p.Ser891Ala RET gene mutations in medullary thyroid cancer: Phenotypical and genealogical characterization of 28 apparently unrelated kindreds and founder effect uncovering in Northern Italy
Abstract
Applying genetic screening in medullary thyroid cancer (MTC) patients we identified an unexpectedly high frequency of c.2671T>G, p.Ser891Ala RET mutation carriers. Our aim was to: (a) deeply characterize the clinical expression of this mutation, (b) identify the presence of a founder effect in our region. Genetic analysis was performed in 251 relatives from 28 Ser891Ala kindreds, among 108 p.Ser891Ala asymptomatic carriers, 64 were submitted to thyroidectomy: mean age for 10 subjects presenting C-cells hyperplasia was 30.2 ± 13.7 years, raising to 37.9 ± 10.3 in 14 subjects with micro-MTC and to 55.0 ± 14.7 years in 39 subjects with MTC. Age-related progression across histopathological groups CCH/microMTC and MTC were statistically significant: genetic screening in Ser891Ala families could be safely postponed at the age of 14. To investigate the hypothesis of a common ancestor for Ser891Ala mutation we genotyped for 18 polymorphic microsatellite markers encompassing RET locus all subjects belonging to Ser891Ala families and we identified a founder effect, estimating the age of a common ancestor, dating back to 1493 AD. Ethnographic data collected in historical archives support laboratory results; the high prevalence of this mutation in our region could suggest the hypothesis of a population study to realize a preventive intervention in a rare neoplastic disease.
1 INTRODUCTION
Medullary thyroid carcinoma (MTC) is a rare disease, which arises from the parafollicular, calcitonin-secreting cells of the thyroid gland and accounts for about 5% of all thyroid cancers. At the time of first clinical presentation, the disease is sporadic in most cases, however, at least 20–25% of MTC occurs in the context of MEN2 syndrome, related to a germline mutation in RET proto-oncogene (NM_020975.4). This hereditary neoplastic syndrome is characterized by at least two clinical variants as the result of the variable aggregation of different pathological phenotypes: (a) MTC, pheochromocytoma (PHEO) and primary hyperparathyroidism (PHPT) in MEN2A; (b) MTC, PHEO and a variety of developmental abnormalities (including marfanoid habitus, mucosal neuromas, hindgut hypergangliosis and thick corneal nerves) in MEN2B (Eng et al., 1996). Nevertheless, familial MTC may also appear as an isolated phenotype termed familial medullary thyroid cancer (FMTC). The phenotypical heterogeneity of MEN2 syndromes is largely dependent on the underlying genetic abnormality in the RET gene: Substitution at cysteine 634 codon is usually associated to full expression of classical MEN2A while the mutation in noncysteine codons is mostly associated to FMTC.
The increasing adoption of RET genetic screening in patients diagnosed with MTC has allowed to identify an unexpectedly high prevalence of genetically determined MTC also in apparently sporadic, nonsyndromic forms. High prevalence of FMTC in apparently sporadic cases is linked to the clinical course of disease in lower risk RET mutations characterized by later age of onset when compared to thyroid disease in MEN2A/MEN2B. Guidelines on prophylactic thyroidectomy in mutation carriers subjects have been recently revised (Wells et al., 2015), and timing for surgery is actually less strict for noncysteine at lower risk mutations than in previous Consensus Statements (Brandi et al., 2001) and 2009 ATA Guidelines (Kloos et al., 2009). However, also in this context, the importance of detecting RET germline mutations cannot be underestimated, since the extension of genetic analysis in relatives and identification of at risk subjects really allows to perform a preventive action in malignant neoplastic disease (Machens & Dralle, 2015). Actually, the strongest independent clinical predictors of survival in MTC are stage and age at the time of diagnosis. Patients with tumors confined to the thyroid gland have at 10-year survival rate of 95–96%, survival rate decreases to 75.5–77% in patients with nodal involvement, and only 40–44% patients with distant metastases at diagnosis survive more than 10 years (respectively citation, Randle et al., 2017; Roman, Lin, & Sosa, 2006). This study describe our 18 years’ experience of RET genetic screening in a large unselected series of consecutive MTC patients observed at a single institution in Northern Italy: We observed an unexpected prevalence of c.2671T>G, p.Ser891Ala substitution in exon 15, compared to prevalence observed in population-based studies from other countries in Europe where Ser891Ala accounts for 4.1% of all RET mutation in France (Lebeault et al., 2017) and 4.4% in Germany (Machens, Lorenz, Weber, & Dralle, 2018). Purpose of our study was: (a) to deeply characterize this mutation, evaluating clinical phenotype, stage of thyroid disease at time of therapeutic/prophylactic thyroidectomy in a large series of subjects, (b) to explore and date back in time, a putative Ser891Ala founder mutation in a distinct geographic region in Northeast Italy.
