A comprehensive molecular study identified 12 complementation groups with 56 novel FANC gene variants in Indian Fanconi anemia subjects
Abstract
Fanconi anemia (FA) is a rare autosomal or X-linked genetic disorder characterized by chromosomal breakages, congenital abnormalities, bone marrow failure (BMF), and cancer. There has been a discovery of 22 FANC genes known to be involved in the FA pathway. This wide number of pathway components makes molecular diagnosis challenging for FA. We present here the most comprehensive molecular diagnosis of FA subjects from India. We observed a high frequency (4.42 ± 1.5 breaks/metaphase) of chromosomal breakages in 181 FA subjects. The major clinical abnormalities observed were skin pigmentation (70.2%), short stature (46.4%), and skeletal abnormalities (43.1%), along with a few minor clinical abnormalities. The combination of Sanger sequencing and Next Generation Sequencing could molecularly characterize 164 (90.6%) FA patients and identified 12 different complementation groups [FANCA (56.10%), FANCG (16.46%), FANCL (12.80%), FANCD2 (4.88%), FANCJ (2.44%), FANCE (1.22%), FANCF (1.22%), FANCI (1.22%), FANCN (1.22%), FANCC (1.22%), FANCD1 (0.61%) and FANCB (0.61%)]. A total of 56 novel variants were identified in our cohort, including a hotspot variant: a deletion of exon 27 in the FANCA gene and a nonsense variant at c.787 C>T in the FANCG gene. Our comprehensive molecular findings can aid in the stratification of molecular investigation in the diagnosis and management of FA patients.
1 INTRODUCTION
Fanconi anemia (FA) is a rare, autosomal recessive, X-linked bone marrow failure (BMF) disorder with a prevalence of 1 in 160,000 live births (www.orpha.net). A higher incidence of FA has been reported in Afrikaners, Spanish Gypsies, and Ashkenazi Jews (Rosenberg et al., 2011). FA is characterized by major clinical anomalies; skin pigmentation, short stature, skeletal anomalies such as hypoplastic or supernumerary thumb, malformed forearms and hands, ocular findings—strabismus eyes, and microphthalmia. The less frequently observed are high arched palate, micrognathia, organ malformations (Glanz & Fraser, 1982). BMF is one of the major hallmarks of FA. FA patients are reported to have an early-onset of hematological malignancies such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) (Mamrak et al., 2017). The cellular phenotype of the FA is that the high frequency of chromosomal breakages is observed in cells treated with alkylating agents such as Mitomycin C (MMC) and Diepoxybutane (DEB) (Auerbach, 2015; Bhattacharjee & Nandi, 2017). However, spontaneous chromosomal breaks without treatment of alkylating agents have also been reported (Auerbach, 2015). Till date biallelic mutations in 21 genes [FANCA (MIM# 607139), FANCC (MIM#613899), FANCG (MIM#602956), FANCE (MIM# 613976), FANCF (MIM# 613897), FANCL (MIM# 608111), FANCM (MIM# 609644), FANCI (MIM# 611360), FANCD2 (MIM# 613984), FANCD1 (MIM# 600185), FANCJ (MIM# 605882), FANCN (MIM#610832), FANCO (MIM# 613390), FANCP (MIM# 613951), FANCQ (MIM# 615272), FANCR (MIM# 617244)/RAD51 (MIM# 179617), FANCS (MIM# 113705), FANCT (MIM# 616435), FANCU (MIM# 617247)/XRCC2 (MIM# 600375), FANCV (MIM# 617243)/MAD2L2 (MIM# 604094)/REV7, FANCW/RFWD3 (MIM# 614151)] and one X-linked gene [FANCB (MIM# 300515)] have been identified to cause FA phenotype (Bagby, 2018; Chandrasekharappa et al., 2013). However, FANCA, FANCG, FANCC have been reported to be common FA complementation groups. FA proteins function in the FA pathway and involve in the repair of DNA interstrand crosslinks (Wang & Smogorzewska, 2015). In this study, we describe a large cohort of FA complementation groups in the Indian population and also the genotype–phenotype correlation of FA.
2 MATERIALS AND METHODS
2.1 Editorial policies and ethical considerations
The present study was approved by the ethical committee of the ICMR–NIIH for Research on Human Subjects, and all participants gave their written consent to participate. In the case of children, assent was taken from the parents.
2.2 Recruitment of subjects
The study was carried out in 181 FA patients referred from all over the country for the diagnosis of FA. The age group ranging from 2 months to 34 years and positive for chromosomal breakage were included in the study. The average age at diagnosis was 8.9 years. All the clinical details were recorded in an intricately designed proforma, including age, clinical presentation, hematological profile, parental age, parental consanguinity, reproductive history, past history of infection, exposure to radiation, and so forth. The peripheral blood samples were collected in heparin and EDTA vacutainer from the subjects with the informed written consent.
2.3 Chromosomal breakage study
Whole blood cultures were set up in RPMI 1640 complete media stimulated with Phytohemagglutinin (PHA) and incubated for 72 h at 37°C. The cells were then induced with Mitomycin MMC (40 ng/ml) after 48 h (Oostra et al., 2012) and arrested with colchicine (50 µg/ml) at 68th hour metaphase stage, followed by hypotonic solution treatment with 0.075M potassium chloride (KCL). The cells were then fixed with Carnoy's fixative (3:1 methanol: glacial acetic acid) and were dropped on pre-chilled slides and stained with Giemsa stain. Fifty to hundred metaphases were analyzed under a bright-field microscope in each case, and the chromosomal breaks and radial forms were scored and compared with the negative control (or non-FA). Chromatid and chromosome breaks and acentric fragments were scored as one break. Dicentric and ring chromosomes were scored as two breaks. The number of breaks in the radial configurations was scored as the number of chromosomes involved in the configuration. For each patient, the chromosome damage was scored as the number of breaks per cell. A score above one break per cell was considered as being positive and selected for the study.
2.4 FANCD2 monoubiquitination by Western blot
The Western blot for detection of FANCD2 monoubiquitination was carried according to the procedure described previously (Solanki et al., 2016). Briefly, the peripheral blood mononuclear cells (PBMC) were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (Sigma) and 2 mM glutamine, penicillin, and streptomycin. Cultures were stimulated with PHA and, after 24 h, induced with MMC (40 ng/ml) and incubated in a CO2 incubator for 72 h. The cells were lysed with lysis buffer and were heated for 5 min at 96°C and loaded on 3%–8% gradient tris—acetate gel for electrophoresis. Proteins were transferred to nitrocellulose using the iBlot1Dry Blotting System (Invitrogen). The nitrocellulose membrane was blocked with 5% nonfat dried milk in TBS-T (50 mM Tris–HCl, 150 mM NaCl, 0.1% Tween20) and incubated overnight with primary anti-FANCD2 mouse monoclonal antibody diluted 1:300 followed by incubation with the secondary antibody linked to horseradish peroxidase and with ECL PLUS kit (GE Healthcare). The short or small band (S) of 155 kDa refers to the non-ubiquitinated protein, and the long or large (L) band of 162 kDa refers to the monoubiquitinated FANCD2 protein.
