Unusual association of a unique CAG interruption in 5′ of DM1 CTG repeats with intergenerational contractions and low somatic mosaicism
Funding information: Contract grant sponsors: Institut National de la Santé et de la Recherche Médicale; Université Paris Descartes; AFM Telethon (16331 and 19757); National Research Agency (ANR-10-IAHU-01).
Communicated by Garry R. Cutting
Abstract
Myotonic dystrophy type 1 (DM1) is a dominant multisystemic disorder associated with high variability of symptoms and anticipation. DM1 is caused by an unstable CTG repeat expansion that usually increases in successive generations and tissues. DM1 family pedigrees have shown that ∼90% and 10% of transmissions result in expansions and contractions of the CTG repeat, respectively. To date, the mechanisms of CTG repeat contraction remain poorly documented in DM1. In this report, we identified two new DM1 families with apparent contractions and no worsening of DM1 symptoms in two and three successive maternal transmissions. A new and unique CAG interruption was found in 5′ of the CTG expansion in one family, whereas multiple 5′ CCG interruptions were detected in the second family. We showed that these interruptions are associated with maternal intergenerational contractions and low somatic mosaicism in blood. By specific triplet-prime PCR, we observed that CTG repeat changes (contractions/expansions) occur preferentially in 3′ of the interruptions for both families.
1 INTRODUCTION
Several human neurological, neurodegenerative, and neuromuscular disorders are associated with unstable DNA microsatellite repeats including tri-tetra-penta-nucleotide tracts (Usdin, House, & Freudenreich, 2015). More recently, an unstable hexanucleotide repeat in the C9ORF72 gene (MIM# 614260) was found associated to amyotrophic lateral sclerosis with frontotemporal dementia (ALS-FTD) (DeJesus-Hernandez et al., 2011; Renton et al., 2011). The first expanded repeats identified were the trinucleotide repeats (TNR) CAG/CTG and CGG/GCC that are associated, respectively, to spinal and bulbar muscular atrophy, Huntington disease (HD), some types of spinocerebellar ataxias (SCAs), myotonic dystrophy type 1 (DM1), and Fragile X syndrome (FXS) (Usdin et al., 2015). DM1 is a dominant inherited disorder caused by one of the most unstable expanded CTG/CAG repeats, and it is located in the 3′ untranslated region (3′UTR) of the DMPK gene (MIM# 605377) encoding a protein kinase (Brook et al., 1992; Mahadevan et al., 1992). CTG repeat expansions produce toxic CUG transcripts that accumulate in the nucleus, form RNA foci and disturb the function of nuclear proteins including MBNL and CELF proteins (Sicot & Gomes-Pereira, 2013). Clinically, DM1 is highly variable, shows a wide range of symptoms and different ages of onset. The adult form typically presents progressive distal muscle weakness, myotonia, cardio-respiratory problems but also ocular, endocrine, gastrointestinal, and cognitive dysfunction. The disease is progressive and often leads to significant disability. The congenital form of DM1 (CDM) is characterized by general hypotonia, respiratory distress, and feeding problems at birth and is often associated with delayed motor development and intellectual disability. Typically, CDM cases are associated with maternal transmissions (Harper, 2001).
In the general population, the CTG repeat length at the DM1 locus varies from 5 to 37 repeats and remains stable across generations and in tissues. Once above ≥40 repeat tracts, the repeats become progressively more and more unstable. Intergenerational mutation rates may be as high as 100% per generation, with a striking tendency toward further repeat gains (about 90%) (Ashizawa & Harper, 2006). Intermediate alleles with 37–49 CTG tracts have been identified in unaffected parents of individuals with DM1 and have a tendency to expand (Martorell, Monckton, Sanchez, Lopez De Munain, & Baiget, 2001). Behavior of the CTG repeat between generations depends on repeat length and on the sex of the transmitting parent (Ashizawa, Dunne, Ward, Seltzer, & Richards, 1994; Brunner et al., 1993; Lavedan et al., 1993). Two large collaborative studies have estimated contraction frequencies at 10% in paternal transmissions and only 3%–4% in maternal transmissions (Ashizawa et al., 1994; Salehi et al., 2007). Typically, the CTG repeat length is positively correlated with symptoms severity and negatively correlated with age of onset, resulting in anticipation particularly obvious in DM1 (De Antonio et al., 2016; Harper, Harley, Reardon, & Shaw, 1992).
In DM1 families, inter-tissue variability of the CTG repeat length can be observed in fetuses at 13–16 weeks and the number of CTG repeats continue to expand throughout life after birth causing age-dependent somatic mosaicism (Martorell et al., 1998; Martorell, Johnson, Boucher, & Baiget, 1997; Martorell, Martinez, Carey, Johnson, & Baiget, 1995; Wong, Ashizawa, Monckton, Caskey, & Richards, 1995). CTG repeat instability has been reported in various human tissues, including peripheral blood lymphocytes and muscles. In adult DM1 patients, the mutant allele size is typically larger in skeletal muscle than in peripheral blood leucocytes (PBLs) of the same individual (Anvret et al., 1993; Ashizawa, Dubel, & Harati, 1993; Thornton, Johnson, & Moxley, III, 1994; Zatz et al., 1995). The somatic mosaicism is particularly high in DM1 and progresses with age toward larger repeat lengths with different rates of expansion between tissues, possibly correlated with the progression of symptoms (Morales et al., 2012).
