Altered closed state inactivation gating in Kv4.2 channels results in developmental and epileptic encephalopathies in human patients
Abstract
Kv4.2 subunits, encoded by KCND2, serve as the pore-forming components of voltage-gated, inactivating ISA K+ channels expressed in the brain. ISA channels inactivate without opening in response to subthreshold excitatory input, temporarily increasing neuronal excitability, the back propagation of action potentials, and Ca2+ influx into dendrites, thereby regulating mechanisms of spike timing-dependent synaptic plasticity. As previously described, a de novo variant in Kv4.2, p.Val404Met, is associated with an infant-onset developmental and epileptic encephalopathy in monozygotic twin boys. The p.Val404Met variant enhances inactivation directly from closed states, but dramatically impairs inactivation after channel opening. We now report the identification of a closely related, novel, de novo variant in Kv4.2, p.Val402Leu, in a boy with an early-onset pharmacoresistant epilepsy that evolved to an epileptic aphasia syndrome (Continuous Spike Wave during Sleep Syndrome). Like p.Val404Met, the p.Val402Leu variant increases the rate of inactivation from closed states, but significantly slows inactivation after the pore opens. Although quantitatively the p.Val402Leu mutation alters channel kinetics less dramatically than p.Val404Met, our results strongly support the conclusion that p.Val402Leu and p.Val404Met cause the clinical features seen in the affected individuals and underscore the importance of closed state inactivation in ISA channels in normal brain development and function.
1 INTRODUCTION
The Kv4.2 protein is widely expressed in the brain where it serves as the pore-forming subunit in voltage-gated, A-type inactivating K+ channels (ISA) localized to the cell bodies and dendritic arbors of neurons (Jerng et al., 2004; Rhodes et al., 2004; Serôdio & Rudy, 1998). Kv4.2 is the sole Kv4 subunit expressed in hippocampal CA1 pyramidal cells in rodents (Chen et al., 2006; Menegola & Trimmer, 2006). ISA channels modify neuronal excitability in response to recent electrical activity, controlling the back propagation of action potentials and Ca2+ influx into the dendritic arbor, thereby regulating mechanisms of spike timing-dependent plasticity (Chen et al., 2006; Hoffman et al., 1997; Migliore et al., 1999; Ramakers & Storm, 2002; Truchet et al., 2012; Watanabe et al., 2002). ISA channels confer these properties on neurons due to closed state inactivation gating (Bähring & Covarrubias, 2011; Jerng et al., 2004). In contrast to N-type and C-type inactivation, which are coupled to pore opening, closed state inactivation is coupled to voltage sensor conformational changes between closed states that are evoked by depolarization (Bähring & Covarrubias, 2011; Fineberg et al., 2012; Jerng et al., 2004). As a result, ISA channels are able to inactivate without opening during subthreshold depolarizations triggered by excitatory synaptic input (Jerng & Pfaffinger, 2014; Jerng et al., 2004; Johnston et al., 2000). This inactivation temporarily increases neuronal excitability. During this interval, suprathreshold excitation increases back propagation of action potentials and Ca2+ influx into dendrites, facilitating changes in synaptic strength (Chen et al., 2006; Hoffman et al., 1997; Migliore et al., 1999; Ramakers & Storm, 2002; Truchet et al., 2012; Watanabe et al., 2002).
Closed state inactivation is mediated by interactions between the S4/S5 linker, located between the voltage sensor and pore domains, and residues in transmembrane segment S6 that form and operate the cytoplasmic gate that occludes ion flow through the pore in the closed state (Figure 1a) (Barghaan & Bähring, 2009; Jerng et al., 1999; Wollberg & Bähring, 2016). In Kv4 channels, this pore gate also prevents ion conduction in the inactivated state (Bähring & Covarrubias, 2011; Fineberg et al., 2012, 2016; Lin et al., 2018). A de novo mutation in S6, which changes valine 404 to methionine (p.Val404Met), is associated with infant-onset epilepsy, autism, and intellectual disability in monozygotic twin boys (Lee et al., 2014). Valine 404 is located in the pore gate and is critically involved in physical coupling between the voltage sensor and pore domains (Barghaan & Bähring, 2009; Jerng et al., 1999; Lin et al., 2018; Wollberg & Bähring, 2016). In a previous report, Lin et al. (2018) showed that the p.Val404Met mutation causes dominant and dramatic changes in deactivation and closed state inactivation gating. They proposed that these changes in channel function underlie the clinical phenotype by disrupting the regulation of neuronal excitability and synaptic plasticity (Lin et al., 2018).