2.5 Molecular screening of FANC genes
2.5.1 DNA and RNA isolation
The total RNA and genomic DNA were extracted from peripheral blood collected in EDTA using QIAamp RNA blood Mini Kit (Qiagen cat no.52304) and QIAamp DNA Blood Mini Kit (Qiagen cat no. 51104), respectively. The concentration and purity of these samples were determined with a NanoDrop™ spectrophotometer.
2.5.2 Molecular screening of FANC genes
2.5.2.1 Direct sequencing
The major complementation groups (FANCA, FANCG, and FANCC) were studied by sequencing their entire coding region by polymerase chain reaction (PCR) amplification of complementary DNA (cDNA) and Sanger sequencing. The primers used are given in Table S3.
2.5.2.2 Detection of large deletions in FANCA gene by multiplex ligation-dependent probe amplification (MLPA)
The MLPA was performed for FANCA gene using SALSA MLPA kits (P031 & P032 FANCA, MRC Holland). The target DNA (patients DNA) was denatured for 5 min at 98°C, and probe mix was added and was heated for 1 min at 98°C and incubated at 60°C overnight. The mixture was incubated at 54°C for 15 min after adding ligase and subsequently inactivated at 98°C for 5 min. The ligation product was added to the PCR mix. The PCR reaction was carried out for 35 cycles (30 s at 95°C, 30 s at 60°C, and 60 s at 72°C). The fragments were analyzed on an ABI model 3130 capillary sequencer (Applied Biosystems) using gene scan-TAMRA 500 size standards (Applied Biosystems).
2.5.2.3 Next-generation sequencing (NGS)
Clinical exome (CES)/targeted exome sequencing (TES) was performed using a custom capture kit at the Med-Genome Labs Pvt Ltd, India. The libraries were prepared and sequenced on the Illumina sequencing platform with a mean coverage of 80–100X. The sequences were then aligned to the human reference genome (GRCh37/hg19) using the BWA program and analyzed using Picard and GATK version 3.6. Gene annotation of the variants was performed using the VEP program. Common variants were filtered based on allele frequency in 1000Genome Phase 3, ExAC, EVS, dbSNP147, 1000 Japanese Genome, and an internal Indian population database. Clinically relevant variants were then annotated using the literature and a set of diseases databases—ClinVar, OMIM, GWAS, HGMD, and SwissVar. The variants' effect was calculated using multiple algorithms such as PolyPhen-2, SIFT, Mutation Taster2, Mutation Assessor, and LRT. Further, these variants were classified on the basis of ACMG guidelines. The list of genes covered in the panel is given in Table S4.
2.6 Bioinformatics analysis
Primers were designed using the online available Primer 3 software version 4.1.0 (https://primer3.ut.ee/). Novel missense mutations were confirmed for their pathogenesis using online bioinformatics tools such as PolyPhen-2 (genetics.bwh.harvard.edu/pph2/), SIFT (http://sift.jcvi.org/), and Mutation Taster (www.mutationtaster.org/). Novel splice region mutations were confirmed for their potential effect on the splicing process of transcripts using Human splicing finder version 3.1 (http://www.umd.be/HSF3/). The Ensemble genome browser (https://asia.ensembl.org/index.html) and NCBI (https://www.ncbi.nlm.nih.gov/) were used for FANC gene references and Rockefeller University Fanconi anemia mutation database (http://www2.rockefeller.edu/fanconi/) was used for the confirmation of reported mutations in FANC gene. Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) tool was used for alignment of the sequenced data with reference genome for mutation screening.
2.7 Genotype–phenotype analysis
Each patient was classified for the presence or absence of at least one physical abnormality. Furthermore, they were classified by the presence or absence of VACTERL-H association (>3 of 8 VACTERL-H features) and presence or absence of PHENOS (>4 of 6 PHENOS features).
Classical congenital abnormalities described in VACTERL-H are Vertebral, Anal, Cardiac, Tracheoesophageal fistula, Esophageal atresia, Renal, upper Limb, and Hydrocephalus, and in PHENOS are Skin Pigmentation, Small Head, Small Eyes, Nervous System, Otology, Short Stature (Fiesco-Roa et al., 2019).
3 RESULTS
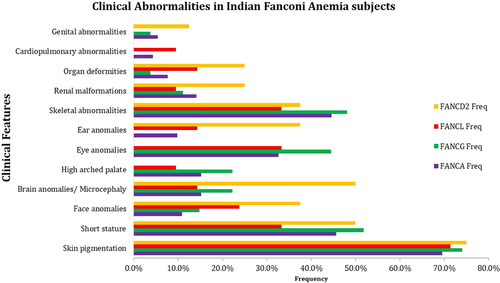
The study was carried out in 181 (n = 181) FA subjects presented with a high frequency of chromosomal breakages. The average chromosomal breakage was 4.42 ± 1.5 breaks/cell. The age group was ranging from 2 months to 34 years at the time of diagnosis, with a mean age of 8.9 years. The male: female ratio in our study was 1.2:1, with 99 males and 82 females. Parental history revealed 48.1% of subjects were born to consanguineous marriages. Hematological profile of FA subjects showed reduced hemoglobin (Hb) (6.6 g/dl), White blood count (WBC) (3.6 × 109/L), and Plateletcount (58 × 109/L) (Table S1). The clinical features are categorized as major (68.6%) and minor (31.4%) abnormalities. The overall clinical features were skin pigmentations (70.17%), short stature (46.41%), skeletal abnormalities (43.09%), eye anomalies (30.94%), brain anomalies (19.34%), renal defects (13.81%), facial anomalies (13.3%), high arched palate (14.9%), ear anomalies (11.6%) organ malformations (8.84%), genital abnormalities (6.63%), and cardiopulmonary abnormalities (6.08%).