TNR instability is probably caused by the formation of unusual DNA triplet structures such as slipped-DNAs during different metabolic processes. Data from mouse models demonstrated that TNRs are unstable only when transcribed, suggesting that the transcription through TNRs is necessary to destabilize triplet repeats (Mangiarini et al., 1997). The formation of expansion is highly dependent on DNA repair, transcription, R-loop formation, DNA replication, and cis- elements such as chromatin packaging and both length and purity of TNR sequences (Polyzos & McMurray, 2017; Santoro, Masciullo, Silvestri, Novelli, & Botta, 2016; Usdin et al., 2015). Interrupted TNR tracts were first described in the 1990s in FXS (Eichler et al., 1994). In FXS, AGG interruptions were identified in the FMR1 allele (MIM# 309550) carrying less than 55 CGG repeats and the number of AGG interruptions is negatively correlated with the risk of full mutation supporting the role of interrupted sequences in the stabilization of TNR repeat loci (Eichler et al., 1994; Nolin et al., 2013; Yrigollen et al., 2014). CAT, CAA, and GAG interruptions are exclusively found in unexpanded alleles for SCA1, SCA2, and Friedreich ataxia (FRDA) and are associated with the stability of CAG and GAA unexpanded repeat tracts, respectively (Choudhry, Mukerji, Srivastava, Jain, & Brahmachari, 2001; Chung et al., 1993; Montermini et al., 1997). In FRDA, GGA interruptions in an expanded GAA sequence were also reported in one individual with late-onset ataxia (Ohshima et al., 1999). In SCA8, various interruptions such as a single CTT or multiple CCG and CTA tracts were found in the expanded CTG repeat alleles and were not clearly associated with a stabilization of CTG array (Hu et al., 2017; Moseley et al., 2000). In DM1, rare DMPK intermediate alleles revealed various CCG interruptions within CTG repeats (Braida et al., 2010; Leeflang & Arnheim, 1995; Musova et al., 2009). Interestingly, Leeflang and Arnheim (1995) observed that a DMPK allele with a (CTG)4(CCGCTG)16CTG pattern is more stable than an allele carrying 27 pure CTG repeats in single sperm analysis. Different DM1 cohort studies have revealed that repeat interruptions are observed in 3%–4% of French, 5% of Czech or 4%–8% of Italian DM1 patients (Botta et al., 2017; Braida et al., 2010; Musova et al., 2009; Santoro et al., 2013). CCG, GGC, and CTC interruptions have been first reported at the 3′ end of the CTG repeat expansion in DM1 patients (Botta et al., 2017; Braida et al., 2010; Musova et al., 2009; Pesovic et al., 2017; Santoro et al., 2013). Recently, multiple CCG interruptions in the 5′ end of the CTG repeat sequence were described in four DM1 patients from two distinct DM1 families. These results show that CCG interruptions are not exclusively located in the 3′end of the CTG repeat expansion (Botta et al., 2017; Dryland, Doherty, Love, & Love, 2013). All these interruptions were associated with stabilization or contraction of the expanded CTG repeat tracts through maternal and paternal transmissions (Botta et al., 2017; Braida et al., 2010; Musova et al., 2009). In addition, using mathematical models, Braida et al. (2010) showed that the degree of somatic mosaicism is low in an atypical DM1/Charcot Dutch family compared with the expected somatic mosaicism for the corresponding age of sampling and progenitor allele length (Higham & Monckton, 2013). Different studies have shown that formation of secondary DNA structures, nucleosome positioning, methylation status and transcription level can be affected by the AGG, CAT, GGA, and CCG interruptions described in SCA1, FXS, FRDA, and DM1 (Jarem, Huckaby, & Delaney, 2010; Mulvihill, Nichol Edamura, Hagerman, Pearson, & Wang, 2005; Pearson et al., 1998; Sakamoto et al., 2001; Santoro et al., 2015; Volle & Delaney, 2013). However, the role of interruptions in the mechanisms of TNR stabilization/contraction remains elusive, especially for DM1.
The aim of our study is to gain additional insights on the mechanisms of CTG repeat contractions observed in some DM1 families. We therefore characterized the sequences and dynamics of CTG repeat tracts across generations. For the first time, we studied the somatic mosaicism in blood from two unusual DM1 families that exhibit apparent intergenerational contractions of the CTG repeat compared with DM1 patients carrying pure repeat and matched in age at sampling and in inherited CTG repeat length. Our data suggest that a single nucleotide change in the 5′ end of the CTG repeat array is sufficient to prevent expansions across generations and to decrease somatic mosaicism.