1.1 Patients and methods
Through the last 18 years (1999–2018), we have performed genetic screening in 204 patients with the diagnosis of MTC, most of them (195) had an apparently sporadic form of the disease. For each index case proven to carry a pathogenic mutation, we extended the genetic counselling and analysis to first degree relatives and to other consanguineous components of the kindred.
1.2 Mutational analysis of the RET proto-oncogene
Germline DNA was extracted from total blood. DNA was amplified with intronic primers flanking exons 5, 8, 9, 10, 11, 12, 13, 14, 15, 16, and 18. Polymerase chain reaction (PCR) products were purified, sequenced and analysed on a 310 DNA analyser (Life Technology Corporation, Carlsbad, CA). Since the last 3 years genetic analysis is performed by Next-Generation Sequencing (Ion Torrent PGM, Life Technology Corporation).
1.3 Clinical phenotype assessment
For each MTC index patient carrying RET germline mutation (RET+) a careful search for the phenotypic characteristics of MEN2 was enforced. The baseline screening for RET+ relatives included biochemical assays of plasma calcitonin and PTH (electrochemiluminescence immunoassay with cut off value of 15 pg/ml for calcitonin and normal range of 11–67 pg/ml for PTH), calcium, measurement of 24-hr urinary excretion of catecholamines/metanephrines, and neck ultrasound evaluation.
1.4 Thyroidectomy and histological evaluation
Indications to thyroidectomy and relative surgical procedures for relatives carrying pathogenic germline RET mutation followed current guidelines (Brandi et al., 2001; Kloos et al., 2009; Machens, Lorenz, & Dralle, 2009) and more recently (Elisei et al., 2013; Wells et al., 2015). Thyroidectomy was defined as “prophylactic” when subjects had basal calcitonin < 20 pg/ml (Costante et al., 2007) or peak calcitonin after injection of pentagastrin (0.5 µg/kg, body weight) below 100 pg/ml (Costante et al., 2007; Niccoli et al., 1997). The entire thyroid was sectioned at 3–5 mm intervals and macroscopically evident lesions were described in terms of size, shape, localization, and appearance. The diagnosis was based on the observation of the typical histological findings and immunohistochemical analysis; according to World Health Organization (WHO), medullary tumors with the greatest dimension lower or equal to 10 mm were defined as microcarcinomas (micro-MTC). C-cell hyperplasia (CCH) was defined by the presence of at least three microscopic foci containing more than 50 C-cells in one low-power field (×100, magnification; LiVolsi, 1997). Serial haematoxylin–eosin sections, Periodic acid–Schiff (PAS) stain and immunostaining for Type IV collagen were useful, in some cases for the distinction between CCH and early medullary carcinoma.
1.5 Data analysis
Data analysis was performed using SPSS version 25.0 (SPSS, Inc., Chicago, IL). We adopted t test for not paired samples. P = 0.05 was regarded to be statistically significant.
1.6 Microsatellite genotyping and haplotype construction
A total of 251 subjects belonging to 28 Ser891Ala families were genotyped for 18 polymorphic microsatellite repeat markers encompassing the RET locus according to the genetic and physical map available at http://genome.ucsc.edugene: 12 microsatellite markers in 5′ and six microsatellite markers in 3′ region, covering a region of 67 cM (38.3 cM at 5′and 28.8 cM at 3′, according to the LDB Genetic Map; Figure 1).
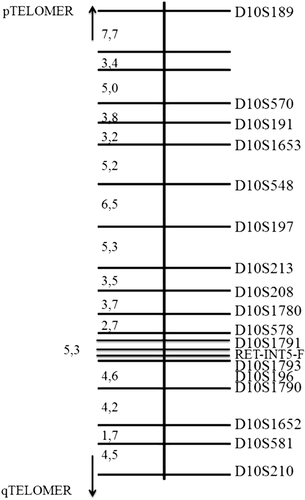
Microsatellite markers used for haplotype reconstruction
Haplotype reconstruction was performed by the use of multipoint nonparametric linkage analysis software Merlin v1.0.0 of Easy Linkage v5.02 (Abecasis, Cherny, Cookson, & Cardon, 2002; Hoffmann & Lindner, 2005) and checked with GeneHunter 2.1 (Lewinson, 1995). Merlin is suitable for quite larger pedigrees and lets the user know when the inference on haplotype reconstruction is uncertain. We assigned the phase only when it was unequivocal, using Aplo-Painter (Thiele & Nürnberg, 2005) for visualization of haplotypes produced by linkage programs.