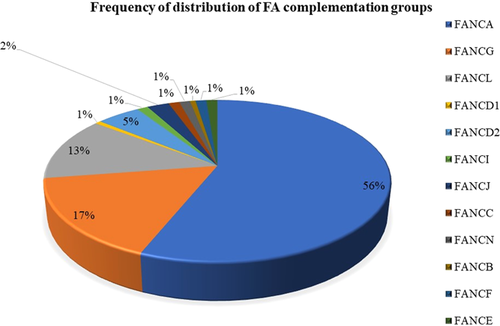
Comprehensive molecular study of 181 FA subjects revealed mutations in 164 (90.6%) FA subjects and 328 mutant alleles in 12 different complementation groups were identified (Figure 2). The major FA complementation groups in our study were FANCA (N = 92, 56.1%), FANCG (N = 27, 16.5%), and FANCL (N = 21, 12.8%) followed by the minor complementation groups FANCD2 (N = 8, 4.9%), FANCJ (N = 4, 2.4%), FANCI (N = 2, 1.2%), FANCC (N = 2, 1.2%), FANCE (N = 2, 1.2%), FANCF (N = 2, 1.2%), FANCN (N = 2, 1.20%), FANCD1 (N = 1, 0.6%), and FANCB (N = 1, 0.6%). The frequencies of homozygous and compound heterozygous mutations were 86.58% (N = 142) and 13.41% (N = 22) respectively. The common mutations of our study were missense mutations (n = 32, 26.66%), deletions (n = 27, 22.5%) and nonsense mutations (n = 23, 19.16%). The splice variants (n = 16, 13.33%), duplications (n = 4, 3.33%) and synonymous mutations (n = 1, 0.83%) were observed in lower frequencies (Table 1).
| Upstream complex defect | Downstream complex defect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutations | FANCA | FANCG | FANC L | FANCB | FANCC | FANCE | FANCF | FANC I | FANCN | FANCD2 | FANC J | FANCD1 | Total (n) |
Large deletions (N = 36) |
26 | - | - | - | - | - | - | - | - | - | 1 | - | 27 |
Missense (N = 40) |
18 | 2 | - | - | - | 1 | - | 1 | 3 | 4 | 3 | - | 32 |
Nonsense (N = 38) |
9 | 7 | - | - | 2 | - | 1 | 2 | - | 1 | 1 | - | 23 |
| Microdeletions (N = 20) | 11 | 3 | - | - | - | - | 1 | - | - | 1 | - | 1 | 17 |
Duplication (N = 6) |
3 | 1 | - | - | - | - | - | - | - | - | - | - | 4 |
| Synonymous mutation leading to aberrant splicing (N = 21) | 0 | - | 1 | - | - | - | - | - | - | - | - | - | 1 |
Splice Donor site (N = 18) |
4 | 2 | - | 1 | - | - | - | - | - | 2 | - | - | 9 |
| Splice Acceptor site (N = 7) | 5 | - | - | - | - | - | - | - | - | 2 | - | - | 7 |
Novel variants (N = 77) |
30 | 9 | - | 1 | 1 | 1 | 1 | 2 | - | 9 | 1 | 1 | 56 |
Reported mutations (N = 109) |
46 | 6 | 1 | - | 1 | - | 1 | 1 | 3 | 1 | 4 | - | 64 |
| Total no of mutations identified in 164 patients | 76 | 15 | 1 | 1 | 2 | 1 | 2 | 3 | 3 | 10 | 5 | 1 | 120 |
- Note: Total of 164 subjects with biallelic mutations was identified. 142 subjects were with homozygous mutations and 22 had compound heterozygous mutations. Variants in compound heterozygous patients are considered as different mutations therefore a total of 186 variants were identified from the 164 patients. Patients harboring the same mutations are reported as a single variant therefore 120 variants were identified.
- Bold typeface, novel mutations; N, number of patients, n, number of mutations.
In the present study, a high frequency (n = 56,46.6%) of novel variants were identified in 10 of the 12 different FA complementation groups (Table 1). Among the FANCA subjects (N = 92), 76 pathogenic mutations were identified, out of which 30 were accounted as novel variants. Homozygous mutations were observed in 80 FANCA subjects, while 12 subjects had compound heterozygous mutations. The most commonly observed mutations were deletions (n = 37/76) including large deletions (n = 26) and micro-deletions (n = 11). Other mutations observed in our study were missense mutations (n = 18/76), nonsense mutations (n = 9/76), and splice variants (n = 9/76). We also observed two hotspot variants in the FANCA gene, a novel homozygous deletion of exon 27 in 6 patients and a reported missense variant in exon 29 at c.2786A>C in 4 patients (Figure S1a,b). The pathogenic mutations (n = 15) identified in the second most frequent complementation group FANCG (N = 27), include 7 nonsense mutations, 2 missense mutations, 3 micro-deletions, 2 splice donor site mutations, and 1 duplication. Of these 15 pathogenic variants identified in FANCG patients, 9 were novel variants. A novel nonsense variant in exon 7 of the FANCG gene at c.787C>T was observed in nine unrelated patients making it a novel hotspot variant observed in our cohort (Figure S1c).
A reported synonymous mutation c.1092 G>A in exon 13 in the FANCL complementation group causing aberrant splicing was identified in 21 (12.8%) of the FA subjects. FANCD2 mutations were identified in 8 subjects; and 10 pathogenic variants (9 novel variants and 1 reported variant) were identified. These variants include 4 missense variants, 1 nonsense variant, 2 splice donor site variants, 2 splice acceptor site variants, and 1 deletion.FANCJ complementation group was identified in 4 (2.4%) FA subjects with 5 pathogenic variants (4 reported mutations and 1 novel variant). Two patients harbored the same homozygous nonsense mutation, one had a compound heterozygous mutation (a deletion and a missense mutation), and the other one had 2 missense compound heterozygous mutations. Two FA subjects each from FANCC, FANCE, FANCF, FANCI, and FANCN complementation groups were detected. One patient from each FANCD1 (microdeletion-c.484delG) and FANCB (splice donor site mutation—c.2165+2T>G) were identified in our cohort (Tables 2 and S2). The mutations were not identified in 17 (9.39%) FA subjects in our current study. Molecular analysis of the parents revealed heterozygous mutations in all the subjects.