2 MATERIALS AND METHODS
2.1 DM1 samples
DM1 patients from families A and B were recruited by the Genetics department of the Necker-Enfants Malades Hospital and DM-Scope registry. DM1 patients with pure repeats were selected from DM-scope registry (Dogan, Puymirat, & Bassez, 2015). The study was performed in collaboration with the Genethon Biobank (BACG) which has been approved by the French data protection authority (National Commission on Informatics and Liberty [CNIL] and the French Ministry of research, N° AC-2007-18, (06/25/2008). Blood samples were collected according the following consent procedure that was approved by the national ethic committee CCTIRS (Advisory Committee on Information Processing in Material Research in the Field of Health): adult patients or the legal guardians received an information letter presenting the study. A written informed consent was required before collecting blood samples and sending them to the Genethon Biobank. Post mortem heart of DM1 fetus was sampled at autopsy twenty years ago and no law required approvals at the time of this sample collection (Michel, Huguet-Lachon, & Gourdon, 2015). The length of the CTG repeats, the age of sampling and symptoms are listed in the Table 1 and Supp. Table S1 for each individual. Lymphoblastoid cell lines (LCLs) were available from the A.3, A4.1, and A4.2 individuals. PBLs from the A4.1 and A4.2 individuals were also available.
| Interruption | DM1 features | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Sex | Normal allele length | Expanded allele length (nCTG) | 5′ | Middle | 3′ | Interruption pattern (5′–3′) | Age of consultation and sampling (yo) | DM1 symptoms |
| A1 | F | 21 | 170 | Yes | No | No | (CTG)31(CAG)1(CTG)x-170 | 69 | Severe muscular weakness -Feeding difficulties - Nasogastric enteral feeding 72 yo: death |
| A2 | F | 5 | 150 | Yes | No | No | (CTG)31(CAG)1(CTG)x-150 | 49 | Severe muscular weakness – Myotonia -Heart conductive defect - Implantable cardiovector defrillator 58 yo: Heart attack |
| A3 | F | 5 | 140 | Yes | No | No | (CTG)29(CAG)1(CTG)x-140 | 32 | Asymptomatic |
| A4.1 | F | 5 | 125 | Yes | No | No | (CTG)31(CAG)1(CTG)x-125 | 13 | Asymptomatic |
| A4.2 | F | 21 | 130 | Yes | No | No | (CTG)31(CAG)1(CTG)x-130 | 7 | Asymptomatic |
| A4.3 | – | 21 | 125 | Yes | No | No | (CTG)30(CAG)1(CTG)x-125 | – | Therapeutic abortion |
| B1 | F | 13 | 365 | Yes | ND | No | (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CGG)1(CTG)n | 58 | Severe muscular weakness - Feeding difficulties - Nasogastric enteral feeding |
| B2 | F | 11 | 310 | Yes | ND | No | (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CGG)1(CTG)n | 33 | No muscular weakness-Myotonia |
| B3.1 | – | 13 | 300 | Yes | ND | No | (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CGG)1(CTG)n | – | Therapeutic abortion |
| B3.2 | – | 13 | 235 | Yes | ND | No | (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CGG)1(CTG)n | – | Therapeutic abortion |
| B3.3 | – | 5 | 250 | Yes | ND | No | (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CGG)1(CTG)n | – | Therapeutic abortion |
2.2 Estimation of inherited CTG repeat length in DM1 patients
To estimate the inherited CTG repeat length in DM1 patients, 5 ng of DNA from blood or trophoblast was amplified in a 25 μl reaction using 0.4 μM ST300F (5′-GAACTGTCTTCGACTCCGGG-3′) and ST300R (5′-GCACTTTGCGAACCAACGAT-3′) primers, 1 × Custom master mix (Thermo Fischer Scientific, Vilnius, Lithuania) and 0.04U Thermoperfect Taq polymerase (Peak International Product b.v, Eerbeek, The Netherlands). The following cycling conditions were used: 5 min at 96°C; 45 sec at 96°C, 30 sec at 60°C and 3 min at 72°C (30 cycles); 1 min at 60°C and 10 min at 72°C (1 cycle). PCR product was mixed with DNA-loading dye and subjected to electrophoresis on a 1.5% agarose gel at 120 V for 1–2 hr. The size of the PCR products was measured using Bio-Rad's Image Lab software. The approximate number of triplet repeats can be obtained by subtracting 361 bp (corresponding to the size of 5′ and 3′ flanking regions) to the PCR product length divided by 3. To complete our analysis, PCR was performed with 101 (5′- CTTCCCAGGCCTGCAGTTTGCCCATC-3′) and 102 (5′-GAACGGGGCTCGAAGGGTCCT GTAGC-3′) primers (Seznec et al., 2000). The approximate number of triplet repeats can be obtained by subtracting 112 bp (corresponding to the size of 5′ and 3′ flanking regions) to the PCR product length divided by 3.