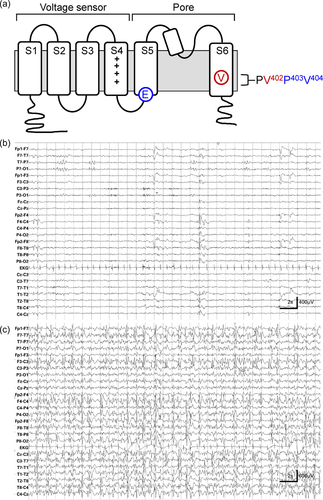
Recently, we identified a related de novo variant located two amino acids away in S6, valine 402 changed to leucine (p.Val402Leu), in a male child with intractable, early-onset pharmacoresistant seizures that evolved to an epileptic aphasia syndrome and comorbid developmental delays (KCND2 NM_012281.2:c.1204 G>C). Valine 402 is located in a highly conserved proline–valine–proline (PVP) sequence that bends to open the pore gate (Figure 1a) (Holmgren et al., 1998; Webster et al., 2004). We now report that the p.Val402Leu variant has qualitatively similar but less dramatic kinetic effects on closed state inactivation gating compared to the p.Val404Met variant. We conclude that gain of function changes in closed state inactivation in Kv4.2 are capable of inducing a developmental and epileptic encephalopathy (DEE), including an epileptic aphasia syndrome.
2 MATERIALS AND METHODS
2.1 Editorial policies and ethical considerations
All animal procedures were approved by the Chancellor's Animal Research Committee at the University of California, Los Angeles. The use of cloned human DNA sequences was approved by the Institutional Biosafety Committee at the University of California, Los Angeles.
2.2 Identification of p.Val402Leu variant and clinical tests
Genomic DNA was isolated from a blood sample in EDTA that was submitted to GeneDx in 2016. Test methods and result reporting for GeneDX EpiXpanded Panel can be found at info_sheet_EpiXpanded_Panel.pdf (genedx.com). The EpiXpanded Panel evaluates for variants in approximately 1400 genes that have been linked to epilepsy (40009_EpiXpanded-Panel-Gene-List-table-02.pdf [genedx.com]). We used the KCND2 cDNA reference transcript NM_012281.2.
2.3 Molecular biology
Plasmids encoding human wild-type Kv4.2 and the p.Val404Met mutant subunit have been described previously (Lee et al., 2014; Lin et al., 2018). A plasmid clone of human KChIP3a was kindly provided by Manuel Covarrubias, Thomas Jefferson University, Philadelphia, PA. The p.Val402Leu mutation was generated in the wild-type clone using the In-Fusion Cloning Kit (Takara Bio). The mutation was verified by sequencing. cRNA was generated in vitro using the mMessage mMachine T7 Ultra kit (Thermo Fisher Scientific).
2.4 Channel expression and functional analysis
For functional analysis of channel activity, RNA encoding wild-type Kv4.2 or the mutant p.Val402Leu was mixed at a 1:1 molar ratio with RNA encoding KChIP3a (Lin et al., 2018). The RNA mixture, 5–20 ng total, was injected into Xenopus laevis oocytes. Channel function was analyzed within 5 days postinjection using a two-electrode voltage clamp as previously described (Lin et al., 2018; Papazian et al., 1991; Timpe et al., 1988). K+ currents were recorded at 18 ± 2°C. Electrodes contained 3 M KCl and had resistances of 0.3–1.0 MΩ. The bath solution contained 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.4. Linear leak and capacitive currents were subtracted using a P/−4 protocol. Data were analyzed using the Clampfit module of pClamp software (Molecular Devices) and Origin software (Origin Labs).
2.5 Statistical analysis
Data are provided as mean ± SEM. Statistical significance was assessed by one-way analysis of variance followed by Tukey's post hoc test. Values were considered to differ significantly for p < 0.05.
2.6 Simulations
Simulations of experimental data recorded from p.Val402Leu channels were generated using IonChannelLab (De Santiago-Castillo et al., 2010) as described previously (Lin et al., 2018). Simulations were based on a kinetic gating model for Kv4.2 expressed with KChIP3a (Lin et al., 2018).
3 RESULTS
3.1 p.Val402Leu mutation in Kv4.2 is associated with a DEE
This child, a former 35-week premature infant of a twin gestation complicated by fetal demise, first came to medical attention at approximately 2 years of age due to concerns about hypotonia and gross motor delays. He did not roll over until 10 months of age but was walking by 18 months. Expressive language developed appropriately, but he did receive speech language therapy starting at 2 years due to difficulties with articulation. Due to continuing developmental concerns, he underwent neuropsychological testing at ~3 years leading to diagnosis of a nonverbal learning disorder and sensory and visual processing disorders. He had negative testing for Fragile X and a normal chromosomal microarray.