| Gene | Exon | cDNA position | Protein change | Type of mutation | Polyphen /SIFT/mutation taster 2 | ACMG guidelines | No. of alleles | No. of patients | Reference | LOVD ID/CLINVAR ID/RSID |
|---|---|---|---|---|---|---|---|---|---|---|
| FANCA | 1 | c.15G>A | p.Trp5Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | Mori et al. (2019) | |
| FANCA | 4 | c.416_417 del | p.Val139GlyfsTer41 | Microdeletion | Damaging | Pathogenic | 2 | 1 | FANCA_000024 | |
| FANCA | 11 | c.964C>T | p.His322Tyr | Missense | Damaging | VUS | 2 | 1 | Monies et al. (2017) | rs772768595 |
| FANCA | 13 | c.1169T>G | p.Leu390Arg | Missense | VUS | 1 | 1 | |||
| FANCA | 14 | c.1344T>G | p.Tyr448Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | ||
| FANCA | 19 | c.1750 del | p.Leu584SerfsTer21 | Microdeletion | Damaging | Pathogenic | 2 | 1 | ||
| FANCA | 20 | c.1795delT | p.Ser599fsTer5 | Microdeletion | Damaging | VUS | 2 | 1 | ||
| FANCA | 25 | c.2265dupA | p.Arg756ThrfsTer | dup | Damaging | Pathogenic | 2 | 1 | ||
| FANCA | 26 | c.2368delC | p.His790ThrfsTer33 | Microdeletion | Damaging | Pathogenic | 2 | 1 | ||
| FANCA | 27 | c.(3505+1_3506-1)_(2601+1_2607-1) | del | VUS | 11 | 6 | ||||
| FANCA | 27 | c.2559_2564delAGATAC | p.Arg853fsTer11 | del | Damaging | Likely pathogenic | 2 | 1 | ||
| FANCA | 28 | c.2656G>T | p.Glu886Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | ||
| FANCA | 29 | c.2786A>C | p.Tyr929Ser | Missense | Damaging | VUS | 9 | 5 | Arthur et al. (2014) | |
| FANCA | 30 | c.2894_2895delCT | p.Pro965ArgfsTer8 | Microdeletion | Damaging | VUS | 2 | 1 | Nie et al. (2020) | |
| FANCA | 32 | c.3142_3147 del | p.Leu1048_Phe1049 del | del | Likely pathogenic | 1 | 1 | |||
| FANCA | 33 | c.3282G>C | p.Arg1084P | Missense | Damaging | Pathogenic | 1 | 1 | ||
| FANCA | 38 | c.3788T>C | p.Phe1263S | Missense | Damaging | VUS | 1 | 1 | ||
| FANCA | 38 | c.3792_3794 del | p.Leu1265del | Microdeletion | Damaging | VUS | 2 | 1 | ||
| FANCA | 41 | c.4085T>A | p.Leu1362Ter | Nonsense | VUS | 1 | 1 | |||
| FANCA | 42 | c.4185dupG | p.Ile1396GlnfsTer29 | dup | Damaging | Pathogenic | 5 | 3 | ||
| FANCA | 4 to 17 | c.(283+1_284-1)_(1626+1_1627-1) del | del | Pathogenic | 2 | 1 | ||||
| FANCA | 12 to 43 | c.(1018+1_1019-1)_*580del | del | Pathogenic | 2 | 1 | ||||
| FANCA | 11- 30 | c.(873+1_874-1)_(2981+1_2982-1)del | del | Pathogenic | 2 | 1 | ||||
| FANCA | 11 to 22 | c.(893+1_894-1)_(2014+1_2015-1) | del | Pathogenic | 1 | 1 | ||||
| FANCA | 16 to 29 | c.(1470+1_1471-1)_(2852+1_2853-1) del | del | Pathogenic | 2 | 1 | ||||
| FANCA | 29 to 43 | c.(2852+1_2853-1)_*580del | del | Pathogenic | 1 | 1 | ||||
| FANCA | 18 to 27 | c. (1625+1_1626-1)_(2601+1_2602-1) | del | Pathogenic | 1 | 1 | ||||
| FANCA | 22-32 | c.(1900+1_1901-1)_(3239+1_3240-1)del | del | Likely pathogenic | 2 | 1 | ||||
| FANCA | 4-8 | c.(283+1_284-1)_(709+1_710-1) del | del | Likely pathogenic | 2 | 1 | LOVD FANCA_000056 | |||
| FANCA | 8-10 | c.(709+1_710-1)_(893+1_894-1)del | del | Likely pathogenic | 2 | 1 | ||||
| FANCA | IVS 32 | c.3239+1dupG | p.Ile1081AspfsTer35 | dup | Damaging | VUS | 1 | 1 | ||
| FANCA | IVS 32 | c.3239+2 T>G | p.Glu1023AspfsTer35 | Splice donor site | Damaging | Likely pathogenic | 2 | 1 | Bogliolo et al. (2020) | |
| FANCA | IVS 34 | c.3409-2A>C | 3' Splice site | Splice site acceptor | VUS | 2 | 1 | |||
| FANCA | IVS 9 | c.826+2T>C | Splice donor site | Damaging | VUS | 2 | 1 | |||
| FANCA | 39 | c.3884T>C | p.Leu1295Ser | Missense | VUS | 1 | 1 | |||
| FANCA | IVS 21 | c.1901-3C>G | (3' splice site) | Splice site acceptor | VUS | 1 | 1 | |||
| FANCB | 9 | c.2165+2T>G | Splice donor site | Damaging | VUS | 2 | 1 | |||
| FANCC | 2 | c.70C>T | p.Gln24Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | ||
| FANCD1 | 6 | c.484delG | p.Ser163ValfsTer9 | Microdeletion | Damaging | Pathogenic | 2 | 1 | ||
| FANCD2 | 7 | c.473C>G | p.Pro158Arg | Missense | Damaging | VUS | 2 | 1 | ||
| FANCD2 | 15 | c.1222C>T | p.Arg408Ter | Missense | 1 | 1 | ||||
| FANCD2 | 38 | c.3796_3798del | p.Leu1266del | Microdeletion | Damaging | VUS | 2 | 1 | ||
| FANCD2 | 38 | c.3817C>T | p.Arg1273Ter | Nonsense | Damaging | 1 | 1 | |||
| FANCD2 | IVS 8 | c.571-3C>G | 3' splice site | Splice site acceptor | Damaging | VUS | 2 | 1 | ||
| FANCD2 | IVS 30 | c.2976+5G>A | In-frame deletion of 39 amino acid | Splice donor site | VUS | 3 | 2 | |||
| FANCD2 | IVS16 | c.1413+2T>A | Splice donor site | Damaging | VUS | 1 | 1 | |||
| FANCD2 | IVS 3 | c.206-1G>T | Splice site acceptor | Damaging | Likely pathogenic | 2 | 1 | |||
| FANCD2 | 25 | c. 2361 T>G | p. Phe787Leu | Missense | VUS | 1 | 1 | |||
| FANCD2 | 44 | c.4338T>G | p.Ser1446Arg | Missense | VUS | 1 | 1 | |||
