DGAT2 Mutation in a Family with Autosomal-Dominant Early-Onset Axonal Charcot-Marie-Tooth Disease
Contract grant sponsors: Korean Health Technology R&D Project (HI12C0135 and HI14C3484); National Research Foundation (2014R1A2A2A01003164, 2014R1A2A2A01004240, and 2013R1A1A1059056), Republic of Korea.
Communicated by Jürgen Horst
ABSTRACT
Charcot-Marie-Tooth disease (CMT) is the most common inherited peripheral neuropathy and is a genetically and clinically heterogeneous disorder. We examined a Korean family in which two individuals had an autosomal-dominant axonal CMT with early-onset, sensory ataxia, tremor, and slow disease progression. Pedigree analysis and exome sequencing identified a de novo missense mutation (p.Y223H) in the diacylglycerol O-acyltransferase 2 (DGAT2) gene. DGAT2 encodes an endoplasmic reticulum-mitochondrial-associated membrane protein, acyl-CoA:diacylglycerol acyltransferase, which catalyzes the final step of the triglyceride (TG) biosynthesis pathway. The patient showed consistently decreased serum TG levels, and overexpression of the mutant DGAT2 significantly inhibited the proliferation of mouse motor neuron cells. Moreover, the variant form of human DGAT2 inhibited the axonal branching in the peripheral nervous system of zebrafish. We suggest that mutation of DGAT2 is the novel underlying cause of an autosomal-dominant axonal CMT2 neuropathy. This study will help provide a better understanding of the pathophysiology of axonal CMT and contribute to the molecular diagnostics of peripheral neuropathies.
Introduction
Charcot-Marie-Tooth disease (CMT) is a group of genetically and clinically heterogeneous disorders of the peripheral nervous system, which is characterized by progressive degeneration of the muscles of the extremities and a loss of sensory function. More than 70 genes have been reported to result in a CMT phenotype [Rossor et al., 2013; Tazir et al., 2014]. CMT is conventionally classified into the demyelinating type with motor nerve conduction velocity of <38 m/s, axonal type with ≥38 m/s [Harding and Thomas, 1980], and intermediate type.
Further genetic and phenotypic classification of CMT2 has grown to more than 20 subtypes [Murphy et al., 2012]. Causative genes have been continuously updated to be the novel underlying cause of CMT2, such as: DYNC1H1 (MIM #600112), DHTKD1 (MIM #614984), HINT1 (MIM #601314), MT-ATP6 (MIM #516060), MARS (MIM #156560), HARS (MIM #142810), HADHB (MIM #143450), TFG (MIM #602498), and DNAJB2 (MIM #604139) [Weedon et al., 2011; Pitceathly et al., 2012; Xu et al., 2012; Zimoń et al., 2012; Gonzalez et al., 2013; Hong et al., 2013; Vester et al., 2013; Gess et al., 2014; Tsai et al., 2014]. Despite novel gene identification, considerable CMT2 patients still wait for a test to uncover genetic cause. CMT2-relevant genes function in varied processes, including cellular components along microtubules (DYNC1H1), mitochondrial dynamics and morphology (MFN2, and GDAP1), the structural scaffold of nerve cells (NEFL) and nucleus (LMNA), transcription preinitiation complex assembly (MED25), endocytosis (DNM2); regulation of the cell adhesion-signaling pathway, self ubiquitylation (LRSAM1), protein synthesis (GARS, AARS, MARS, and HARS), calcium channels (TRPV4), chaperoning (HSPB1, HSPB8, and DNAJB2), and carbohydrate and fatty acid metabolism—Kreb's cycle (DHTKD1, MTATP6, and HADHB).
Recently, zebrafish have been widely used to model human disorders including CMT. For example, MFN2 (MIM #608507) and LRSAM1 (MIM #610933), causative genes of CMT2, were manipulated to mimic the pathological phenotype of CMT in zebrafish [Weterman et al., 2012; Chapman et al., 2013]. Particularly, the MFN2 model of loss of function revealed an axonal transport defect in the neuromuscular junction (NMJ) in zebrafish [Chapman et al., 2013].