At age 4½ years, he was diagnosed with focal epilepsy after presenting with a prolonged seizure. The seizure commenced with repetitive gagging evolving to unresponsiveness, loss of tone, and deviation of the eyes to the left for which he was acutely treated with lorazepam with seizure resolution. In retrospect, seizures characterized by repetitive gagging followed by postictal sleep commenced before 1 year of age. His electroencephalogram (EEG) demonstrated the presence of independent, bilateral spike waves in the occipital region. Head imaging (computed tomography and magnetic resonance imaging) was normal. Subsequent gene panel testing (GeneDx EpiXpanded Panel) was positive for a heterozygous de novo variant c.1204 G>C in exon 2 of the KCND2 gene (NM_012281.2:c.1204 G>C) resulting in the replacement of valine at position 402 by leucine (p.Val402Leu). This variant is not observed in large population cohorts (1000 Genomes Project Consortium et al., 2015; Exome Variant Server [http://evs.gs.washington.edu/EVS]; Lek et al., 2016). Neither parent harbored this variant nor were other variants reported including GRIN2A, a gene previously linked to epileptic aphasia syndromes (Carvill et al., 2013).
Despite treatment with levetiracetam, topiramate, lamotrigine, and clobazam, he continued to have frequent focal unaware seizures with repetitive movements of the mouth and tongue (i.e., orofacial automatisms). At age 8½ years, at which time he was being treated with a combination of lacosamide, rufinamide, and valproate, he developed a worsening epileptic encephalopathy complicated by developmental regression including loss of expressive language, being fearful of sounds, loss of toileting skills, worsening oromotor coordination, food aversions, and tremoring of the hands. EEGs obtained at that time (Figure 1b,c) revealed frequent multifocal spike waves involving the left > right hemisphere which became nearly continuous during slow wave sleep with a spike wave index of >85%. He was diagnosed with Continuous Spike Wave during Sleep syndrome, which has symptomatic overlap with Landau–Kleffner Syndrome (McVicar & Shinnar, 2004), and treated with a high dose diazepam protocol (Sánchez Fernández et al., 2013) resulting in seizure cessation and improved expressive language (i.e., speaking in short sentences and expressing needs), following commands, and improved social interactions. At age 9 years, in the setting of a reduced diazepam dose, he once again regressed with word-finding difficulties and slowed language processing, loss of toileting skills, oral aversions, and hand tremors. EEG demonstrated recurrence of the continuous spike waves during sleep leading to initiation of treatment with acetazolamide. With the addition of the acetazolamide, he regained lost skills.
A repeat EEG obtained at 9½ years was normal in the wake and sleep states. When last seen at 11 years, he remained seizure free on rufinamide and acetazolamide as well as a reduced dose of valproate. In addition, he was being treated with venlaflaxine, clonidine, and extended release amphetamine for obsessive compulsive behaviors and attention deficit hyperactivity disorder. He had a slight psychomotor delay, was conversant, and was making progress at school with accommodations.
3.2 p.Val402Leu mutation slows decay of macroscopic Kv4.2 current and alters voltage dependence of activation and inactivation gating
To investigate the effects of the p.Val402Leu mutation on Kv4.2 function, the mutant subunit or wild-type Kv4.2 was expressed with KChIP3a in Xenopus oocytes for analysis using a two-electrode voltage clamp (Papazian et al., 1991; Timpe et al., 1988). Incorporation of auxiliary KChIP subunits into Kv4.2 channels removes N-type inactivation, making it feasible to study closed state inactivation in isolation (An et al., 2000; Beck et al., 2002; Pioletti et al., 2006; Wang et al., 2007). In contrast to closed state inactivation, the vestigial N-type inactivation in Kv4 channels is not thought to make a significant contribution to the physiological functions of ISA channels (Bähring & Covarrubias, 2011; Jerng et al., 2004).
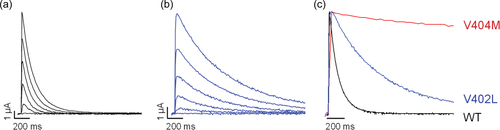
In response to a series of depolarizing voltage pulses, p.Val402Leu mutant channels produced robust currents in oocytes (Figure 2). Decay of the macroscopic current, which reflects inactivation of the channel after opening, was slowed substantially compared to the wild-type current (Figure 2a,b). However, the effect was not as large as previously reported for p.Val404Met channels (Figure 2c) (Lin et al., 2018).