2 RESULTS
2.1 Clinical presentation in MTC subjects
We examined 204 patients (68 males and 136 females) with histologically proven diagnosis of MTC; median age at the diagnosis was 56.6 years (range, 18–87). At the first clinical presentation, four patients had evidence of familial/syndromic disease, five nonsyndromic patients had positive family history (one relative with a previous diagnosis of “sporadic” MTC). One hundred and ninety-five subjects had a sporadic clinical presentation of disease (Table 1).
| Family ID | Sex | Clinical presentation | Agea | Mutation | Phenotype | MTC pTNM | Gene carriers/ screened relatives |
|---|---|---|---|---|---|---|---|
| #1 | F | Fa | 58 | Ex10 Cys609Tyr | MTC | T3mN1bM0 | 12/24 |
| #2 | M | Sp | 65 | Ex10 Cys611Phe | MTC | T2N0M0 | 1/5 |
| #3 | F | Sp | 35 | Ex10 Cys618Ser | MTC | T2N1bM0 | 3/3 |
| #4 | F | Fa/Sy | 36 | Ex10 Cys618Ser | MTC–PHEO (40 ys) | T2N0M0 | 2/2 |
| #5 | F | Sp | 30 | Ex10 Cys620Arg | MTC | T4N1bM0 | 1/5 |
| #6 | F | Sp | 41 | Ex10 Cys620Ser | MTC | T1bmN0M0 | 1/1 |
| #7 | F | Sy | 18 | Ex11 Cys634Tyr | MTC–PHEO (31 ys) | T3N0M0 | 1/6 |
| #8 | M | Sy | 49 | Ex11 Cys634Trp | MTC–PHEO (56ys) | T2mN1M0 | 0/3 |
| #9 | F | Fa | 27 | Ex11 Cys634Phe | MTC | T1N0M0 | 2/2 |
| #10 | M | Fa/Sy | 42 | Ex11 Cys634Phe | PHEO (33ys)–MTC–PHPT (54ys) | T2N0M0 | 1/3 |
| #11 | M | Sp | 59 | Ex11 Asp631Asnb | MTC | T1N0M0 | 2/2 |
| #12 | F | Fa | 54 | Ex11 Lys666Glu | MTC | T1M0N0 | 0/3 |
| #13 | F | Sp | 42 | Ex13 Val778Ile | MTC | T2N1bM1 | 2/6 |
| #14 | F | Sp | 75 | Ex13 Leu790Phe | MTC | T1mN0M0 | 0/2 |
| #15 | F | Sp | 51 | Ex13 Leu790Phe | MTC | T3N1bM0 | 0/0 |
| #16 | F | Sp | 52 | Ex14 Val804Met | MTC | T1mN0M0 | 2/4 |
| #17 | F | Sp | 48 | Ex14 Val804Met | MTC | T1N1M0 | 0/1 |
| #18 | F | Sp | 58 | Ex14 Val804Met | MTC | T1mN0M0 | 0/2 |
| #19 | M | Sp | 76 | Ex14 Val804Met | MTC | T1N0M0 | 0/3 |
| #20 | F | Sp | 38 | Ex14 Val804Met | MTC | microT1mN0M0 | 4/6 |
| #21 | M | Sp | 51 | Ex14 Val804Met | MTC | T2mN1bM0 | 0/3 |
| #22 | M | Sp | 71 | Ex14 Val804Met | MTC | T3mN1bM0 | 11/42 |
| #23 | F | Sp | 33 | Ex15 Ser891Ala | MTC | T1mN0M0 | 14/31 |
| #24 | F | Sp | 68 | Ex15 Ser891Ala | MTC | T2N1bM0 | 5/22 |
| #25 | F | Sp | 66 | Ex15 Ser891Ala | MTC | T2N0M0 | 4/6 |
| #26 | F | Sp | 64 | Ex15 Ser891Ala | MTC | T2mN1M0 | 0/2 |
| #27 | M | Sp | 41 | Ex15 Ser891Ala | MTC | T3mN1b | 0/0 |
| #28 | F | Fa | 29 | Ex15 Ser891Ala | MTC | microT1mN0M0 | 19/33 |
| #29 | F | Sp | 63 | Ex15 Ser891Ala | MTC | T3mN1bM0 | 0/0 |
| #30 | M | Sp | 65 | Ex15 Ser891Ala | MTC | T4mN0M0 | 3/7 |
| #31 | F | Sp | 60 | Ex15 Ser891Ala | MTC | T4mN1bM1 | 5/13 |
| #32 | F | Sp | 65 | Ex15 Ser891Ala | MTC | T2mN1M0 | 1/1 |
| #33 | M | Sp | 66 | Ex15 Ser891Ala | MTC | T1mN0M0 | 3/17 |
| #34 | F | Sp | 87 | Ex15 Ser891Ala | MTC | T2mN1M0 | 3/17 |
| #35 | F | Sp | 56 | Ex15 Ser891Ala | MTC | T1N0M0 | 4/13 |
| #36 | M | Sp | 46 | Ex15 Ser891Ala | MTC | T4N1aM0 | 9/16 |
| #37 | F | Sp | 44 | Ex15 Ser891Ala | MTC | T2mN1M0 | 0/1 |
| #38 | F | Sp | 60 | Ex15 Ser891Ala | MTC | T1N0M0 | 0/0 |
| #39 | F | Sp | 62 | Ex15 Ser891Ala | MTC | T1mN0M0 | 0/1 |
| #40 | F | Sp | 49 | Ex15 Ser891Ala | MTC | T1mN0M0 | 11/21 |
| #41 | F | Sp | 68 | Ex15 Ser891Ala | MTC | T3mN1bM0 | 8/14 |