Whole-exome sequencing (WES) has recently been introduced to identify underlying causes in CMT [Choi et al., 2012]. This study performed WES for an early-onset autosomal-dominant CMT2 family, and identified a mutation in the DGAT2 gene (MIM #606983; NM_032564.4). This study also revealed that the overexpression of mutant DGAT2 induced an axonal defect in the peripheral nervous system of zebrafish.
Materials and Methods
Patients and Clinical Studies
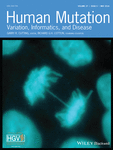
We studied seven members of a Korean axonal CMT family with two affected individuals (family ID: FC490; Fig. 1A). In addition, 500 healthy controls were recruited after careful clinical examinations. Clinical, electrophysiological, histological, and MRI studies were performed by the method of Nakhro et al. (2013). Written informed consent was obtained from all participants according to the protocol approved by the IRB for Kongju National University and Sungkyunkwan University.
Measurement of Proliferation and Triglyceride Levels
To measure cell proliferation, NSC34 cells were cultured in 24-well plates (2×105 cells) for 24 hr, and were then transfected with control and DGAT2 vectors. The proliferation of the cell was determined every 24 hr. The total triglyceride (TG) levels of the cells were determined using a Triglyceride Quantification Kit (Abcam, Cambridge, UK). Fibroblasts from normal controls and the patient were used to determine the cellular TG level.
Western Blotting
Protein expression in NSC34 cells was determined by standard Western blotting. Anit-myc Ab (Abcam), antiactin Ab, antimouse secondary Ab, and antirabbit secondary Ab (Sigma, St. Louis, MO) were used to detect the corresponding proteins. Expression and localization of DGAT2 proteins were analyzed by immunocytochemistry using an anti-myc antibody after transfection into HEK293 cells.
DNA Preparation and Testing for 17p12 Duplication
DNA was purified from blood using a QIAamp blood DNA purification kit (Qiagen, Hilden, Germany). Patient samples were prescreened for 17p12 duplication, which is a major genetic cause of CMT by using hexaplex microsatellite PCR [Choi et al., 2007].
Exome and Transcriptome Sequencing and Filtering of Variants
Exome was captured from four samples (two affected: II-2, III-1; two unaffected: I-1, I-2) using the Human SeqCap EZ Human Exome Library v3.0 (Roche/NimbleGen, Madison, WI), and sequenced by the HiSeq 2000 Genome Analyzer (Illumina, San Diego, CA). We first selected functionally significant variants (missense, nonsense, exonic indel, and splicing site variants) from the WES data, and then filtered out polymorphic variants registered in the dbSNP (http://www.ncbi.nlm.nih.gov) 1000 Genomes Pproject Database (http://www.1000genomes.org/), or Exome Variant Server (http://evs.gs.washington.edu/EVS/) except for very rare variants (minor allele frequencies of less than 0.01). Since the proband's parents were not affected, we chose de novo variants in the proband from the selected pool. Then, we further isolated variants that were presented in the proband's affected son (III-1). For the candidate mutation(s), the genomic evolutionary rate profiling (GERP) scores were determined by the GERP++ program (http://mendel.stanford.edu/SidowLab/downloads/gerp/index.html). For transcriptome analysis, polyA RNA was extracted from distal sural nerve biopsies, and the cDNA library was prepared using the TruSeq RNA library kit (Illumina).
Construction of DGAT2 Vector and Transfection
Total mRNA was purified from HEK293 cells using the RNeasy Mini kit (Qiagen) and cDNA was synthesized using the superscript reverse transcriptase (Invitrogen, Carlsbad, CA) and primers of DGAT2-F (5′-CCATGAAGACCCTCATAGCC) and DGAT2-R (5′-GCTCAGTTCACCTCCAGGAC). After PCR amplification, the product was cloned into a pCR2.1-TOPO vector and moved into the pCMV-myc vector (Clontech, Mountain View, CA). Site-directed mutagenesis was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and primers of DGAT2-MutF (5′-CGGGACACCATAGACCATTTGCTTTC) and DGAT2-MutR (5′-GAAAGCAAATGGTCTATGGTGTCCCG). NSC34 cells (2×105) were transfected with pCMV-myc and cloned DGAT2 DNA using a Lipofectamine 2000 reagent (Invitrogen).