2.3 5′ Triplet prime PCR
To analyze the purity of the CTG repeat tract at the 5′ end of the CTG repeat array, triplet prime PCR (TP-PCR) was performed on both strands of the CTG repeat (Warner et al., 1996). 5′ of the CTG repeat was amplified using the primer upstream of the CTG repeat DM-C-FAM (5′FAM-AACGGGGCTCGAAGGGTCCT-3′) and P3R (5′-TACGCATCCCAGTTTGAGACG-3′) and P4CAG (5-’TACGCATCCCAGTTTGAGACGCAGCAGCAGCAGCAGCA-3′) primers (Warner et al., 1996). 20–100 ng of DNA from blood or trophoblast was amplified in a 25 μl reaction using 0.4 μM of FAM and P3R primers and 0.04 μM of P4CAG primer, 1 × Custom master mix (Thermo Fischer Scientific, Vilnius, Lithuania) and 0.06 U Thermoperfect Taq polymerase (Peak International Product b.v, Eerbeek, The Netherlands). The following cycling conditions were used: 5 min at 94°C; 60 sec at 94°C, 1 min 30 sec at 68°C and 2 min at 72°C (30 cycles) and 10 min at 72°C (1 cycle). The amplified product (1–3 μl) was analyzed using 3500XL genetic analyzer (Applied Biosystems, Foster city, CA, USA) and Gene Mapper software. GeneScan 600 LIZ dye size standard v2.0 is used in this experiment (Thermo Fisher Scientific, Courtaboeuf, France). The method analyzing the purity of the CTG repeat tract at the 3′ end of the CTG repeat array is detailed in Supplementary Material.
2.4 Sequencing
PCR using ST300F and ST300R was performed to sequence normal and expanded CTG repeat tracts. PCR products were purified using NucleoSpin Gel and PCR Clean-Up according to manufacturer recommendation (Macherey Nagels, Hoerdt, France). PCR products were sequenced either by direct sequencing using primer ST300F and the BigDye Terminator v3.1 cycle sequencing kit (Thermo Fischer Scientific, Courtaboeuf, France) or cloning approach. Purified PCR products were cloned using TOPO-TA cloning kit (Thermo Fischer Scientific, Courtaboeuf, France) and each clone was sequenced using primers M13F, M13R and BigDye Terminator v3.1 cycle sequencing kit. Direct sequencing PCR reactions involved 1 cycle of denaturation 96°C (5 min) and 30 cycles of 96°C (30 sec), 58°C (15 sec), 60°C (45 sec). Sequence reaction products were sequenced using 3500xL Genetic analyzer (Applied Biosystem, Foster city, CA, USA) and analyzed using Macvector software.
2.5 Specific TP-PCR
TP-PCRs targeted to the alleles with CAG interruption or multiple CCG interruptions were performed using the primer upstream of the CTG repeat DM-C-FAM (5′FAM-AACGGGGCTCGAAGGGTCCT-3′) and P3R (5′-TACGCATCCCAGTTTGAGACG-3′) and primer binding interruptions. CAG interruption was targeted using ST-P4-5′CAG(CTG)i (5′-TACGCATCCCAGTTTGAGACGCAGCAGCAGCAGCAGCTG-3′) and CCG interruption was targeted using ST-P4-5′CAG(CCG)i (5′-TACGCATCCCAGTTTGAGACGCAGCAGCGGCAGCAGC-3′). Specific TP-PCR was carried out under TP-PCR conditions.
2.6 Small pool PCR
Small pool PCR (SP-PCR) amplifications and PCR product electrophoretic analyses were performed using the methods previously described (Gomes-Pereira, Bidichandani, & Monckton, 2004). Blood DNA samples were digested with HindIII enzyme and SP-PCR was performed using DM-C and DM-BR primers (Monckton, Wong, Ashizawa, & Caskey, 1995). The DNA was denatured by heating to 96°C (5 min). PCR reactions involved 28 cycles of 96°C (45 sec), 68°C (45 sec), and 70°C (3 min) with a chase of 1 min at 68°C and 10 min at 70°C. SP-PCR products were loaded on the same 1,5% agarose gel to compare instability between DM1 patients from families A and B and their DM1 respective matched DM1 patients and ran at 160 V for 16–20 hr. The PCR products were then transferred to GeneScreen Plus Hybridization transfert Membrane (Perkin Elmer, Villebon sur Yvette, France) and detected by hybridization using a nonradioactive method as described in Tome, Nicole, Gomes-Pereira, and Gourdon (2014).
2.7 RNA isolation and reverse transcription
RNAs were extracted from LCLs and PBLs cells as described in Huguet et al. (2012). Briefly, cells were homogenized in Trizol (Thermo Fischer Scientific, Courtaboeuf, France) using a tissue lyser and total RNA was extracted using PureLink RNA miniKit (Ambion, Thermo Fischer Scientific, Courtaboeuf, France) according to the manufacturer's protocol. To analyze the expression of DMPK transcripts, cDNA was synthesized using Superscript II reverse transcriptase (Thermo Fischer Scientific, Courtaboeuf, France) and random hexamer primers from 1ug of RNA from LCLs. To investigate the expression of antisense or sense DMPK transcripts, cDNA was synthesized using Superscript III reverse transcriptase (Thermo Fischer Scientific bought Lifectechnologies) and strand specific primers (Primer sens LK-RTDMPK3S 5′-CGACTGGAGCACGAGGACACTGACGCCTGCCAGTTCACAACCGCTCCGAGCGT-3′ and primer antisens LK-AMH 5′-CGACTGGAGCACGAGGACACTGACGCCTGCCAGTTCACAACCGCTCCGAGCGT-3′) from 1 μg of RNA from LCLs and from 125 ng from PBLs according to manufacturer's protocol. RNAs were tested with retrotranscriptase (RT+) and without retrotranscriptase (RT-) to check DNA genomic contamination.