The steady state properties of closed state inactivation were characterized using a prepulse inactivation protocol (Figure 3). The membrane was stepped for 1 s from the holding voltage of −110 mV to prepulse voltages ranging from −130 to −10 mV before a test pulse to +60 mV (Figure 3a). Peak current amplitude during the test pulse was normalized to the maximal value measured in the absence of a prepulse and plotted versus prepulse voltage (Figure 3b). The voltage dependence of closed state inactivation was shifted ~14 mV in the hyperpolarized direction with a small change in slope compared to the wild-type channel. This shift was nearly identical to that previously reported for p.Val404Met channels (Lin et al., 2018). However, unlike p.Val404Met, the ability of p.Val402Leu channels to inactivate was not significantly compromised at depolarized voltages that lead to pore opening (Lin et al., 2018).
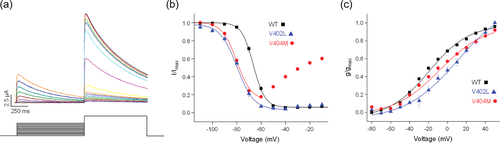
p.Val402Leu also altered the steady state properties of activation. The voltage dependence of channel opening was shifted ~30 mV in the depolarized direction with a small change in slope compared to the wild-type channel (Figure 3c). The shift in pore opening in p.Val402Leu channels was larger than the ~8 mV shift observed in p.Val404Met channels (Figure 3c) (Lin et al., 2018). Because Kv4 channels need not open before inactivating, the voltage dependence of inactivation and channel opening are not coupled (Bähring & Covarrubias, 2011).
3.3 Kinetics of current decay in p.Val402Leu channels are intermediate to wild-type and p.Val404Met channels
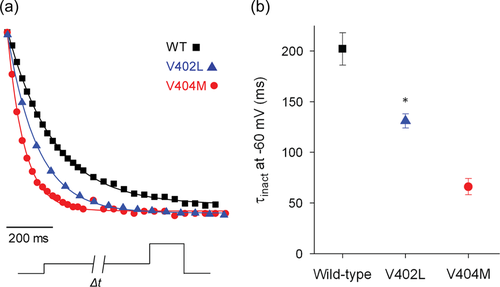
To compare access of wild-type and p.Val402Leu channels to the inactivated state after the pore has opened, a single exponential function was fitted to the decay of macroscopic currents elicited by depolarizing pulses (Figure 4a). Values of the estimated time constant of inactivation, τdecay, were plotted versus voltage (Figure 4b). Decay of the current was significantly slower in p.Val402Leu mutant channels than in wild-type over the entire tested voltage range. Values of τdecay were 3- to 5.3-fold larger in mutant channels than in wild-type.
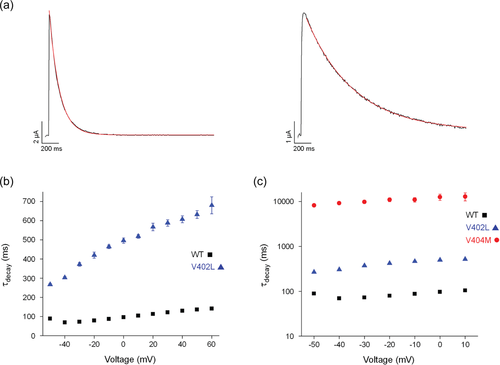
Despite the substantial slowing of decay in p.Val402Leu channels, it was significantly less than the slowing caused by the p.Val404Met mutation, as is evident when the data are shown on a log scale (Figure 4c). Values of τdecay were ~25- to 30-fold larger in p.Val404Met channels than in p.Val402Leu channels over the tested voltage range (Lin et al., 2018).
3.4 Onset of inactivation from closed state is accelerated by p.Val402Leu mutation
From the open state, entry into the inactivated state requires closing of the pore gate, which blocks ion conduction during closed state inactivation in Kv4 channels (Bähring & Covarrubias, 2011; Fineberg et al., 2012, 2016; Lin et al., 2018). The slowing of macroscopic current decay in Kv4.2 channels could therefore be due to a decreased rate of pore closing or due to slower transitions between closed states and the inactivated state (Bähring & Covarrubias, 2011; Fineberg et al., 2012, 2016; Lin et al., 2018). In p.Val404Met channels, the rate of deactivation is dramatically slowed. In addition, p.Val404Met increases the stability of the inactivated state as indicated by faster inactivation directly from the closed state and slower recovery from inactivation (Lin et al., 2018). These results indicate that the p.Val404Met mutation slows macroscopic current decay by decreasing the rate of pore closing.