Microinjection of mRNA into Zebrafish Embryos
Zebrafish (AB strain) were raised, staged, and maintained according to standard protocol [Westerfield, 2007]. Wild-type and mutant (Y223H) human DGAT2 genes were subcloned into the pCS2+ vector. The mRNAs of the DNA constructs were synthesized in vitro using the mMESSAGE mMACHINE SP6 kit (Ambion, Austin, TX). 100 pg/nl of each mRNA were injected into one or two cell-stage embryos by microinjection.
Immunohistochemistry of Zebrafish Larvae
The zebrafish larvae collected at 3 and 15 days postfertilization (dpf) were fixed in 4% paraformaldehyde for 2 hr at room temperature (RT) and permeabilized in ice-cold acetone for 7 min. The fixed larvae were blocked in solution (1% DMSO, 1% BSA, 0.5% Triton X-100, and 5% normal goat serum in 0.1 M PBS) for 1 hr at RT, followed by incubation with Alexa 647-conjugated α-BTX (1:100; Molecular Probes, Eugene, OR) for 30 min at RT. The larvae were washed three times in PBDT buffer (1% DMSO, 1% BSA, 0.5% Triton X-100) for 15 min per wash and incubated with primary mouse monoclonal anti-SV2 antibody (1:200; Developmental Studies Hybridoma Bank, Boston, MA) at 4°C overnight. The larvae were washed six times with PBST (0.5% TritonX-100 in 0.1 M PBS) for 15 min per wash and incubated in Alexa Fluor 488 goat antimouse antibody (1:250; Life Technologies, Grand Island, NY) for 2 hr at RT. The larvae were mounted onto slides with 70% glycerol in 0.1 M PBS and fluorescent images were taken using a LSM700 fluorescence confocal microscopy (Zeiss, Jena, Germany).
Analysis of Neuronal Axons in Zebrafish Trunks
The confocal images were processed and analyzed using the Zeiss ZEN imaging software (Zeiss). Trunk neuronal fascicles and branched axons from a fascicle were analyzed in 3 and 15 dpf zebrafish larvae, respectively. The analyzed axonal fascicles and branches were quantified using the Image J plugin Neuron J (NIH, Bethesda, MD). For quantification, the branches per fascicle within one somite of zebrafish were counted in the dorsal and ventral region separately.
Results
Clinical Manifestations
Patient 1
A 38-year-old man (II-2; Fig. 1B) was born from unaffected parents. Frequent falling with ataxia and weakness of the distal lower limbs was first noticed at 8 years of age. He experienced tremors in both hands at 12 years. When he was 37, gait difficulty slowly progressed and he began to use an ankle foot orthosis. A neurological examination at 38 years revealed distal muscle weakness and atrophies, predominantly in the lower limbs without proximal muscle involvements. Bilateral pes cavus, broad-based gait, and atrophic changes of intrinsic foot and calf muscles were noted, but scoliosis was not observed. Sensitivity to pinprick, touch, position, and vibration decreased. Vibration and position senses were more severely disturbed than pain and touch. Sensory ataxia and a positive Romberg sign were present. Deep tendon reflexes were absent, and no pathological reflexes were found. He had a mild category of physical disability with CMT neuropathy score (CMTNS) of 10.
Serum TG level slightly decreased (63.3 ± 1.7 mg/dl) compared with age-matched controls (normal values of 38-year-old men: 125 ± 65 mg/dL). However, serum levels of total cholesterol and LDL-cholesterol were normal with 172 mg/dL (reference value: 130–240 mg/dL) and 106 mg/dL (reference value: <120 mg/dL), respectively. Serum creatine kinase was elevated at 348 IU/l (reference value: <185 IU/l). Blood glucose, HbA1C, lactate, and pyruvate levels were normal.