2.8 PCR
The expression of the DMPK transcripts was analyzed using DM-C and DM-BR primers. The expression of sense DMPK transcripts was analyzed using LK3 (5′-GGAGCACGAGGACACTGA-3′) and 102 (5′-GAACGGGGCTCGAAGGGTCCTTGTAGC-3′) primers. cDNAs were amplified in a 25 μl reaction using 0.4 μM of primers, 1 × buffer, 0.5 mM MgCl2, 10% Glycerol, 0.5 mM dNTP and 0.04U Thermoperfect Taq polymerase (Peak International Product b.v, Eerbeek, The Netherlands). The following cycling conditions were used: 5 min at 96°C; 45 sec at 96°C, 30 sec at 68°C and 3 min at 72°C (30 cycles); 1 min at 68°C and 10 min at 72°C (1 cycle). The expression of antisense DMPK transcripts was analyzed using LK3 and antiN3H3 (5′-TGCGAACCAACGATAG-3′) primers. cDNAs were amplified in 25 μl reaction using 0.4 μM of primers, 1 × buffer, 1 mM MgCl2, 10% glycerol, 0.5 mM dNTP and 0.04U Thermoperfect Taq polymerase (Peak International Product b.v, Eerbeek, The Netherlands). The following cycling conditions were used: 5 min at 96°C; 30 sec at 96°C, 30 sec at 60°C and 1 min at 72°C (30 cycles); 1 min at 60°C and 10 min at 72°C (1 cycle).
2.9 Bisulfite-sequencing PCR
DNA (50–200 ng) from blood of DM1 patients was bisulfite treated using the Imprint DNA modification Kit (Sigma–Aldrich, Lyon, France) according to one step modification procedure. Bisulfite reaction time is adapted for DNA template contains high GC content or secondary structure (150 min). Bisulfite-treated DNA was quantified by Qubit fluoremetric quantification (Thermo Fischer Scientific, Courtaboeuf, France) and amplified as described in Barbe et al. (2017). Upstream and downstream of the CTG repeat tracts (CTCF1 and CTCF2 regions, respectively) were amplified by nested and hemi-nested PCR (Barbe et al., 2017). For the first PCR, 2.5–5 ng of bisulfite-treated DNA was amplified in a 25 μl reaction using 0.4 μM primers, 1 × Custom master mix (Thermo Fischer Scientific, Courtaboeuf, France) and 0.04U Thermoperfect Taq polymerase (Peak International Product b.v, Eerbeek, The Netherlands). The following cycling conditions were used: 5 min at 94°C; 30 sec at 94°C, 30 sec at 55°C and 30 sec at 72°C (40 cycles); 5 min at 72°C (1 cycle). PCR products (3—6 μl) from the first round were amplified following the same conditions of the first PCR. Amplicons were resolved by running 2% agarose gel electrophopheresis. The length of PCR products obtained from CTCF1 and CTCF2 regions is 294 and 169 bp, respectively. PCR products were purified using NucleoSpin Gel and PCR Clean-Up according to manufacturer recommendation (Macherey Nagels, Hoerdt, France). Purified PCR products were cloned using TOPO-TA cloning kit (Thermo Fischer Scientific, Courtaboeuf, France) and each clone (15–60 ng) was sequenced using primers M13R and BigDye Terminator v3.1 cycle sequencing kit. Sequence reaction products were sequenced using 3500xL Genetic analyzer (Applied Biosystem, Foster city, CA, USA) and analyzed using quantification tool for methylation analysis (QUMA). Samples showing less that 95% of bisulfite conversion and more that 10% of DNA mismatches were excluded of our analyses. At least 20 clones sequencing were performed to quantify the level of the methylation of each CpG site.
3 RESULTS
3.1 Apparent CTG repeat contractions in successive maternal transmissions
Among 51 French DM1 families followed in the Genetics department of the Necker-Enfants Malades Hospital (corresponding to about 150 transmissions), two families (families A and B) showed maternal intergenerational CTG repeat contractions (Figure 1). PCR amplification of the CTG repeat array using blood DNA revealed a decrease of CTG repeat length across three generations and five maternal transmissions in family A (Figure 1B). In family B, no apparent intergenerational expansion was observed in four maternal transmissions across two successive generations (Figure 1). In addition, despite adult symptoms reported in the mothers A1, A2, and B1 from the first generations in both families, no congenital, juvenile or childhood forms of DM1 were observed after two to three generations and four maternal transmissions suggesting no worsening of DM1 symptoms across generation in these two families (Table 1).