| FANCE | 2 | c.491T>C | p.Leu164Pro | Missense | Damaging | VUS | 4 | 2 | ||
| FANCF | 1 | c.594-595 del GA | p.Asn199GlnfsTer66 | Microdeletion | Damaging | Pathogenic | 2 | 1 | ||
| FANCG | 4 | c.346C>T | p.Gln116Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | ||
| FANCG | 7 | c.787C>T | p.Gln263Ter | Nonsense | Damaging | Pathogenic | 18 | 9 | ||
| FANCG | 10 | c.1252 G>T | p.Glu418Ter | Nonsense | Damaging | Likely pathogenic | 4 | 2 | ||
| FANCG | 10 | c.1375C>T | p.Gln459Ter | Nonsense | Likely pathogenic | 2 | 1 | |||
| FANCG | 10 | c.1385C>A | p.Ala462Asp | Missense | Damaging | VUS | 2 | 1 | ||
| FANCG | 11 | c.1468G>T | p.Glu490Ter | Nonsense | Damaging | Pathogenic | 4 | 2 | ||
| FANCG | 12 | c.1501C>T | p.Gln501Ter | Nonsense | Damaging | Pathogenic and VUS | 4 | 3 | ||
| FANCG | 12 | c.1572 G>A | p.Trp524Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | ||
| FANCG | IVS8 | c.1076+3_1076+7 del GAGGT | Microdeletion | Damaging | VUS | 2 | 1 | |||
| FANCI | 5 | c.295del | p.His99IlefsTer10 | Nonsense | Damaging | Pathogenic | 1 | 1 | ||
| FANCI | 37 | c.3907G>T | p.Glu1303Ter | Nonsense | Pathogenic | 1 | 1 | |||
| FANCJ | 1 | deletion | del | Pathogenic | 1 | 1 | ||||
| FANCA | 5 | c.(426+1_427-1)_(522+1_523-1)del | del | Pathogenic | 2 | 1 | Morgan et al. (1999) | FANCA_000027 | ||
| FANCA | 6 | c.(522+1_523-1)_(596+1_597-1)del | del | Pathogenic | 4 | 2 | Morgan et al. (1999) | FANCA_000076 | ||
| FANCA | 11 | c.987_990delTCAC | p.His330AlafsTer4 | Microdeletion | Damaging | Pathogenic | 5 | 3 | Morgan et al. (1999) | FANCA_000080 |
| FANCA | 11 | c.(893+1_894-1)_(1006+1_1007-1) | del | Pathogenic | 6 | 3 | Solanki et al. (2016) | |||
| FANCA | 13 | c.1144C>T | p.Gln382Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | N. Li et al. (2018), Q. Li et al. (2017), Sun et al. (2017) | rs769718381 |
| FANCA | 14 | c.1235C>T | p.Ala412Val | Missense | VUS | 2 | 1 | |||
| FANCA | 14 | c.1273G>C | p.Glu425His | Missense | Damaging | Likely pathogenic | 2 | 1 | Solanki et al. (2016) | |
| FANCA | 14 | c.1303C>T | p.Arg435Cys | Missense | Damaging | Pathogenic | 1 | 1 | Adachi (2002), Tachibana et al. (1999), Yagasaki et al. (2004) | rs148473140 |
| FANCA | 14 | c.1304G>A | p.Arg435His | Missense | Damaging | Likely pathogenic | 2 | 1 | Moghrabi et al. (2009) | FANCA_000599 |
| FANCA | 24 | c.2182C>T | p.Gln728Ter | Nonsense | Damaging | Likely pathogenic | 4 | 2 | Solanki et al. (2016) | |
| FANCA | 26 | c.2500delC | p.Leu834fsTer1 | Microdeletion | damaging | Likely pathogenic | 4 | 2 | Solanki et al. (2016) | |
| FANCA | 27 | c.2574C>G | p.Ser858Arg | Missense | Damaging | Pathogenic | 4 | 2 | Savino et al. (2003), Tamary et al. (2000), Wijker et al. (1999) | FANCA_000182 |
| FANCA | 28 | c.2749C>T | p.Arg917Ter | Nonsense | Damaging | Pathogenic | 3 | 2 | Kimble et al. (2018), Moghrabi et al. (2009), Solanki et al. (2016) | rs1060501880 |
| FANCA | 29 | c.2786A>C | p.Tyr929Ser | Missense | Damaging | VUS | 9 | 5 | ||
| FANCA | 29 | c.2851C>T | p.Arg951Trp | Missense | Damaging | Pathogenic | 4 | 2 | Gille et al. (2012), Solanki et al. (2016) | FANCA_000205 |
| FANCA | 29 | c.2852G>A | p.Arg951Gln | Missense | Damaging | Pathogenic | 1 | 1 | Gille et al. (2012), Solanki et al. (2016) | FANCA_000205 |
| FANCA | 30 | c.(2852+1_2853-1)_(2981+1_2982-1)del | del | Pathogenic | 4 | 2 | Yagasaki et al. (2004) | FANCA_000413 | ||
| FANCA | 31 | c.(2981+1_2982-1)_(3066+1_3067-1)del | del | Pathogenic | 2 | 1 | Wijker et al. (1999) | FANCA_000210 | ||
| FANCA | 32 | c.3189G>A | p.Trp1063Ter | Nonsense | Damaging | Likely pathogenic | 2 | 1 | Moghrabi et al. (2009), Solanki et al. (2016) | VCV001076167 |
| FANCA | 32 | c.3239G>A | p.Arg1080Gln | Missense | Pathogenic | 3 | 2 | FANCA_000561 | ||
| FANCA | 36 | c.3538G>A | p.Val1180Met | Missense | Damaging | VUS | 2 | 1 | rs372706571 | |
| FANCA | 37 | c.3677C>G | p.Ser1226Ter | Nonsense | Damaging | Likely pathogenic | 2 | 1 | Solanki et al. (2016) | |
| FANCA | 37 | c.3679G>C | p.Ala1227Pro | Missense | Damaging | VUS | 2 | 1 | Solanki et al. (2016) | |
| FANCA | 37 | c.3745delC | p.Leu1249TrpfsTer9 | Microdeletion | Damaging | Likely pathogenic | 2 | 1 | rs1555535472 | |
| FANCA | 37 | c.3760delGA | p.Glu1254GlyfsTer23 | Microdeletion | Pathogenic | 2 | 1 | Arthur et al. (2014), Solomon et al. (2015) | FANCA_000264 | |
| FANCA | 39 | c.3926_3929delCAGA | p.Thr1309Argfs52 | Microdeletion | Damaging | Pathogenic | 2 | 1 | FANCA_000520 | |
| FANCA | 42 | c.4199G>C | p.Arg1400Pro | Missense | Damaging | Likely pathogenic and VUS | 1 | 1 | Pilonetto et al. (2017) | FANCA_000649 |
| FANCA | 1 to 43 | c.-4617_*580del | del | pathogenic | 2 | 1 | Flynn et al. (2014) | FANCA_000693 | ||