| #42 | M | Sp | 38 | Ex15 Ser891Ala | MTC | T2mN0M0 | 0/0 |
| #43 | F | Sp | 54 | Ex15 Ser891Ala | MTC | T2mN0M0 | 2/2 |
| #44 | F | Sp | 69 | Ex15 Ser891Ala | MTC | T2N1aM0 | 0/1 |
| #45 | F | Sp | 71 | Ex15 Ser891Ala | MTC | T2N1aM0 | 0/0 |
| #46 | F | Fa | 25 | Ex15 Ser891Ala | MTC | microT1N0M0 | 0/0 |
| #47 | F | Sp | 65 | Ex15 Ser891Ala | MTC | T1mN0M0 | 7/11 |
| #48 | F | Sp | 44 | Ex15 Ser8 PHEO 91Ala | MTC | T3mN1bM0 | 3/10 |
| #49 | F | Sp | 83 | Ex15 Ser891Ala | MTC | T3mN1bM0 | 1/2 |
| #50 | F | Sp | 53 | Ex15 Ser891Ala | MTC | T1mN1bM0 | 6/10 |
| #51 | M | Sp | 30 | Ex16 Met918Val | MTC | T1N0M0 | 0/0 |
- Note. F: female; Fa: familiar presentation; M: male; pTNM: pathologic TNM staging; Sp: sporadic presentation; Sy: syndromic presentation.
- a At the time of MTC diagnosis.
- b VUS (variant undetermined significance).
2.2 Germline RET mutations and phenotype in index cases
The genetic analysis revealed heterozygotic germline RET variants in 51/204 (25%) subjects, all except one, previously described as pathogenic mutations. Median age at diagnosis was 52.9 ± 14.15 (range, 18–87). All nine patients with clinical evidence of familial/syndromic MTC harbored a pathogenic RET gene mutation; an unexpected germline mutation was found in 42/195 (21.5%) clinically “sporadic MTC”. Most of our patients had mutation in noncysteine codons. Val804Met, the RET mutation with the highest prevalence in previous Italian series, was identified in seven patients (13.7%); most of index cases in our population, 28 out of 51 (54.9%), had exon 15 Ser891Ala mutation (Figure 2). In one patient #11, we identified a RET gene variant of undetermined significance (VUS). Genotypic and phenotypic features in index patients, together with kindred expansion, are summarized in Table 1.
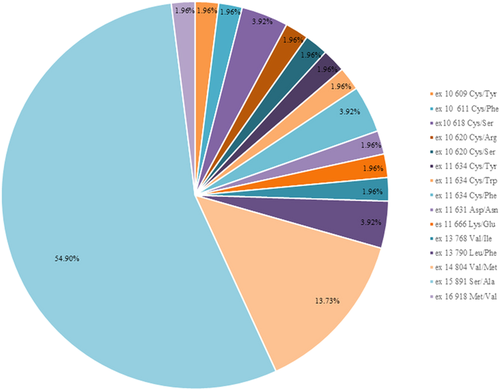
RET mutation distribution in 51 index patients
2.3 Expanding pedigrees in Ser891Ala kindred: Germline RET mutations and phenotype in relatives
Genetic screening was expanded to 251 relatives of Ser891Ala index cases with the identification of 108 RET+ subjects. In Table 2 we report the results of presurgical evaluation and histological findings of the 64 subjects submitted to thyroidectomy at our institution: Families are numerated according to the index case. According to presurgical evaluation, 28 patients were operated on prophylactic thyroidectomy, 36 patients had the therapeutic intervention. At the histological analysis, all subjects, except one, had evidence of cell C disease, ranging from CCH (10 subjects), to micro-MTC (14 subjects), or MTC (39 subjects). Only thyroid specimens from a 7 years child showed normal thyroid tissue. Mean age of patients was respectively: 30.2 ± 13.7 for CCH, 37.9 ± 10.3 for micro-MTC and 55.0 ± 14.70 for MTC. The mean age of the subjects with MTC was significantly higher than the mean age of subjects with CCH (P < 0.001) or micro-MTC (P < 0.001; Figure 3). Four micro-MTC and 33 MTC were multifocal, six patients had nodal metastasis (mean age, 59.5; range, 31–77). One 57 years old asymptomatic Ser891Ala mutation carrier had developed metastatic liver involvement (histological evidence) at the time of genetic diagnosis.