On neurological and electrophysiological examinations, his parents (I-1 and I-2) did not exhibit any neuromuscular symptoms.
Patient 2
The son of the proband (III-1) was able to walk at the age of 1 year. Dorsiflexion weakness in the great toe and frequent falling were first noticed at 5 years of age. A neurological examination at 6 years revealed predominant weakness in the distal muscle of the lower limbs. He showed mild distal lower limb weakness of bilateral ankle dorsiflexion, but showed normal flexibility in the upper limbs. Like his father, he had sensory ataxia and a positive Romberg sign. Deep tendon reflexes were decreased, and no pathological reflexes were found.
Electrophysiological Features
Electrophysiological examinations of the two affected individuals were consistent with an axonal neuropathy (Table 1). They showed prolonged motor latencies in both the median and ulnar nerves, and compound muscle action potentials of the peroneal and tibial nerves were not elicited. Sensory nerve action potential amplitudes in the sural nerves were absent or decreased bilaterally, and decreased in the median and ulnar nerves. Needle electromyography revealed a neurogenic pattern of muscle degeneration. Visual-evoked potentials and brainstem auditory-evoked potentials were normal.
| Patients | Patient 1 (II-2) | Patient 2 (III-1) | Normal values | ||
|---|---|---|---|---|---|
| Age at onset (yrs) | 8 | 5 | - | ||
| Age at exam (yrs) | 38 | 6 | - | ||
| Side | Right | Left | Right | Left | - |
| Median motor nerve | |||||
| TL (ms) | 5.0* | 4.2* | 2.8 | 3.1 | < 3.9 |
| CMAP (mV) | 12.3 | 13.0 | 9.6 | 10.0 | > 6.0 |
| MNCV (m/s) | 54.2 | 54.5 | 54.8 | 57.1 | > 50.5 |
| Ulnar motor nerve | |||||
| TL (ms) | 3.1* | 3.6* | 2.1 | 2.1 | <3.0 |
| CMAP (mV) | 14.7 | 15.2 | 11.9 | 11.6 | >8.0 |
| MNCV (m/s) | 54.2 | 55.6 | 60.0 | 57.1 | >51.1 |
| Peroneal nerve | |||||
| TL (ms) | A* | A* | 3.5 | 3.0 | <5.3 |
| CMAP (mV) | A* | A* | 5.3 | 4.6 | >1.6 |
| MNCV (m/s) | A* | A* | 47.8 | 43.1 | >41.2 |
| Tibial nerve | A* | A* | |||
| TL (ms) | A* | A* | 2.8 | 2.7 | <5.4 |
| CMAP (mV) | A* | A* | 10.4 | 13.9 | >6.0 |
| MNCV (m/s) | A* | A* | 46.5 | 45.0 | >41.1 |
| Median sensory nerve | |||||
| SNAP (μV) | 5.5* | 6.9* | 14.5 | 11.8 | >8.8 |
| SNCV (m/s) | 31.2* | 30.7* | 33.3* | 32.3* | >39.3 |
| Ulnar sensory nerve | |||||
| SNAP (μV) | 4.7* | 4.9* | 13.0 | 14.2 | >7.9 |
| SNCV (m/s) | 27.0* | 28.8* | 33.7* | 30.2* | >37.5 |
| Sural nerve | |||||
| SNAP (μV) | A* | A* | 9.3 | 11.5 | >6.0 |
| SNCV (m/s) | A* | A* | 30.0* | 30.7* | >32.1 |
- Asterisks (*) indicate abnormal values.
- A, absent potential; TL, terminal latency; CMAP, compound muscle action potential; MNCV, motor nerve conduction velocity; SNAP, sensory nerve action potential; SNCV, sensory nerve conduction velocity.
Length-Dependent Muscle Atrophies in Lower Limb
Muscle atrophies in the lower limbs were observed in the proband. T1-weighted MRIs demonstrated moderate to severe atrophy in the calf muscles, and was predominant in the distal areas. At the thigh level, the semitendinosus muscles, adductor muscles, vastus medialis, and intermedius muscles were relatively sparing compared with the lower limbs (Fig. 1C). In the lower legs, the soleus (arrow), gastrocnemius, and peronei (arrowhead) muscles showed moderate to severe impairment; however, the tibialis anterior and posterior muscles revealed mild fatty infiltration (Fig. 1D).
