Mutations in SNRPB, Encoding Components of the Core Splicing Machinery, Cause Cerebro-Costo-Mandibular Syndrome
Contract grant sponsor: Séverine Bacrot has been supported by a Fondation pour la Recherche Médicale (FRM) funding (DEA20130727667). Niklas Feldhahn was supported through a Bennett fellowship by Leukaemia & Lymphoma Research (LLR).
Communicated by Christophe Béroud
[Correction added on 28 January 2015, after online publication on 11 December 2014: the Abstract was corrected.]
ABSTRACT
Cerebro-costo-mandibular syndrome (CCMS) is a developmental disorder characterized by the association of Pierre Robin sequence and posterior rib defects. Exome sequencing and Sanger sequencing in five unrelated CCMS patients revealed five heterozygous variants in the small nuclear ribonucleoprotein polypeptides B and B1 (SNRPB) gene. This gene includes three transcripts, namely transcripts 1 and 2, encoding components of the core spliceosomal machinery (SmB′ and SmB) and transcript 3 undergoing nonsense-mediated mRNA decay. All variants were located in the premature termination codon (PTC)-introducing alternative exon of transcript 3. Quantitative RT-PCR analysis revealed a significant increase in transcript 3 levels in leukocytes of CCMS individuals compared to controls. We conclude that CCMS is due to heterozygous mutations in SNRPB, enhancing inclusion of a SNRPB PTC-introducing alternative exon, and show that this developmental disease is caused by defects in the splicing machinery. Our finding confirms the report of SNRPB mutations in CCMS patients by Lynch et al. (2014) and further extends the clinical and molecular observations.
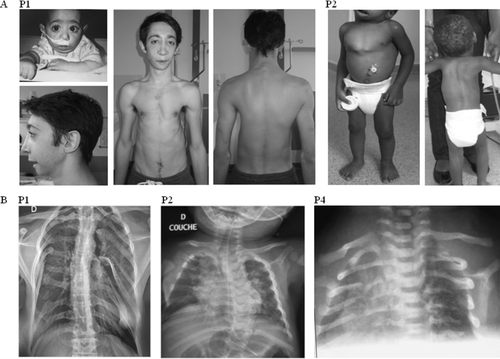
The cerebro-costo-mandibular syndrome (CCMS; MIM #117650) is a developmental disorder combining Pierre Robin sequence and posterior rib defects (“rib gaps”). Since its original description by Smith [Smith et al., 1966], more than 80 cases have been reported. Orofacial anomalies include microretrognathia, glossoptosis, and soft palate cleft, usually U-shaped cleft but V-shaped cleft is possible. A variety of rib defects have been reported ranging from a few dorsal rib gaps to complete absence of rib ossification. X-rays show apparent discontinuity between the ossified ribs, as if they were split into two parts. This may lead to “flail chest,” a paradoxical movement of the thoracic wall. At birth, respiratory difficulties often lead to severe hypoxia, which may be responsible for intellectual disability. Other features have been occasionally reported, including pre- and postnatal growth retardation, microcephaly, vertebral anomalies, spina bifida, and absence of external ear canals. The majority of cases are sporadic but both autosomal dominant and recessive inheritances have been described and males and females are equally affected. The etiology of CCMS is hitherto unknown. Conserved oligomeric Golgi subunit 1 (COG1) variants have been identified in two patients with a CCMS-like phenotype [Zeevaert et al., 2009] and a few other candidate genes have been excluded in CCMS, namely MYF5, GSC, RUNX2, and TCOF1 [Su et al., 2010].
In order to identify the gene responsible for CCMS, five patients were recruited. All five fulfilled inclusion criteria namely Pierre Robin sequence and rib gap defects, without intellectual disability (Fig. 1, Supp. Table S1). They were all of French origin and no consanguinity was noted (three males aged 3–19 years and two females aged 22–35 years). Four cases were isolated and one was a familial form (patient 5 has an affected daughter), supporting autosomal dominant inheritance. Informed consents for participation to the study, sample collection, and photograph publication were obtained via protocols approved by our local ethics committee.
We first considered the nucleic acid binding protein 2 (NABP2; MIM #612104) and NABP1 (MIM #612103) genes as candidate genes, based on the phenotype of the NABP2 KO murine model. This model displays an almost complete lack of ribcage ossification leading to perinatal death, shortened mandible often associated with cleft palate, and limb outgrowth defect, closely reminiscent of the CCMS phenotype [Feldhahn et al., 2012; Gu et al., 2013; Shi et al., 2013]. Both genes were excluded by direct sequencing (data not shown).