| Family ID | Sex | Thyroidectomy Proph/Ther | Age at thyroidectomy | Presurgery calcitonin, pg/ml (<15) | PTH pg/ml (range, 11–67) | Cathecolamines and/or metanephrines | MTC pTNM |
|---|---|---|---|---|---|---|---|
| #23 | F | T | 61 | 217 | 173 | Normal | T1mN0M0 |
| F | T | 65 | 126 | 55 | Normal | T1mN0M0 | |
| M | T | 72 | 240 | 63 | Normal | T1N1M0 | |
| F | P | 58 | 14 | 22 | Normal | T1mN0M0 | |
| F | P | 36 | 5 | 68 | Normal | CCH | |
| M | P | 33 | 5 | 52 | Normal | microT1N0M0 | |
| F | P | 45 | 25 | 58 | Normal | T1amN0M0 | |
| #24 | F | T | 77 | 481 | 92 | - | T3mN1M0 |
| F | T | 63 | 80 | 56 | Normal | microT1mN1M0 | |
| F | T | 74 | 102 | 52 | Normal | T1mN0M0 | |
| M | T | 50 | 28 | 37 | Normal | T1mN0M0 | |
| M | T | 46 | 83 | 33 | Normal | T1mN0M0 | |
| #25 | M | P | 38 | <5 | 42 | Normal | microT1N0M0 |
| M | P | 34 | <5 | 103 | Normal | microT1N0M0 | |
| #28 | M | P | 7 | - | - | - | Normal |
| M | P | 37 | 7 | 46 | Normal | microT1N0M0 | |
| M | P | 33 | <10 | 32 | Normal | microT1mN0M0 | |
| M | P | 72 | 19 | - | - | T1N0M0 | |
| F | T | 77 | 1,057 | - | Normal | T2N0M0 | |
| F | T | 52 | 94 | 28 | Normal | T1N0M0 | |
| M | T | 39 | 39 | 33 | Normal | microT1N0M0 | |
| F | P | 41 | - | - | Normal | T1N0M0 | |
| F | T | 85 | - | - | Normal | T2mN0M0 | |
| F | T | 49 | 844 | - | Normal | T2mN0M0 | |
| #30 | M | T | 31 | 153 | 57 | Normal | T1N1bM0 |
| F | T | 57 | 345 | - | - | T3mN1bM1 | |
| #31 | F | T | 57 | 1,226 | 39 | Normal | T1mN1aM0 |
| M | P | 27 | 16,7 | 50 | Normal | CCH | |
| M | T | 34 | 145 | 41 | Normal | T1N0M0 | |
| M | T | 32 | 20,6 | 35 | Normal | T1mN0M0 | |
| #32 | M | P | 37 | <5 | 30 | Normal | T1N0M0 |
| #33 | F | P | 27 | 7 | - | Normal | microT1N0M0 |
| M | T | 59 | 29 | 21 | Normal | T1mN0M0 | |
| #34 | F | T | 59 | 198.8 | 60 | Normal | T1mN0M0 |
| #35 | F | P | 71 | 12 | 47 | Normal | T1mN0M0 |
| F | P | 66 | 7 | 53 | Normal | T1mN0M0 | |
| F | P | 44 | 13 | 45 | Normal | microT1mN0M0 | |
| F | T | 44 | 92 | 109 | Normal | T1mN0M0 | |
| #36 | F | P | 17 | <5 | 59 | Normal | CCH |
| F | P | 20 | <5 | 100 | Normal | CCH | |
| F | P | 17 | <5 | 86 | Normal | CCH | |
| #40 | F | T | 29 | 123 | 64 | Normal | T1mN0M0 |
| M | T | 48 | 9 | 52 | Normal | T1mN0M0 | |
| M | P | 27 | <5 | 23 | Normal | microT1mN0M0 | |
| F | P | 47 | <5 | 33 | Normal | CCH | |
| F | P | 57 | 7 | 38 | Normal | CCH | |
| F | P | 55 | <5 | 36 | Normal | T1mN0M0 | |
| F | T | 34 | 25 | 45 | Normal | T1mN0M0 | |
| F | P | 33 | <5 | 49 | - | microT1N0M | |
| #41 | M | T | 67 | 154 | 109 | - | T1mN0M0 |
| F | T | 57 | 228 | 56 | Normal | T3mN0M0 | |
| F | T | 37 | 246 | 58 | Normal | T1mN0M0 | |
| M | T | 30 | 33 | 66 | Normal | microT1N0M | |
| M | T | 56 | 154 | 109 | Normal | microT1mN0M0 | |
| F | T | 71 | 2,000 | - | Normal | T1mN0M0 | |
| #46 | F | T | 60 | 76 | 53 | Normal | T1mN0M0 |
| M | P | 41 | 7 | 33 | High | CCH | |
| F | T | 63 | 93 | 55 | Normal | T1mN0M0 | |
| M | T | 28 | 24 | - | Normal | microT1N0M0 | |
| #48 | F | P | 25 | <5 | 37 | Normal | CCH |
| M | P | 15 | <5 | 90 | Normal | CCH | |
| #49 | F | T | 57 | 92 | - | Normal | T1mN0M0 |
| M | T | 56 | 71 | 37 | Normal | T1mN0M0 | |
| #50 | F | T | 50 | 190 | - | Normal | T1mN0M0 |
- Note. Missing values are indicated by hyphens.