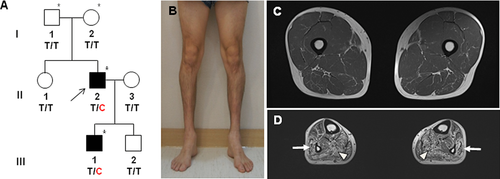
Light microscopic examination of longitudinal and cross-sections of nerve fibers showed slightly decreased size of nerve fascicles, with a mild thickening of perineurium and proliferated perineurial cells. Semithin transverse sections showed focal subperineurial edema, loss of large myelinated fibers (MFs) with medium-sized and small MFs remaining, scattered thinly MFs, and rarely noted regenerating axonal clusters (Fig. 2A). The remaining MFs were slightly decreased and the average diameter was shorter than the age- and sex-matched control as well as left-shifted unimodal distribution pattern (Fig. 2B). The MF% area (5.9%) was smaller than the control (27.1%); however, the g-ratio (0.66 ± 0.08) was similar (0.66). Electron microscopic examination revealed scattered myelinated and unmyelinated axons with swelling or vacuolization of axoplasm and membranous structures. The presence of MFs with a small axonal diameter and thick myelin, and clusters of Schwann cell cytoplasmic processes, including collagen pockets with no axons, was compatible with an axonopathy (Fig. 2C). Degenerating MFs with myelin breakdown and axon were rarely noted (Fig. 2D), but demyelinated axons or onion bulb formation were not observed.
Identification of a Mutation in DGAT2
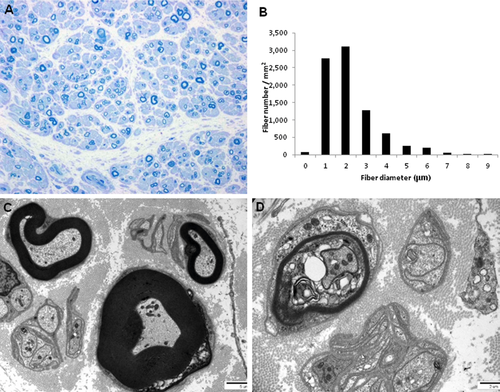
The mean sequencing yield of four WES was approximately 8.83 Gbp/sample with a 91.25% coverage rate (≥10X) (Supp. Table S1). We first used dbSNP142 and 1000 Genome project to filter out all the reported SNPs from the exomes. From the novel or uncommon functionally significant variants, we selected variants that were only present in the affected members. This was easy to do because the proband's parents (I-1, I-2) were both normal, whereas the proband and his son were affected (II-2, III-1). These filtering process narrowed down the search to a single candidate mutation, c.667T>C (p.Y223H) in DGAT2 gene. The DGAT2 mutation was cosegregated in the extended members of the family (Figs. 1A and 3A). The mutation lies in a well-conserved N-acetyltransferase superfamily (NAT-SF) domain (Fig. 3B). The GERP of the mutation site was calculated to a high score of 5.99. The p.Y223H DGAT2 mutation was submitted at the Leiden Open Variation Database (http://www.lovd.nl/DGAT2).
Transcriptome analysis for healthy control samples (N = 3) revealed a relatively high level of DGAT2 expression in the distal sural nerve compared with DGAT1 (MIM #604900) and MFN2 (Fig. 3C). All other functionally significant variants observed in peripheral neuropathy-related genes were considered to be nonpathogenic polymorphisms, because they were found in controls or not segregated with affected individuals in the family that was examined (Supp. Table S2).
Decreased Enzyme Activity and Inhibition of Cell Proliferation by DGAT2 Mutant
When TG levels of the fibroblast originated from the patient (II-2), and sex- and age-matched controls (30–37 years old) were measured, the level was significantly lower than those of control fibroblasts (Fig. 3D). This result was consistent with the decreased serum TG level in the proband.