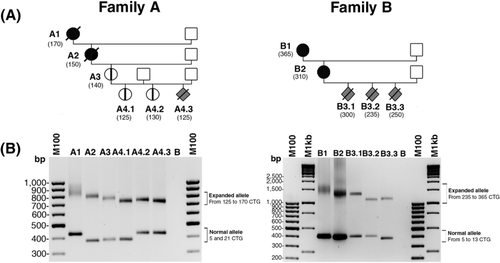
3.2 Identification of a unique CAG in the 5′ end of the CTG repeats in family A
In order to characterize the nature of the expanded CTG repeat sequences in family A, we performed TP-PCR both at the 5′ and 3′ ends of the CTG repeat, on blood DNA from different members of this family (Figure 2B and Supp. Figure S1A). By this technique, we covered all CTG repeat expansions in DM1 members that carry less than 150 CTG repeat tracts. For all DM1 patients, a distinct gap is observed in the contiguous peak pattern of the 5′ TP-PCR products whereas the peak pattern of the 3′ TP-PCR products is regular (Figure 2B and Supp. Figure S1A). The peak pattern revealed the presence of interruption(s) at the 5′ end of the CTG repeat expansion in all DM1 members. The 3′ end and the middle part of the CTG repeat sequence showed no interruption (Supp. Figure S1A). Surprisingly, we did not detect a clear gap using 3′ TP-PCR that also covers the full CTG repeat tracts, probably due to lower fluorescent signal at the end of the 3′ TP-PCR products. In order to identify the 5′ interruption(s) in this family, we combined cloning and direct sequencing of the CTG repeat expansions amplified by PCR using two primers located upstream and downstream of the repeat. For the first time in DM1 families, we identified a unique CAG triplet closer to the 5′ end of the CTG repeat (Figure 3A). Digestions of the CTG repeat PCR products with AluI enzyme (that recognizes AGCT motif) confirmed the presence of a CAG repeat interruption (Supp. Figure S2). By haplotype analysis at the DM locus in members of family A, we observed that this interrupted CTG repeat expansion is associated with the common DM1 haplotype (Haplotype A) as described in Neville, Mahadevan, Barcelo, and Korneluk (1994) (data not shown).

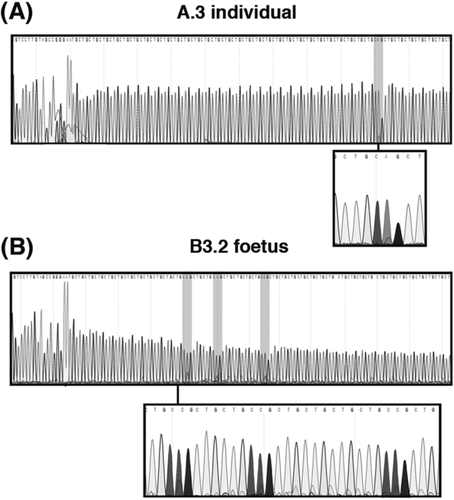
3.3 Detection of three nonconsecutive CCG interruptions in the 5′ end of the CTG repeat in family B
In family B, carrying more than 235 triplet repeats, bidirectional TP-PCR revealed the presence of interruptions at the 5′ end of the CTG repeat expansion in five members (four transmissions and two generations; Figure 2C). In contrast, the 3′ end of the repeat appeared free of interruption (Supp. Figure S1C). The TP-PCR method did not allow the characterization of the middle of the expanded repeats in this family. By cloning and sequencing the CTG repeat tracts, we identified three nonconsecutive CCG interruptions in the 5′ of the expansion. All the DM1 members in this family carry a new identical motif (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CCG)1 in the 5′ end of the tracts that have not been described before (Figure 3B). The presence of CCG interruptions was confirmed by digestion using AciI enzyme (Supp. Figure S3).
3.4 CTG repeat instability predominantly occurs in 3′ of the interruptions
In order to localize directly the interruptions in the CTG repeats from both families A and B, we combined direct sequencing and specific 5′ TP-PCR with one primer covering the interruption and a second primer located upstream of the CTG repeat array (Figure 4 and data not shown). By specific TP-PCR, we observed a major peak corresponding to the interrupted DMPK allele products in family A (Figure 4A). DM1 individuals A1 and A2 carry the CAG repeat interruption at the 32nd trinucleotide position, A3, A4.1, and A4.2 at the 30th position and A4.3 at the 31st position. This observation suggests that small contractions and expansions (−2 or +1) can occur in 5′ of the interruption but the majority of the repeat length difference between individuals arose in 3′ of the interruptions. In family B, as expected, 5′ specific TP-PCR showed three distinct peaks corresponding to three nonconsecutive CCG repeat interruptions (Figure 4B). The motif (CTG)11(CCG)1(CTG)2(CCG)1(CTG)4(CCG)1 was found at the same position in the five DM1 individuals indicating that the CTG repeat length changes between individuals occurred in 3′ of the interruptions.

3.5 5′ Interruptions decrease the level of somatic mosaicism in blood
In order to analyze the CTG repeat somatic mosaicism in blood, SP PCR was performed using blood DNA from A2, A3, and B2 individuals (Figure 5). DM1 patients with pure CTG repeat expansions and matched in age and CTG repeat lengths as close as possible were used for comparison (Supp. Table S1). The extent of somatic mosaicisms appeared lower for A2, A3, and B2 individuals compared with the respective matched DM1 patients with pure repeats, despite the heterogeneity observed between these DM1 patients (Figure 5). These results indicate that 5′ interruptions are associated with a lower rate of somatic instability. However, CTG repeat expansions can still be observed for A2, A3 and B2 individuals suggesting that the CTG repeat length could increase over time (Figure 5). SP-PCR experiments performed at two time points over a period of about 10 years revealed no detectable increase of the somatic mosaicism in A3 (Figure 6A). However, individual B2 showed a small shift of the mosaicism toward expansions with age (Figure 6B). Classical PCR amplifications with the different ages at sampling support the apparent intergenerational contractions in family A and at least stabilization of the repeat over maternal transmission in family B (Figure 6C and D).