- F: female; M: male; P: prophylactic thyroidectomy; PTH: parathyroid hormone; T: therapeutic thyroidectomy.
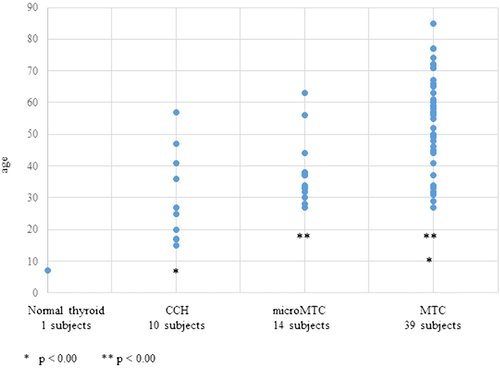
Relation between C-cell disease progression and age in 64 p.Ser891Ala RET + relatives. CCH: C-cell hyperplasia; MTC: medullary thyroid cancer
Among 90 subjects screened, the biochemical and instrumental evaluation revealed the presence of asymptomatic PHEO in a 41 years old subject; this patient was submitted to left adrenalectomy and prophylactic thyroidectomy (CCH at histological examination).
No one among Ser891Ala subjects (probands and relatives) had clinical PHPT, we obtained PTH value for 79 subjects, among them only 10 had mild PTH increase: Calcium levels were always in the normal range and ultrasound evaluation did not identify enlargement of parathyroid glands.
Overall considering all Ser891Ala RET+ subjects, probands, and relatives in our series: (a) thyroid disease progression is strongly associated to age; age-related progression across histopathological groups 10-CCH (30.2 ± 13.7 years), 14 micro-MTC (37.9 ± 10.3 years), and 67-MTC (55.8 ± 14.8 years) is statistically significant. Nodal involvement, including probands and relatives, was present in 18 subjects (12 probands and 6 relatives), with a mean age of 60.4 (standard deviation [SD] ± 12.9). In our population age difference between MTC without nodal involvement (54.3 ± 14.9) and with nodal involvement (60.4 ± 12.9), is not statistically significant (P = 0.13), probably due to the little sample of subjects with positive lymph nodes. (b) Penetrance for PHEO is lower than 1% in this series of Ser891Ala mutation carriers, whereas previous data reported a penetrance of 4–10% (Schulte et al., 2010; Wells et al., 2015), (c) penetrance for PHPT is consistent with previous figures (12%, in our series), but with very low clinical impact.
2.4 Founder effect
2.4.1 The founder hypothesis and genealogical reconstruction
To investigate the hypothesis of a common ancestor for Ser891Ala mutation in our population we choose two parallel and complementary strategy based on laboratory work and archival research. The laboratory strategy was first to identify the existence of a common haplotype, including the mutation, shared by Ser891Ala kindreds and afterward to identify the age of the putative common ancestor. The accurate archive research on Church and Municipal Registries was conducted to confirm the likelihood of the results obtained from laboratory study.
2.4.2 Haplotype reconstruction and age estimation of founder effect
The reconstruction of the complex haplotype covering a great region (67 cM) around RET gene locus allowed to identify the presence of a common haplotype among Ser891Ala kindreds: The dimension of the shared haplotype was expression of different level of genetic relationship among families (Figure 4 ).