To investigate whether p.Val402Leu alters the rate of transitions between the closed and inactivated states, we applied prepulses of varying durations to measure the kinetics of inactivation directly from the closed state (Figure 5). The membrane was stepped from the holding voltage of −100 to −60 mV for different durations before a test pulse to +20 mV. The peak current amplitude recorded during the test pulse was normalized to the maximal amplitude in the absence of a prepulse and plotted versus prepulse duration (Figure 5a). A single exponential component was fitted to the data to estimate the time constant of inactivation onset at −60 mV, τinact (Figure 5a,b). The kinetics of inactivation were accelerated in p.Val402Leu channels, with a time constant that was intermediate between that in wild-type and p.Val404Met channels (Figure 5b). Because the p.Val402Leu mutation increases the rate of inactivation directly from the closed state, slowing of macroscopic current decay must reflect slower closing of the pore gate.
Taken together our results indicate that the p.Val402Leu mutation has qualitatively similar but quantitatively less dramatic effects on Kv4.2 channel kinetics compared to the p.Val404Met mutation (Lin et al., 2018).
3.5 p.Val402Leu and p.Val404Met mutations alter Kv4.2 function by similar mechanisms
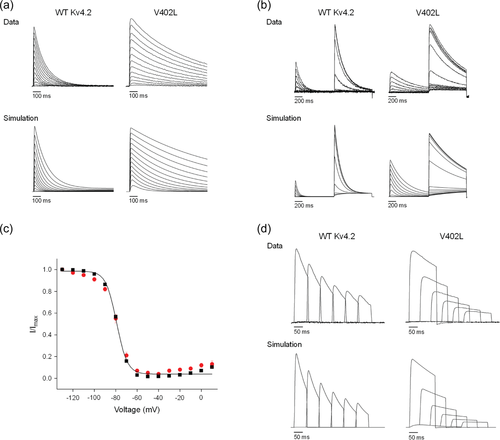
Previously, Lin et al. (2018) simulated the kinetics of Kv4.2 currents using a gating scheme with five closed states, five parallel inactivated states, and one open state (Supporting Information: Figure S2). In this model, the pore gate must close for the channel to inactivate, in accord with the available experimental data (Bähring & Covarrubias, 2011; Fineberg et al., 2012, 2016; Lin et al., 2018). To describe the coupling between voltage sensor conformational changes and entry into the inactivated state, the kinetics of inactivation onset and recovery are modified by factors of fn and 1/fn, respectively (Supporting Information: Figure S2). Lin et al. (2018) found that the functional properties of the p.Val404Met mutant channels could be reasonably well simulated by increasing the rate of pore opening, dramatically decreasing the rate of pore closing, increasing the rate of inactivation onset, and decreasing the rate of recovery from inactivation compared to wild-type Kv4.2 channels. Given the qualitatively similar but quantitatively less dramatic effects of the p.Val402Leu mutation on channel function, we investigated whether changes in the same kinetic parameters could reasonably simulate the functional properties of p.Val402Leu mutant channels (Supporting Information: Table S1). We found that intermediate values of these parameters were able to reproduce the main features of p.Val402Leu mutant channels, including the kinetics of macroscopic current decay (Figure 6a), the properties of closed state inactivation (Figure 6b,c), and the kinetics of inactivation onset at −60 mV (Figure 6d). These results support the conclusion that the p.Val402Leu and p.Val404Met mutations affect Kv4.2 function by similar mechanisms, primarily by increasing the forward bias of opening and the stability of the inactivated state.