| FANCA | 1 to 8 | c.(?_-42)_(792+1_793-1)del | del | Pathogenic | 2 | 1 | Gille et al. (2012) | FANCA_000033 | ||
| FANCA | 15 to 29 | c.(1359+1_1360-1)_(2778+1_2779-1)del | del | Pathogenic | 2 | 1 | Morgan et al. (1999) | FANCA_000643 | ||
| FANCA | 16 to 17 | c.(1470+1_1471-1)_(1626+1_1627-1)del | del | Pathogenic | 2 | 1 | Morgan et al. (1999) | FANCA_000109 | ||
| FANCA | 1to22 | c.(?_−42)_(1900+1_1901-1)del | del | Likely pathogenic | 2 | 1 | Morgan et al. (1999) | FANCA_000005 | ||
| FANCA | 8-27 | c.(709+1_710-1)_(2601+1_2602-1)del | del | Pathogenic | 2 | 1 | Morgan et al. (1999), Shukla et al. (2013) | FANCA_000592 | ||
| FANCA | 1 to 30 | c.(?_−1)_(2981+1_2982-1) del | del | Likely pathogenic | 2 | 1 | Wijker et al. (1999) | FANCA_000011 | ||
| FANCA | 7 to 11 | c.(596+1_597-1)_(1006+1_1007-1)del | del | Likely pathogenic | 1 | 1 | ||||
| FANCA | IVS 31 | c.3066+1G>T | p.Ser994ArgfsTer3 | Splice donor site | Damaging | Likely pathogenic | 6 | 3 | Solomon et al. (2015) | rs587783028 |
| FANCA | IVS 32 | c.3240-1G>A | Splice site acceptor | Likely pathogenic | 2 | 1 | Levran et al. (2005), Moghrabi et al. (2009), Zhang et al. (2015) | VCV000847757 | ||
| FANCA | IVS 39 | c.3934+2T>C | p.Ser1277ThrfsTer51 | Splice donor site | Damaging | Pathogenic | 6 | 4 | Yagasaki et al. (2004) | FANCA_000456 |
| FANCA | IVS 8 | c.793-3C>G | 3' splice site | Splice site acceptor | Damaging | VUS | 2 | 1 | Zhang et al. (2015) | FANCA_000101 |
| FANCA | IVS 9 | c.827-1G>A | 3' Splice site | Splice site acceptor | Damaging | Pathogenic | 2 | 1 | Yagasaki et al. (2004) | FANCA_000063 |
| FANCA | 33 | c.3263C>T | p.Ser1088Phe | Missense | VUS | 1 | 1 | |||
| FANCC | 15 | c.1642C>T | p.Arg548Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | Aftab et al. (2017), Ten Foe et al. (1996), Murer-Orlando et al. (1993) | rs104886457 |
| FANCF | 1 | c.496C>T | p.Gln166Ter | Nonsense | Damaging | Pathogenic | 2 | 1 | Chandrasekharappa et al. (2004) | FANCF_000010 |
| FANCG | 1 | c.77A>G | P.Gln26Arg | Missense | Probably damaging | VUS | 1 | 1 | Auerbach et al. (2003), Demuth et al. (2000), Morgan (2005) | rs200677800 & FANCG_000070 |
| FANCG | 5 | c.637_643delTACCGCC | p.Tyr213LysfsTer7 | Microdeletion | Damaging | Pathogenic | 2 | 1 | Morgan (2005) | FANCG_000037 |
| FANCG | 7 | c.883dupG | p. Asp295GlyfsTer14 | Duplication | Damaging | Pathogenic | 2 | 1 | Solanki et al. (2017) | FANCG_000069 |
| FANCG | 11 | c.1471_1473delAAAinsG | p. Lys491GlyfsTer9 | Microdeletion | Damaging | Pathogenic | 2 | 1 | Solanki et al. (2017) | FANCG_000054 |
| FANCG | IVS12 | c.1636+7A>G | SKIPPING OF EXON 12 | Splice donor site | Damaging | VUS | 5 | 4 | Auerbach et al. (2003), Demuth et al. (2000) | FANCG_000070 |
| FANCG | IVS9 | c.1143 + 5G > C | p. Arg359SerfsTer22 | Splice donor site | Damaging | Pathogenic | 2 | 1 | FANCG_000068 | |
| FANCI | 18 | c.1813C>T | p.Leu605Phe | Missense | Damaging | VUS | 2 | 1 | FANCI_000041 | |
| FANCJ | 15 | c.2119C>T | p.Arg707Cys | Missense | Damaging | VUS and pathogenic | 1 | 1 | Ghazwani et al. (2016), Levitus et al. (2005) | BRIP1_000005 & BRIP1_000007 |
| FANCJ | 16 | c.2329C>T | p.Arg777Cys | Missense | 1 | 1 | ||||
| FANCJ | 17 | c.2392C>T | p.Arg798Ter | Nonsense | Damaging | Pathogenic | 4 | 2 | Ghazwani et al. (2016) | BRIP1_000007 |
| FANCJ | 20 | c.3103C>T | p.Arg1035Cys | Missense | Damaging | VUS | 1 | 1 | BRIP1_000048 | |
| FANCL | 13 | c.1092G>A | p.Lys364Lys | Synonymous¶ | Damaging | Pathogenic | 42 | 21 | Chandrasekharappa et al. (2013), Donovan et al. (2019) | FANCL_000005 |
| FANCN | 4 | c.560C>A | p.Pro187His | Missense | Probably damaging | VUS | 1 | 1 | rs371582757 & rs1246969208 | |
| FANCN | 5 | c.1739A>G | p.Tyr580Cys | Missense | Damaging | VUS | 1 | 1 | ||
| FANCN | 13 | c.3415A>G | p.Ile1139Leu | Missense | Damaging | VUS | 2 | 1 | rs1249960937 |
- Note: FANCA (ENST00000389301.3, NM_000135, NP_000126); FANCB (ENST00000398334.1, NM_001018113, NP_001018123); FANCC (ENST00000289081.3, NM_000136, NP_000127); FANCD1 (ENST00000544455.1, NM_000059, NP_000050); FANCD2 (ENST00000287647.3, NM_033084, NP_149075); FANCE (ENST00000229769.2, NM_021922, NP_068741); FANCF (ENST00000327470.3, NM_022725, NP_073562). FANCG (ENST00000378643.3, NM_004629, NP_004620); FANCI (ENST00000310775.7, NM_001113378, NP_001106849); FANCJ (ENST00000259008.2, NM_032043, NP_114432); FANCL (ENST00000402135.3, NM_001114636, NP_001108108); FANCN (ENST00000261584.4, NM_024675, NP_078951); cDNA numbering is based on c.1 being the A of the ATG translation initiation codon.
- Bold typeface, novel mutation; plain typeface, known mutation; ¶ denotes synonymous mutation leading to aberrant splicing.