When wild-type and Y223H mutant DGAT2 were transfected into NSC34 cells, both were well expressed with no significant difference (Fig. 3E). Then, changes of cell proliferation were monitored after overexpression in NSC34. The expression of wild-type DGAT2 protein showed no influence on cell growth; however, Y223H mutant significantly inhibited cell proliferation (Fig. 3F).
To further investigate the influence of mutant DGAT2 protein on axonal integrity, we analyzed tubulin acetylation and phosphorylation of neurofilament, which was reported to associate with neurodegeneration and axonal transport [Irobi et al., 2004; Ackerly et al., 2006]. However, we did not observe any abnormality such as decrease of tubulin acetylation and increase of phospho-neurofilament in NSC34 cells transfected with mutant DGAT2. Intriguingly, endoplasmic reticulum (ER) stress markers were decreased by the transfection of the mutant protein in the presence or absence of an ER stress inducer (Supp. Fig. S1).
Human DGAT2 Mutant Disturbs Axon Branching in Zebrafish Peripheral Nervous System
To examine the pathological effect of the mutation in DGAT2 (Y223H), human DGAT2 (Y223H) was overexpressed in zebrafish and the phenotype was compared with those of exogenous human DGAT2 (WT)-expressing larvae and uninjected controls. The developmental morphology of the mutant Y223H-overexpressed larvae was quite normal at both 3 and 15 dpf (Fig. 4A and E). The major phenotype of CMT2 is an axonal defect in the area of the NMJ, and thus we performed immunohistochemistry with an anti-SV2 antibody and an anti-α-Bungarotoxin (BTX) antidote in zebrafish in order to label the NMJ. At 3 dpf of uninjected- and DGAT2 (WT)-overexpressed zebrafish larvae, we could observe a bundle of neuronal axons (fascicle), labeled by anti-SV2 antibody and muscle fibers labeled by anti-α-BTX antidote. However, the mutant Y223H-overexpressed larvae showed an abrogated formation of the fascicles in the trunk at 3 dpf, whereas the muscle fibers persisted without any significant change (Fig. 4B–D; Supp. Fig. S2).
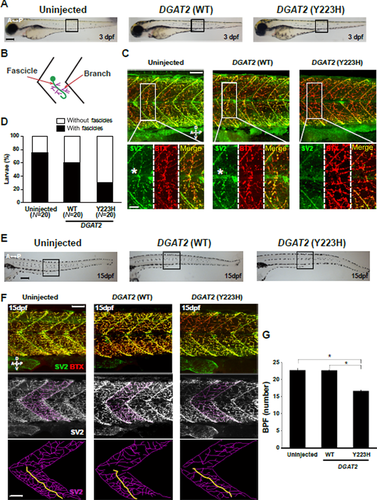
To determine the later effect of the mutant Y223H-induced malformed fascicles in zebrafish, we observed the larvae at 15 dpf. The zebrafish larvae expressing human DGAT2 mutant (Y223H) showed a significant decrease in number of branched axons from the fascicle in the NMJ without any notable defect in the muscular fibers. The numbers of branches per fascicle in the uninjected larvae were 22.7 ± 0.49, 22.65 ± 0.14 in DGAT2 WT-expressing larvae, and 16.65 ± 0.17 in DGAT2 Y223H-expressing larvae (Fig. 4F and G). Taken together, these data suggest that DGAT2 plays an important role in the axon branching of motor neurons, but not for muscle fiber formation in the NMJ.
Discussion
We identified a novel de novo p.Y223H mutation in the DGAT2 from an autosomal-dominant Korean CMT family through pedigree analysis and exome sequencing. The mutation was not found in the healthy controls or in any of the many public human genome variant databases. The mutation was located in the highly conserved NAT-SF domain, and in silico analysis predicted pathogenicity. RNAseq analysis indicated that DGAT2 is more strongly expressed in the distal nerve than DGAT1, although DGAT2 is expressed in most tissues [Stone et al., 2004; Yen et al., 2008].