4 DISCUSSION
4.1 Altered closed state inactivation in p.Val402Leu channels is associated with DEE and epileptic aphasia
Closed state inactivation of Kv4 channels confers on neurons the ability to modulate excitability and synaptic strength in response to recent electrical activity (Chen et al., 2006; Hoffman et al., 1997; Migliore et al., 1999; Ramakers & Storm, 2002; Truchet et al., 2012; Watanabe et al., 2002). Closed state inactivation is strongly coupled to voltage-dependent conformational changes of the voltage sensor domain during transitions between closed states (Bähring et al., 2012; Bähring & Covarrubias, 2011; Fineberg et al., 2012; Jerng et al., 2004). During this process, the voltage sensor adopts an alternative conformation that makes the pore gate “reluctant to open” (Barghaan & Bähring, 2009; Dougherty et al., 2008; Jerng et al., 2004). Structural elements involved in functional coupling between the voltage sensor domain and the gate have been identified. Residues in the S4/S5 linker interact physically with residues near the cytoplasmic end of S6 that form the gate (Barghaan & Bähring, 2009; Holmgren et al., 1998; Long et al., 2005, 2007; Webster et al., 2004). Strong evidence has been presented that the pore gate occludes ion flow in the inactivated state, in addition to its better-known role in blocking conduction in the closed state (Bähring & Covarrubias, 2011; Fineberg et al., 2012, 2016; Lin et al., 2018). As a result, after the pore opens, the gate must reclose for the channel to enter the inactivated state. This is illustrated by previously reported results with the p.Val404Met mutant channel (Lin et al., 2018). p.Val404Met dramatically slows the kinetics of pore closing, substantially impairing access to the inactivated state from the open state. Interestingly, p.Val404Met also stabilizes the inactivated state, increasing the rate of inactivation directly from the closed state and decreasing the rate of recovery from inactivation. These changes in Kv4.2 function are expected to markedly disrupt dynamic control of excitability and synaptic strength in ISA-expressing neurons, making it highly plausible that the mutation is responsible for the observed clinical phenotype of infant-onset seizures, autism, and cognitive impairment (Lee et al., 2014; Lin et al., 2018).
In this study, we tested the hypothesis that the p.Val402Leu mutation, like p.Val404Met, impairs inactivation of Kv4.2 channels after pore opening. Confirming this proposal would add strong support to the conclusion that gain of function changes in ISA inactivation underlie the clinical phenotypes associated with these mutations. Val402 is located in the middle of a highly conserved PVP motif in S6 (residues 401–403) that is critically involved in operation of the pore gate (Figure 1a). The gate is formed by the cytoplasmic ends of S6 from each of the four subunits of the channel, which interact closely in a structure described as the “bundle crossing” to occlude K+ permeation through the pore (Holmgren et al., 1998; Webster et al., 2004). To open the gate, S6 is thought to bend at the PVP motif, splaying the bundle apart, and allowing K+ flow through the pore.
We have now shown that p.Val402Leu alters inactivation gating, impairing access to the inactivated state after the pore has opened. Like p.Val404Met, p.Val402Leu slows decay of the macroscopic current. p.Val402Leu also increases the rate of inactivation directly from closed states. This result is consistent with the idea that p.Val402Leu increases the relative stability of the inactivated state. Both mutations shift the voltage dependence of activation and inactivation gating. Quantitatively, the effects of p.Val402Leu on channel kinetics are not as large as those of p.Val404Met (Lin et al., 2018), which may explain why the clinical phenotype associated with the p.Val402Leu variant is less severe.
Previously, Lin et al. (2018) showed that increased side chain volume that results from replacing valine with methionine is a key factor in altered inactivation and deactivation gating in p.Val404Met mutant channels. Structural modeling based on homology with Shaker-type channels indicates that Val404 in S6 is in atomic proximity with Glu323 in the S4/S5 linker (Heler et al., 2013; Lin et al., 2018; Zhang et al., 2021). Functional evidence indicates that this interaction is a key component in the operation of the pore gate during activation, deactivation, and closed state inactivation gating (Barghaan & Bähring, 2009; Yifrach & MacKinnon, 2002). The second site mutation p.Glu323Asp, which conserves the negative charge of the original glutamate and compensates for the increase in side chain volume at position 404 in the p.Val404Met variant, reverts the inactivation gating properties of the mutant channel to more closely resemble wild-type channels (Lin et al., 2018). Interestingly, the p.Val402Leu mutation increases side chain volume nearly as much as p.Val404Met, but the effects of p.Val402Leu on channel gating are milder than p.Val404Met. This is consistent with the results of thermodynamic double mutant cycle analysis in Kv4.2 channels, which showed that the energetic contribution of V402 to closed state inactivation is substantially less than V404 (Barghaan & Bähring, 2009).
As reported prevously, the male monozygotic twins affected by p.Val404Met also harbor de novo mutations or compound heterozygosity in the genes BICC1, SLC8A2, and GRP124, which limited complete attribution of the clinical presentation to the p.Val404Met mutation (Lee et al., 2014). Although whole exome sequencing was not performed for the child with the p.Val402Leu mutation, he was not found to have any variants in 1386 other genes that have been linked to epilepsy. Therefore, this third child and corresponding alterations in the channel's biophysics provide additional evidence that gain of function changes in closed state inactivation gating in Kv4.2 channels are capable of inducing a DEE. We propose that changes in the regulation of neuronal excitability and synaptic plasticity contribute to the clinical phenotype.