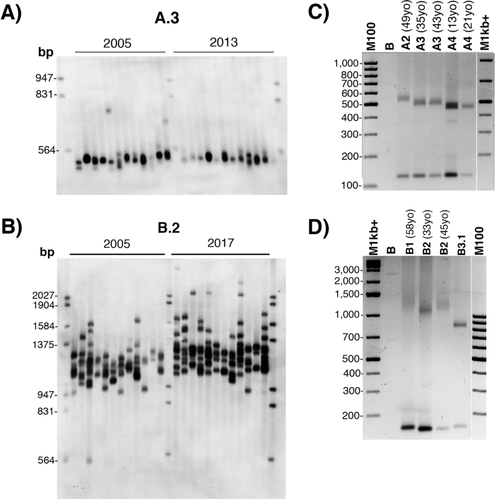
3.6 Expression of DMPK in family A
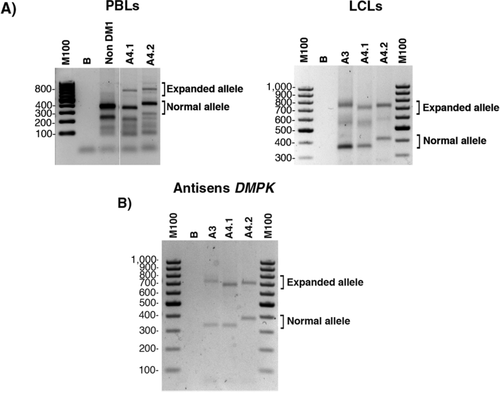
In order to understand how a single nucleotide change in 5′ can induce the unusual behavior of the CTG repeat in family A, we checked if DMPK gene is expressed or not in both direction in available samples from A3, A4.1, and A4.2 by RT-PCR. We observed that normal and expanded DMPK alleles are transcribed in PBLs and in LCLs (Figure 7A). Using specific primers, we observed that antisense transcripts for the normal and expanded DMPK alleles are expressed in LCLs (Figure 7B). An absence of expanded DMPK transcripts cannot explain the atypical dynamic of the CTG repeats observed in family A.
3.7 No differential CpG methylation profile in families A and B
Recently, it was reported that multiple CCG interruptions at the 3′ end of CTG repeat tracts are associated with a hypermethylation of the region downstream of the CTG repeat expansions. To investigate if a unique CAG or multiple CCG interruptions, located at the 5′end of repeat tracts, could also alter the levels of methylation in the flanking regions, we used bisulfite sequencing. We found no difference in the CpG methylation status between A3 and B2 individuals and matched DM1 patients with pure repeats (Figure 8).
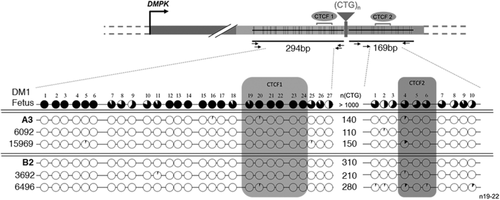
4 DISCUSSION
In this study, we report two unusual French DM1 families showing apparent CTG repeat contractions over three and two maternal successive transmissions corresponding to five and four maternal transmissions in each family, respectively. No juvenile or congenital forms were observed in these two families, although it would have been expected that both forms of DM1 would be transmitted after several successive maternal transmissions (Harper et al., 1992). These observations suggest that one or more genetic factor(s) prevent CTG repeat expansions across maternal transmissions in these families. We describe, for the first time, a unique CAG interruption in 5′ of the expanded CTG repeat in one family and, as previously described in Italian families, three nonconsecutive CCG interruptions (Botta et al., 2017). However, the location and number of CCG interruptions differ between the French and Italian families. In family A, the CAG interruption is associated with the DM haplotype suggesting that pure and interrupted repeat expansions shared the ancestral haplotype as observed in SCA2 (Ramos et al., 2010). This observation suggests that the CAG interruption has arisen as de novo base substitution in this family. This hypothesis is supported by the direct observation of de novo CCG or CTC interruptions across one or several generations in DM1 families (Botta et al., 2017; Braida et al., 2010; Musova et al., 2009; Pesovic et al., 2017). Identification of a unique CAG interruption in one of our French DM1 families with apparent intergenerational contractions suggests that not only multiple interruptions but also unique interruption are sufficient to prevent CTG repeat expansions through several maternal transmissions. We also found that the extent of somatic mosaicism decreases in blood from patients carrying the 5′ unique CAG interruption or the 5′ multiple CCG interruptions. Although somatic mosaicism (frequency of expansions or CTG repeat size changes) is altered in these DM1 patients, no apparent contraction was observed in blood. Moreover, B2 patient clearly showed somatic expansions with age. These findings suggest that the behavior of interrupted CTG repeat expansion differs between germline and soma. TNR instability is sensitive to the level of DNA repair proteins, replication profiles, and transcription (Cleary & Pearson, 2005; Jones, Houlden, & Tabrizi, 2017; Pearson, Nichol Edamura, & Cleary, 2005; Santoro et al., 2016; Tome et al., 2013; Tome et al., 2011). The efficiency of the different processes involved in TNR instability probably varies in somatic and germinal tissues and could be affected by the presence of interruptions in CTG repeat expansions in different ways.