The DGAT2 encodes an acyl-CoA:diacylglycerol acyltransferase that catalyzes the final step of TG biosynthesis [Cases et al., 2001]. The DGAT2 enzyme is a transmembrane protein that localizes on the ER and is associated with lipid droplets, mitochondria-associated membranes, and microtubules [Stone et al., 2009; Kwiatkowska et al., 2012]. Many subtypes of axonal CMT are associated with mitochondrial dysfunction. Despite similar enzyme activity of DGAT2 and DGAT1, they have no sequence homology and locate in different regions of the ER [Shockey et al., 2006; Yen et al., 2008], indicating distinct roles of both enzymes. DGAT2 plays a key role in generating and maintaining the pool of TGs, which have critical functions in multiple physiological processes as the major storage molecules of fatty acids for energy utilization [Ganji et al., 2004; Friedel et al., 2007; Yen et al., 2008]. Of the many axonal CMT-causative genes, PDK3 (MIM #300906), MT-ATP6, and HADHB are involved in a bioenergetic pathway [Pitceathly et al., 2012; Hong et al., 2013; Kennerson et al., 2013].
TG levels in the patient's serum and fibroblast were significantly lowered than controls, which suggests that the mutation in DGAT2 induces loss of enzymatic activity. Overexpression of the Y223H mutant significantly inhibited the cell proliferation of NSC34 cells, implying that the mutant protein has an antiproliferative effect in motor neurons. These results suggest that decreased TG levels might affect any neuronal function. Thus, the mutation in DGAT2 might cause either haploinsufficiency or a dominant negative effect.
Using the zebrafish model, we observed that the expression of DGAT2 mutant impaired axon branching as well as axon fasciculation in neurons of the NMJ. To further address the pathological mechanism of mutant DGAT2, we examined the aberration of localization, mitochondrial activity, axonal transport, and ER stress by overexpression of the mutant protein. However, we could not find the exact pathological mechanism of the mutant protein except reduced ER stress, indicating that the mutant protein did not induce an unfolded protein response.
Knockdown mouse of BSCL2 (MIM #606158) had significantly reduced DGAT2 synthesis [Payne et al., 2008]. BSCL2 was originally identified as a loss-of-function gene for congenital lipodystrophy type 2 [Magré et al., 2001]. In subsequent studies, mutations in BSCL2 have also been reported to cause autosomal-dominant distal hereditary motor neuropathies. At this juncture, a strong correlation of DGAT2 to lipodystrophy can be drawn owing to its imperative influence on the same pathway.
Clinical manifestations of the present axonal CMT family were characterized by early-onset age, sensory ataxia, tremor, and slow disease progression. Lower limb MRIs of patient 1 with a 30 years disease duration revealed mild muscle atrophy and fatty infiltrations in the bilateral calf muscles. However, the lower limb muscles were almost intact at the level of the hips and thighs. These features suggest length-dependent axonal degeneration. PRX- and FGD4-associated demyelinating CMT neuropathies are characterized by slow clinical progression, early-onset, and sensory ataxia. Although they display different types of neuropathies between demyelinating and axonal neuropathies, it is interesting that some clinical features of DGAT2-associated CMT were similar to those of CMT patients with PRX (MIM #605725) and FGD4 (MIM #611104) mutations [Guilbot et al., 2001; Delague et al., 2007; Stendel et al., 2007].
DGAT2 has not been linked to any disease in the OMIM database (http://www.omim.org) thus far; however, there is considerable documentation supporting the function and phenotypic implementation of this gene. Here, we suggest that mutation in DGAT2 is the novel underlying cause of an autosomal-dominant CMT2 phenotype that has been demonstrated by genomic analysis, and in vitro and in vivo studies. We believe this study will help provide a better understanding of the pathophysiology of DGAT2 in relation to axonal neuropathies and contribute to the exact molecular diagnostics of CMT.
Acknowledgments
We would like to thank the patients’ family for their cooperation and sample donation. We also thank Macrogen Inc. (Seoul, South Korea) for exome and transcriptome data analysis.
Disclosure statement: The authors declare no conflict of interest.