored by BML Inc. and Life Technologies Japan Ltd. of Thermo Fisher Scientific Inc. The remaining authors declare no conflict of interest.