4.2 Correlations between genotype and clinical phenotype in children with Kv4.2 mutations
Our conclusions are supported by the recent findings of Bähring and colleagues (Zhang et al., 2021). They identified three additional novel mutations in KCND2 and another occurrence of the p.Val404Met variant in six unrelated individuals who presented with global developmental delays impacting motor behavior, speech, and cognition with the onset of symptoms within the first 6 months of life. The novel mutations are p.Pro403Ala and p.Val404Leu in S6 and p.Glu323Lys in the S4/S5 linker (Figure 1a). As noted above, Glu323 is within atomic proximity of Val404 in the Kv4.2 structure and involved in coupling between the voltage sensor and the pore gate (Barghaan & Bähring, 2009; Heler et al., 2013; Lin et al., 2018; Yifrach & MacKinnon, 2002; Zhang et al., 2021). Children with the p.Pro403Ala and p.Glu323Lys mutations have not had seizures although they do exhibit other neurological symptoms, as detailed by Zhang et al. (2021). In contrast, the two individuals with the p.Val404Leu variant were diagnosed with DEE like the children with the p.Val402Leu and p.Val404Met mutations. Brain malformations were noted in one of the p.Val404Leu cases and the recurrent p.Val404Met case.
To compare our results to those of Zhang et al. (2021), we will focus on experiments in which they coexpressed wild-type or mutant Kv4.2 subunits with KChIP2 because those are most similar to our experiments, in which we coexpressed Kv4.2 subunits with KChIP3a. It is important to note that the incorporation of the dipeptidyl peptidase-like (DPP) subunits DPP6 or DPP10, which are thought to coassemble with Kv4 and KChIP subunits to form ISA channels in neurons (Amarillo et al., 2008; Jerng et al., 2005; Maffie & Rudy, 2008), would modify the functional properties of mutant ISA channels. However, altered inactivation properties remain strongly evident when Kv4.2 variants are coexpressed with KChIP and DPP subunits (Lee et al., 2014; Lin et al., 2018; Zhang et al., 2021).
Functional analysis of the newly identified variants revealed qualitatively similar gain of function changes in closed state inactivation gating in the DEE-associated variants p.Val402Leu, p.Val404Met, and p.Val404Leu. All three slow the decay of the macroscopic current, indicating that they impair access to the inactivated state after the pore opens (Lin et al., 2018; Zhang et al., 2021). p.Val404Met stabilizes the inactivated state (Lin et al., 2018). p.Val402Leu accelerates inactivation from closed states (this study) and p.Val404Leu slows recovery from inactivation (Zhang et al., 2021). Although not definitive, these results are consistent with the idea that p.Val402Leu and p.Val404Leu also stabilize the inactivated state. These gain of function effects predominate in the p.Val404Leu and p.Val404Met mutations. In contrast, p.Val402Leu also has partial loss of function character because the voltage dependence of channel opening is shifted in the depolarized direction, with a value of V½,act 30 mV more positive than in wild-type.
Although p.Pro403Ala does impair access to the inactivated state after the channel opens, it is not associated with DEE (Zhang et al., 2021). This likely reflects the prominent loss of function effects of this mutation, which would substantially reduce channel activity under physiological conditions. p.Pro403Ala dramatically decreases current amplitude, slows opening, and shifts the voltage dependence of opening in the depolarized direction, with a value of V½,act 60 mV more positive than in wild-type Kv4.2. p.Glu323Lys is also a loss of function mutation with significantly decreased current amplitude (Zhang et al., 2021). The effects of p.Glu323Lys on channel kinetics are more modest than the other mutations. These results suggest that Kv4.2 mutations that are predominantly loss of function are associated with global developmental delays and additional consequential symptoms, but do not cause early-onset seizures or a DEE.
Lin et al. (2018) characterized the functional properties of another Glu323 mutation, p.Glu323Asp, which has not been found in patients. p.Glu323Asp shifts the voltage dependence of activation in the depolarized direction. It does not impair access to the inactivated state after the pore opens. If identified in patients, it would not be expected to cause a DEE.