The clinical correlation with the genotype revealed 157 subjects with at least one clinical abnormality irrespective of the complementation group. Comparison of major complementation groups (FANCA, FANCG, FANCL, and FANCD2) revealed no significant difference in the frequency of abnormalities observed. However, slightly severe anomalies were observed in FANCG and FANCD2 subjects (Figure 1). Phenotype analysis by VACTERL-H and PHENOS categorization, revealed 4 (2.2%) patients with VACTERL-H (≥3 out of 8 VACTERL-H features) belonging to FANCI (1/2, 50%), FANCL (1/21, 4.76%), and FANCA (1/95, 1.05%) complementation groups. Fifty-two (n = 52, 28.2%) subjects categorized into PHENOS (≥3 out 6 PHENOS features) were associated with FANCA (28/95, 29.5%), FANCD2 (3/8, 37.5%), FANCF (1/2, 50%), FANCI (1/2, 50%),FANCJ (2/4, 50%), FANCG (8/27, 29.6), and FANCL (7/21, 33.4%). The high arched palate, ear anomalies, and dysmorphic facies were observed as minor clinical abnormalities along with cardiovascular abnormalities and genital abnormalities. The high arched palate was observed in FANCE (1/2, 50%), FANCI (1/2, 50%), FANCJ (1/4, 25%), FANCG (6/27, 24%), FANCA (15/95, 16.3%), and FANCL(2/21, 10%) complementation groups. Ear anomalies were observed in FANCA (10/95, 10.9%), FANCD2 (3/8, 37.5%), FANCJ (2/4, 50%), and FANCL (3/20, 15%). FA dysmorphic facies were observed in FANCA (10/95, 10.9%), FANCD2 (1/8, 12.5%), FANCG (4/27, 16%), FANCI (1/4 25%), and FANCL (4/20, 20%). FANCD1 did not show any particular phenotype, and only organ abnormality was observed in FANCN complementation groups.
4 DISCUSSION
BMF is one of the classical characteristic features of FA subjects (Parinda A. Mehta et al., 2002). Although studies reported FA presentation at adult age, BMF occurs in the first decade of life (Garaycoechea & Patel, 2014). In the present study, the mean age of FA subjects was8.9 years, which is similar to the reported literature (Czechowicz et al., 2020). The clinical diagnosis of FA subjects was challenging as major and minor clinical features have been reported (Alter & Rosenberg, 2013; Faivre et al., 2005). Recently, Fiesco-Roa et al. (2019) described that the most common abnormalities are part of either VACTERL-H or PHENOS in FA subjects. In the present study, the clinical features observed were similar to the frequencies of clinical anomalies reported in the literature (Fiesco-Roa et al., 2019). The most commonly observed clinical abnormalities in our cohort were skin pigmentation, short stature, and skeletal abnormalities. However, though most FA subjects (86.9%) presented with clinical anomalies, the chromosomal breakage analysis is a gold standard for the diagnosis of FA as 93.5% of FA subjects presented with a high frequency of chromosomal breakages in our study(Auerbach, 2015). However, 11(6.4%) FA subjects were without chromosomal breaks. The subjects without chromosomal breakages had typical FA phenotypes, and we could molecularly characterize these patients to have FANC gene mutations. The absence or very low number of chromosomal breakages could be due to undetected somatic mosaicism. The molecular pathology of FA is well understood (Kee & Alan, 2012). To date, 22 genes are known to be associated with FA phenotype (Ceccaldi et al., 2016; Nepal et al., 2017). However, studies on FA complementation groups are not much known from the Indian subcontinent. Our lab is the first to be reporting FA cases from India (Donovan et al., 2019; Shukla et al., 2013; Solanki et al., 2016, 2017). This is the first study in a large cohort (n = 181) of FA subjects from India. Using recent molecular technology, we could identify 12 complementation groups in 164 (90.6%) out of the 181 FA subjects. The major complementation groups in our study are FA-A (56.1%), FA-G (16.5%), and FA-L (12.8%). FANCA gene accounts for more than 65% of all the mutations in FA patients worldwide. The FANCA gene has 43 exons, and the database reported mutations all over the gene. The current study had identified 92 (56.1%) subjects with 76 FANCA pathogenic variants; among these 30 variants were novel variants (Tables 2 and S2). Among these, two mutations, a homozygous deletion of exon 27 and a missense mutation in exon 29 at c.2786A>C were found to be hotspot mutations that need to be studied in a large cohort and should be followed up to understand the progression of the disease. The hotspot mutations in FANCA have been reported in the literature. Hotspot deletions, c.3788_3790delTCT, and c.1115_1118delTTGG were identified in the Brazilian population and Spanish Gypsies, respectively. Also, c. 295C>T, a nonsense mutation was commonly seen in the Spanish gypsies (Castella et al., 2011). The novel founder mutation c.3446_3449dupCCCT has been reported in the FANCA gene in the Romani population (Dimishkovska et al., 2018). In the Korean population, c.2546delC and c.3720_3724delAAACA mutations were reported to be hotspot mutations (Park et al., 2013). An intragenic deletion of exon 12-31 was also reported as a hotspot mutation in the Afrikaner population (Tipping et al., 2001).
According to the literature, the other frequently occurring complementation groups are FANCG and FANCC (Chandrasekharappa et al., 2013; Joenje & Patel., 2001; Wang & Smogorzewska, 2015). In our cohort also we could observe FA-G (N = 27) as the second frequently occurring complementation group. FANCG spans 14 exons, and mutations are reported all over the gene. A novel nonsense variant in exon 7 of FANCG c.787 C>T was seen in 9 FA subjects, making it a hotspot mutation in our cohort. Similar to the FANCA gene, hotspot mutations in FANCG gene have also been reported in other populations as well. A nonsense mutation, c.313G>T and a frameshift deletion, c.1794_1830del was reported in the European population, a splice variant, c.307+1G>C in the Japanese population. A seven base pair deletion mutation, c.637_643delTACCGCC was observed in almost 80% of the sub-Saharan African population (Feben et al., 2015; Gille et al., 2012; Yagasaki et al., 2003). FA-C is reported as one of the major complementation groups in the United States (Verlander et al., 1994), however in other western countries (Spain, France, Italy, and Germany), it is reported at a very low frequency, similar to what we have observed in our cohort. We could identify only 2 cases (1.2%), including a reported nonsense c.1642C>T mutation in exon 15 and a novel mutation, c.70C>T in exon 2 of the FANCC gene. The founder FANCC IVS 4+4A>T mutation has been reported in Ashkenazi Jews and the Pakistani population (Aftab et al., 2017). Although FANCC gene mutation was reported by Makoto et al. in the Japanese population with a mild phenotype, their recent studies showed a much less frequency ofFA-C complementation group in their population (Futaki et al., 2000; Mori et al., 2019). Park et al. in 2013 reported the absence of FANCC in the Korean population (Park et al., 2013). However, in China, Li et al. reported only one patient with FANCC mutations in their cohort (Li et al., 2018). This suggests that FANCC might be present at a very low frequency in the Asian population. However, in our cohort, FA-L was found to be one of the major complementation groups (21, 12.6%). FANCL is reported at a very low frequency in the literature. Commonly reported mutations in FANCL are c.1096_1099dup in exon 14, few deletions, and missense mutations in exon 9, 11, and 12 (Ali et al., 2009; Chandrasekharappa et al., 2013; Meetei et al., 2003; Vetro et al., 2015; Wu et al., 2017). In our cohort, we identified the FANCL mutation, c.1092G>A, causing the skipping of exon 13 and hence forming a truncating protein. Our previous study proved this mutation to be a founder mutation in the south Asian population (Donovan et al., 2019). Hence careful evaluation FANCL c.1092 G>A should be done in all Indian FA patients.