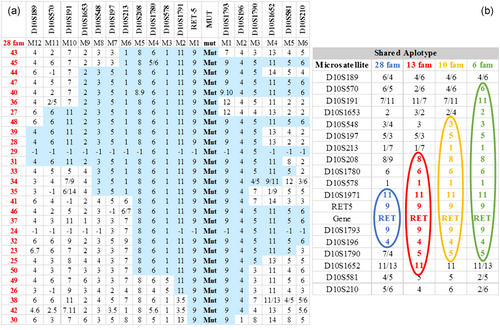
(a) Haplotypes reconstruction of the 28 families involved in the study. The sections highlighted in blue, point out the shared haplotype. (b) Shared haplotype among different groups of kindreds
To estimate the age of the possible common ancestor we used a likelihood-based method, elaborated by Genin (Genin, Tullio-Pelet, Begeot, Lyonnet, & Abel, 2005) which uses information provided by haplotype shared among affected individuals: Genetic distance between RET gene locus and nonshared microsatellites alleles allows to estimate the number of generations dividing our patients from the founder of the mutation. Considering the whole group, composed of 28 families, we could estimate the age of the founder effect 21 generations ago (95% confidence interval, 16–28). Assuming for each generation the conventional time of 25 years, we can calculate the presence of the common ancestor in the periods 1318–1618 AD, presumably around 1493 AD.
When the analysis was restricted to smaller groups of 13, 10, or 6 families, which shared larger haplotypes, we could identify a more recent common ancestor dating back to 1518 AD (Figure 5).

Founder effect estimation for different groups of kindreds; for each subgroup are indicated the number of generations, the confidence interval, and the estimated date of the genetic link. Number within each bar refers to the total group of 28 families, and to the subgroups of 13, 10, and 6 families strongly genetically linked
2.4.3 Pedigrees reconstruction
We conducted a systematic research on Municipal Archives for the last 150 years (1860–2010) and on Parish archives for the age before Italian Unification (1860; Figure 6 a). From these Archives, we retrieved genealogical data of families and we traced the family trees back to the 18th century (Figure 6 b,c). As hypothesised we could confirm the presence of relationships among most of the families (data available from the authors), even if we could not identify a possible single common ancestor. Several factors contributed to make technically unmanageable our task: The presence of a great number of homonym subjects, the difficulty to retrieve unequivocal information for female and difficult interpretation of oldest documents. Anyway, we could confirm that Ser891Ala families actually resident in Plain of Brescia are descendant of immigrant from Seriana Valley in province of Bergamo (Poloni, 2011; Figure 7).
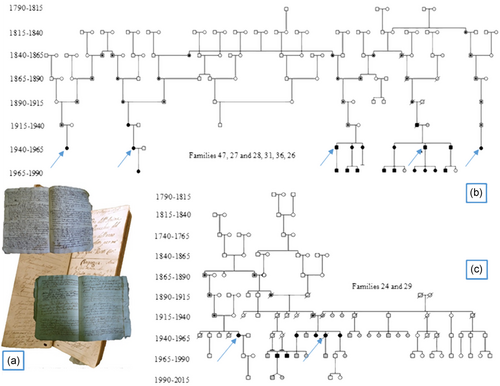
(a) Example of parish registers: register of Baptisms (1619) and Marriages (1786) of a parish in the province of Brescia. (b) Results of research in parish and municipal archives: links between families 47–27, 28–31–36–26 and (c) for families 24–29. Black circle identify the affected patients, the grey ones the nonmutated subjects, those with a black dot in the circle the presumably mutated subjects, the with ones the untested individuals. The arrows show MTC index patients
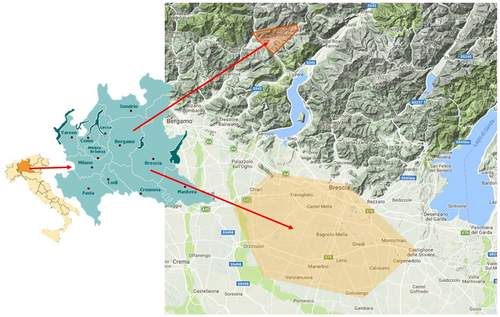
Map of the Bergamo's area, the origin of the p.Ser891Ala mutation, and its diffusion in the plain of Brescia
3 DISCUSSION
In this series of MTC patients examined at a single institution we observed a large majority of sporadic presentations as compared to familial/syndromic forms (95.6% vs 4.4%); despite this, more than 20% of 195 sporadic MTC patients had a pathogenic RET variants, a percentage reported in previous studies including many more familial/syndromic presentations.