Interestingly, mutations in other voltage-gated K+ channel genes, including KCNQ2 and KCNQ3, which encode Kv7.2 and Kv7.3, respectively, and KCNA2, which encodes Kv1.2, are also associated with DEE (Gong et al., 2021; Kessi et al., 2018; Masnada et al., 2017; Miceli et al., 2015; Syrbe et al., 2015). Several variants in these channels are clustered near the beginning of S4 and in the outer pore region. These mutations have gain of function effects due to negative shifts in the voltage dependence of activation, which increase K+ current amplitude and hyperpolarize the membrane. Mutations with combined gain and loss of function properties are also associated with a severe clinical presentation (Masnada et al., 2017). Although the DEE-associated Kv4.2 mutations are located in the voltage sensor/pore coupling region and in and near the cytoplasmic gate of the pore, the suppression of inactivation after channel opening would likewise increase K+ channel amplitude and hyperpolarize the membrane. These results suggest that gain of function mutations in several K+ channels generate seizures by similar mechanisms (Niday & Tzingounis, 2018). In contrast, mutations in Kv7.3 and Kv1.2 that are predominantly loss of function are associated with less severe clinical phenotypes (Gong et al., 2021; Masnada et al., 2017; Miceli et al., 2015; Syrbe et al., 2015).
The mechanisms by which the Kv4.2 variants p.Val402Leu, p.Val404Met, and p.Val404Leu and gain of function mutations in other K+ channels generate early-onset seizures is unknown (Niday & Tzingounis, 2018). Kv4.2 is widely expressed in the mouse brain starting early in development (Alfaro-Ruíz et al., 2019), with the highest expression in the hippocampus, cerebellum, caudate putamen, and thalamus and lower expression in the cortex and septum. In the hippocampus, Kv4.2 is highly expressed in excitatory pyramidal cells with less expression in most types of interneurons (Alfaro-Ruíz et al., 2019). Although it is difficult to predict the effects of these mutations on the emergent properties of neuronal circuits, several plausible mechanisms for seizure generation can be suggested (Niday & Tzingounis, 2018). In excitatory neurons, a negative shift in the voltage dependence of inactivation would be expected to increase the fraction of ISA channels inactivated at rest, increasing cellular excitability. Prolonged opening of ISA channels after one or more action potentials would be expected to hyperpolarize the resting membrane, increasing the availability of voltage-gated Na+ channels, which would also increase excitability. Alternatively, increased ISA current in inhibitory interneurons would be expected to decrease their activity, thus disinhibiting excitable cells in the circuit. Interestingly, Kv4.2 is prominently expressed in a particular subset of hippocampal interneurons called chandelier cells, which make axo-axonic synapses near the axon initial segment in pyramidal neurons (Niday & Tzingounis, 2018; Paul et al., 2017). Synaptic input from chandelier cells inhibits pyramidal cell firing (Woodruff et al., 2011). As a result, suppression of ISA inactivation in chandelier cells would be expected to increase pyramidal cell excitability (Niday & Tzingounis, 2018). In addition, neuroanatomical changes, which were noted by Zhang et al. (2021) in two of their DEE patients, could alter synaptic density, disrupting the excitatory/inhibitory balance, and thereby also contribute to seizure generation (Niday & Tzingounis, 2018). Using a model of the hippocampal circuit, Miceli et al. (2015) investigated the effect of KCNA2 gain of function mutations on pyramidal cell firing. They proposed that hyperexcitability results from changes in interacting components of the neuronal circuit rather than alterations in the intrinsic properties of neurons.
Similar to other children with gain of function variants impacting Kv4.2 (Lee et al., 2014; Zhang et al., 2021), the child discussed in this report initially presented during early childhood with a chief complaint of hypotonia and developmental delays with, in retrospect, presumed seizure onset before 1 year of age. Novel to this child was that his encephalopathy evolved to an epileptic aphasia syndrome responsive to high dose diazepam and acetazolamide with normalization of his EEG and freedom from seizures and improved language development starting at approximately 9 years of age, which has persisted through age 11 years. The effectiveness of benzodiazepines such as diazepam and carbonic anhydrase inhibitors such as acetazolamide in suppressing his seizures results from depression of central nervous system activity by positive allosteric modulation of GABAA receptors, and prevention of low CO2 levels and pH fluctuations, respectively (Ciccone et al., 2021; Ochoa & Kilgo, 2016). While GRIN2A mutations have been linked to epileptic aphasia syndromes (Carvill et al., 2013), these syndromes have not previously been observed in the children with Kv4.2 variants. A similar decrease in seizure frequency was observed in the monozygotic twins reported by Lee et al. (2014) at approximately the same age, which suggests that there may be developmental changes in expression, regulation, or function of Kv4.2 that will need to be explored in future experiments.
ACKNOWLEDGMENTS
We are grateful to Allan Mock and Dr. Meng-chin Lin for assistance and advice. This study was supported by a kind gift from an anonymous donor.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.