In the FA pathway, the downstream proteins are FANCD1 (BRCA-2), FANCD2, FANCJ (BRIP1), FANCN (PALB2), FANCO (RAD51C), FANCP (SLX4), FANCQ (ERCC4), FANCR (RAD51), FANCS (BRCA-1), FANCU (XRCC2), FANCV (REV7), FANCW (RFWD2) (Rodríguez & D'Andrea, 2017). FANCD2 is a crucial protein that plays a central role in the FA pathway (van Twest et al., 2017). The FANCD2 mutations were identified in 8 (4.9%) FA subjects. Though it is one of the important genes in the FA pathway, the mutations are less frequently reported (Steinberg-Shemer et al., 2020). Overall, 90% of the total FA patients belonged to the common 4 complementation groups (FA-A, FA-G, FA-L, and -D2), and the remaining 10% were the other 7 complementation groups (Figure 2). Patients presenting with mutations in FANCJ, FANCN, and FANCD1 have been reported with early onset of cancers; however, we did not observe cancer development in our cohort, but the subjects' follow-up is important to understand the disease development. FA-F and FA-E complementation groups are reported with a low frequency worldwide; similarly, in our cohort, only two patients were detected in both-F and FA-E complementation groups. FANCB is the only gene that is X-linked, and according to the literature, FANCB is identified with a 3% frequency in the Japanese population (Mori et al., 2019). Recently Jung et al. reported FANCB variants associated with severe clinical features resembling the VACTERL-H association (Jung et al., 2020). In our cohort, we could identify only one patient with a hemizygous splice donor site mutation, c.2165+2T>G in the FANCB gene with clinical features such as café-au-lait spots, facial anomalies, and micro-penis.
The variants were not identified in 17 (9.39%) FA subjects though these patients had typical FA features and high chromosomal breakages. The monoubiquitination of FANCD2 was studied in all FA subjects; 7 of these subjects (IN016, IN018, IN024, IN028, IN031, IN053, IN064) had a single non-ubiquitinated FANCD2 protein detected in Western blot analysis, suggesting an upstream complex defect. We could not identify the FANCD2 protein status in 10subjects (IN051, IN060, IN086, IN092, IN108, IN136, IN143, IN147, IN148, IN171) due to technical limitation (Table S5 and Figure S2). The subjects without a molecular diagnosis need to be studied with advanced technology like transcriptome analysis or deep intronic variant analysis using whole-genome sequencing.
Somatic mosaicism has been reported in FA in about 25% of the cases (Ten Foe et al., 1997). Even though the clinical significance is not much known, many researchers state that patients with somatic mosaicism express milder phenotypes. Somatic mosaicism can result in a self-correction in the bone marrow or early progenitor cells and has quite clinical implications (Gregory et al., 2001; Gross et al., 2002; Soulier et al., 2005). However, due to technical limitations, FA patients with somatic mosaicism can be skipped from basic diagnosis. In our study, also we have technical limitations, and hence we could not identify somatic mosaicism in FA patients.
FA complementation groups are known to be associated with multiple phenotypes (Faivre et al., 2000). We analyzed the mutational data with the phenotypes on the basis of the VACTERL-H or PHENOS association. Four patients revealed the VACTERL-H association; these patients belonged to FANCI, FANCL, and FANCA complementation groups. Literature studies also report VACTERL-H association with FANCI and FANCL complementation (Savage et al., 2016; Vetro et al., 2015). Even though FANCD1 and FANCN are known to be associated with a high frequency of clinical abnormality in our cohort, both did not show any particular phenotype; this can be due to the very low frequency of both these complementation groups. The genotype-phenotype correlation has been reported in FA subjects (Fiesco-Roa et al., 2019; Steinberg-Shemer et al., 2020). In our cohort, the genotype-phenotype correlation of FA revealed that skin pigmentation, short stature, and skeletal abnormalities were the common clinical feature among all complementation groups. However, renal anomalies, organ deformities, and genital anomalies were observed with high frequency, while a low frequency of skin pigmentation and short stature were observed in the FA-D2 complementation group (Figure 1). The genotype-phenotype correlation was not possible for other complementation groups due to a smaller number of FA subjects in those complementation groups. The careful examination is important, as FA subjects presented with a spectrum of clinical features and major clinical anomalies may not be present in some of the complementation groups. However, a follow-up study is essential to understand the progression of the disease.
5 CONCLUSION
The molecular study established 12 complementation groups in Indian FA subjects. The major complementation groups were FANCA, G, L, and their genes need to be screened for early diagnosis and management of the disease. The FA family screening is important for the appropriate genetic counseling. Follow-up studies are essential to understand the cancer development in FA subjects.
ACKNOWLEDGMENTS
We would like to thank all the patients for participating in our study. We also thank the pediatricians and hemato-oncologists for the clinical assessment of the FA patients. We thank Prof. Alan D'Andrea (Dana-Farber Cancer Institute, Boston, USA) for his guidance to conduct Fanconi anemia research in our Institute. Thanks also due to Dr. Chandrasekharappa Settara (NIH/NHGRI, Bethesda, USA) for his comments and suggestions on our manuscript. This study was funded by DST/SERB (Grant Number EEQ/2016/000510; B. R. V).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Merin George, Avani Solanki, and Niranjan Chavan contributed to the study conception and design, carried out the experiments, and analyzed the data. Aruna Rajendran, Revathi Raj, Sheila Mohan, Sandeep Nemani, Shailesh Kanvinde, Deendayalan Munirathnam, Sudha Rao, Nita Radhakrishnan, Harsha Prasada, Radha Gulati Ghildhiyal, Mamta Manglani, Chandrakala Shanmukhaiah, Sunil Bhatt, Sowmyashree Ramesh, Anchu Cherian, and Pritesh Junagade performed the clinical assessment, diagnosed the patients, and provided the patients' data. Merin George prepared the manuscript. All the authors have read and approved the manuscript before submission. Babu Rao Vundinti contributed to the study conception and design, analyzed the data, and finalizing the manuscript.
WEB RESOURCES
genetics.bwh.harvard.edu/pph2/
https://asia.ensembl.org/index.html
http://www2.rockefeller.edu/fanconi/
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article and its supplementary information files. The novel variants identified have been reported to ClinVar in a 2 spreadsheet submission (Accession no.: SCV001832555—SCV001832581 and SCV001832582—SCV001832608) and an individual ClinVar submission (Accession no.: SCV001821511).