Interruptions in CTG repeat expansions allowed analysis of the polarity of the CTG repeat tract changes in the two DM1 French families using specific TP-PCR. Small CTG expansions and contractions were observed in 5′ of the unique CAG interruption whereas no change was detected in 5′ of the multiple CCG interruptions. Despite few CTG repeat changes in 5′ of the CAG interruption, CTG repeat changes preferentially occurred in 3′ of the interruptions in both families A and B. Our data indicate that CTG repeat changes (contractions and/or expansions) are polar in DM1 patients carrying interrupted CTG repeat expansion. Interestingly, the 3′ polarity of the tract length change was also observed in yeast carrying a CAG repeat tract interrupted by a unique CAT interruption (Maurer, O'Callaghan, & Livingston, 1998).
Little is known about the mode of action of interruptions on the dynamics of TNRs instability. Studies in different model systems have shown that transcription through CTG/CAG repeats is involved in TNR instability (Dion, 2014). Mangiarini et al. (1997) observed in HD mouse models that expanded CAG repeats are unstable only in the lines expressing the huntingtine transgene. These data support the hypothesis according which transcription of TNR loci is necessary to destabilize the CTG/CAG repeat tracts. More recently, Nakamori, Pearson, and Thornton (2011) have demonstrated that bidirectional transcription of DMPK further destabilizes CTG repeat expansions in transfected human cells in comparison with unidirectional transcription. In our study, we observed that normal and mutated alleles are both transcribed bidirectionally in LCLs and/or PBLs from family A. Therefore, these data suggest that the absence of expanded DMPK transcripts cannot explain the atypical dynamic of the CTG repeats observed in this family. Previous studies have shown that CpG methylation near expanded CTG/CAG repeats change the dynamics of repeat expansions in SCA1 and SCA7 mouse models (Dion, Lin, Hubert, Waterland, & Wilson, 2008; Libby et al., 2008). In addition, Santoro and his colleagues have found that the 3′CCG interrupted expanded alleles were associated with CpG hypermethylation downstream of the CTG repeat expansion compared with pure expanded alleles in patients carrying more than 400 CTG repeats (Santoro et al., 2015). However, we found no difference in the levels of CpG methylation between patients carrying interrupted or pure CTG repeat expansions. These results suggest that methylation in the vicinity of the repeat tract is not the major mechanism involved in the unusual instability observed in families A and B. The differences in the CpG methylation profiles between our two families and the families reported by Santoro et al. could be explained by differences in location, type, and pattern of interruptions and inherited repeat lengths.
In vitro analyses of interrupted TNR showed that interruptions limit the formation of secondary structures formed by TNR in SCA1, FRAXA, and FRDA (Jarem et al., 2010; Pearson et al., 1998; Sakamoto et al., 2001). Pearson et al. (1998) have observed that a unique AGG interruption in the expanded CGG repeat tract is sufficient to weaken the formation of slipped strand DNA structure. Thus, a single CAG interruption in the 5′ end of the CTG repeat expansions could modulate the formation and/or the stability of hairpin structures that could impact the dynamics of instability by altering DNA repair or the replication fork progression. Destabilization of interrupted CTG repeats has been observed in MMR-deficient yeast suggesting that mismatch repair proteins such as MSH2 are important to stabilize the interrupted CTG sequence in this model (Rolfsmeier, Dixon, & Lahue, 2000). We can assume that the mismatch repair proteins may also stabilize the interrupted CTG repeat in humans.
In conclusion, our data describe DM1 patients with a new pattern of CCG interruptions at the 5′ end of the CTG repeat expansion as well as a unique CAG interruption. These two types of interruptions are associated with successive maternal CTG repeat contractions and low somatic instability in DM1. Strikingly, these results suggest that a single A modification in CTG repeat expansions is sufficient to alter the CTG repeat dynamics in DM1 patients by mechanism(s) yet to be discovered.
ACKNOWLEDGMENTS
The authors would like to thank DM1 patients, Banque Genethon-culture cellulaire and colleagues at Imagine Institute for helpful discussion and comments. We specially thank Thierry Larmonier, Eliane Gardais, Safaa Saker-Delye, Pauline Arnaud, Chasserieau Raphaële, Pascal Cintas, Ana-maria Cobo Esteban, Marie-Carmen Cruz, Dalil Hamroun, Armelle Magot, Alexandra Nadaj-Pakleza, Anne-Catherine Aube-Nathier, and Andoni Urtizberea for their active involvement in this project.
DISCLOSURE STATEMENT
The authors declare no conflict of interest.