We have also observed an unexpected distribution of mutations: More than 80% of mutations were located in noncysteine codons. These figures are markedly different from other series reporting an average of 61% of cysteine codon mutations, and also from the ItaMEN Network analysis which also comprises part of our population (49.8 noncysteine mutation; Romei et al., 2010). The main reason for such differences between our and previous studies is attributable to the high prevalence in our series of Ser891Ala mutation (54.9%), a noncysteine codon variant that has been reported in other populations at a much lower frequency: From 2% of all RET mutations in EUROMEN Study Group (Machens et al., 2003), to 9.6% in ITAMEN Study (Romei et al., 2010) and 14,8% in the French Medullary Study Group (Niccoli-Sire et al., 2001). In more recent study, Ser891Ala accounts for 4.4% of all RET mutation in France (Lebeault et al., 2017) and for 4,1% in Germany (Machens et al., 2018).
The most relevant result of this study is the identification and clinical characterization of the largest sample of Ser891Ala RET mutation carriers: We confirmed that also this noncysteine RET mutation is fully penetrant for C-cell disease, but phenotype expression is strongly linked to age, malignant progression of C-Cell disease is rather slow and never occurs before the second decade of life. The younger subject with micro-MTC and MTC were 27 and 25 years old. Our results are consistent with recent data from a large study in more than 500 RET mutation carriers, that first validated for most of unique RET variant the genotype-specific, age-related progression for MTC (Machens et al., 2018).
Recently updated recommendation (Wells et al., 2015) underlines high variability among families in the age of onset and the aggressiveness of the MTC, and suggest that family members in ATA-MOD category, cervical ultrasound examination and measurement of serum calcitonin levels, should begin at 5 years of age. This is a very young age for both genetic screening and surveillance especially in families with late onset cancer disease: Psychological relapses for both children and parents should not be underestimated. Considering results in this large series of patients, we suggest that genetic screening in Ser891Ala could be safely postponed at the age of 14, 10 years before the earlier age of documented malignant disease. Our results confirm that Ser891Ala cannot be considered purely associated with isolated FMTC and careful search for PHEO cannot be omitted. Lifetime screening for PHPT is probably not essential, and after complete hormonal assessment at first evaluation, subsequent follow-up could be performed by annual calcium serum measurement.
Due to the high frequency of this mutation in our population we further investigated and demonstrated the hypothesis of a founder mutation in a distinct geographic region in Northeast Italy: In fact we could notice the regional clustering of the mutated kindred in two areas: the Seriana Valley in province of Bergamo, and the Plain of Brescia (the foothill expansion of the Po Valley, close to the south of Brescia). This is a very interesting clue, as the reconstruction of genealogical tree allowed us to confirm that families actually resident in the Plain of Brescia are descendant of immigrants from Castione della Presolana in Seriana Valley. At the beginning of 17th (16th) century, families dedicated to agriculture and livestock farming left the mountain lands researching, in the closer plain, more favorable working conditions and fertile lands. Citations regarding migration from Bergamo mountains to plain of Brescia go back to 1630–1690 AD (Poloni, 2011), supporting the hypothesis that the Ser891Ala mutation was most likely introduced by a founder mutation from Seriana Valley.
Founder mutation and confined geographical clustering for specific RET mutation have been already described; Martins-Costa et al. (2016) demonstrated the presence of a founder effect for Met918Val mutation in Brazil, also in this study haplotype analysis of the founder effect confirmed ethnographic data collected into historical archives of the region (churches’ archives and oral life history narratives). Also for the Gly533Cys mutation described in a large Brazilian family and in Greece, and rarely reported in other ethnic groups, Cunha et al. (2017) identified the presence of a common ancestor. Genetic screening in Cypriot population identified an unexpectedly high frequency of the Cys618Arg RET mutation: Haplotype analysis in this series of patients allowed to identify the presence of a common haplotype (Fanis et al., 2018). Moreover, the presence of a founder effect for the Cys611Tyr RET mutation in Danish population is probably responsible for the higher incidence of MEN2a in Denmark compared to other countries (Mathiesen et al., 2018).
As a consequence of a founder effect, we actually observe an unexpectedly high prevalence of this mutation in our population, and we should possibly consider the hypothesis of a population study. A similar project has been realized in the Mocheni Area near Trento, where was observed a founder effect for SDHC gene mutation (Schiavi et al., 2012). Population screening focused in at risk areas could give the opportunity to identify mutation carriers and so realize a population preventive intervention in a rare neoplastic disease.
ACKNOWLEDGMENT
The authors thank C. Baronchelli, MD and M. L. Morassi, MD for the excellent contribution in the pathological evaluation of tumor samples.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and design: M. G., A. P., and M. C.; Development of methodology: M. G., A. P., and L. M.; Acquisition of data: M. G., A. P., M. C. T., R. T., F. C ., C. C., I. P., D. L., D. P., and C. C.; Writing, review, and revision: M. G. and A. P.