Two cases (P2 and P3) were then selected for whole exome sequencing analysis. Exome capture was performed with the SureSelect Human All Exon kit (Agilent Technologies, Les Ulis, France) and paired-end sequencing was performed on an Illumina HiSeq, from the Imagine Institute Genomics platform. All the variants were annotated with an in-house-developed annotation software system. We focused our analyses on nonsynonymous variants, splice-acceptor and donor-site variants, and coding indels, not considered benign according to the prediction software SIFT (http://sift.bii.a-star.edu.sg/, cutoff value = 0.05) and Polyphen2 (http://genetics.bwh.harvard.edu/pph2/, HumVar cutoff = 0.447). We also defined variants as previously unidentified if they were absent from all data sets, including dbSNP (http://www.ncbi.nlm.nih.gov.gate2.inist.fr/SNP/, dbSNP 138), Exome variant server (http://evs.gs.washington.edu/EVS/, evs_5400), the 1000 Genomes Project (http://browser.1000genomes.org), and in-house genome data. Under the assumption of a dominant mode of inheritance, only two genes were found to harbor heterozygous missense variants in the two patients, namely small nuclear ribonucleoprotein polypeptides B and B1 (SNRPB; MIM #182282) and OR8H1 gene (Supp. Table S2). The results were confirmed by Sanger sequencing (P2: c.165G>C [p.Arg55Ser] (Polyphen2: 0.76 and SIFT: 0) and P3: c.164G>T [p.Arg55Met] (Polyphen2: 0.91 and SIFT: 0), ENST00000474384). No pathogenic variants of OR8H1 were found in the other three cases. By contrast, the screening of SNPRB in the three other cases led to identification of three heterozygous variants in the same region of SNRPB (P1: c.166G>C [p.Gly56Arg] (Polyphen2: 0.99 and SIFT: 0), P4: c.213+57C>A and P5: c.164G>C [p.Arg55Thr] (Polyphen2: 0.76 and SIFT: 0), ENST00000474384). These variants were not observed in the healthy parents (of patient 1–2 and 4). Data were submitted to the Leiden Open Variation Database (http://databases.lovd.nl/shared/transcripts/23960).
The SNRPB gene encodes two nearly identical core spliceosome components: SmB and SmB′. Interestingly, CCMS variants consistently involved the second intron of the primary transcripts (transcripts 1 and 2: NM_198216 and NM_003091, SNRPB_002 and SNRPB_001 respectively, Ensembl Human Genome server, http://ensembl.org) in a region subject to alternative splicing (AS) (Fig. 2A and B). Retention of this alternative exon results in premature termination codon (PTC), leading to the formation of transcript 3 (SNRPB_003, ENST00000474384), target of nonsense-mediated mRNA decay (NMD). This AS coupled to NMD (AS-NMD) is highly conserved in mammalian genomes (UCSC genome browser, http://genome.ucsc.edu) and allows the SmB/B′ protein self-regulation by promoting inclusion of this PTC-introducing alternative exon in its own pre-mRNA [Saltzman et al., 2008, 2011].
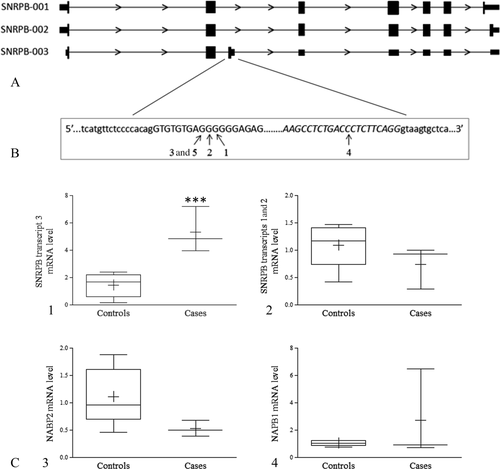
Quantitative reverse transcription PCR (RT-qPCR) analyses of transcripts 1–2 and 3 showed that they are widely expressed in various tissues including osteoblasts and chondrocytes (Supp. Table S3).
The impact of SNRPB variants on specific mRNA amount was then studied by semiquantitative RT-PCR and gel visualization in leukocytes, confirming the presence of transcripts 1–2 and transcript 3 in all individuals (Supp. Fig. S1). Yet, the amount of transcript 3 level was significantly lower than that of coding transcripts but higher in patients compared to controls. RT-qPCR analyses of SNRBP transcripts using the TaqMan Gene Expression Assay protocol (Life Technologies, Saint Aubin, France) revealed significantly higher levels of PTC-containing transcript in patients (P = 0.035), transcript 3 being 4–7.2 times more abundant in patients than controls (Fig. 2C1). No differences in transcripts 1 and 2 levels (Fig. 2C2) and no significant differences in NABP1 and NABP2 transcript levels between patients and controls were noted (Fig. 2C3 and C4).
Here, we report on SNRPB heterozygous mutations in five CCMS cases all of French origin, including one familial form. The variants were not present in the healthy parents, confirming that they occurred de novo, further supporting autosomal dominant inheritance. On a clinical point of view, it is important to highlight the absence of any very severe or lethal case in our cohort.
Interestingly, five mutations in the same region have recently been identified in another series of CCMS patients [Lynch et al., 2014] and three of them are identical to our patients variants (P1, P2, P4).
All five variants identified were located near the PTC-introducing alternative exon splice site and seems to promote its inclusion, as supported by our finding of increased transcript 3 in CCMS cases leukocytes. Among these variants, four (concerning P1, P2, P3, and P5) clustered at the 5′ end of the PTC-introducing exon. This region overlaps with highly conserved exonic splicing silencers (ESSs) [Human Splicing Finder, http://www.umd.be/HSF3/; Desmet et al., 2009]. The fifth mutation (P4), located at the 3′ end of this alternative exon, also lies within splicing regulatory elements [Human Splicing Finder, http://www.umd.be/HSF3/; Desmet et al., 2009]. Interestingly, Saltzman et al. [2011] demonstrated that 12-base segments deletion including these two regions leads to an increase in inclusion of the alternative exon. Moreover, Lynch et al. [2014] reported increased expression of the PTC-containing transcript associated with decreased overall expression of SNRPB in CCMS case fibroblasts. Transcript 3 is essential for SmB autoregulation. Indeed, it accumulates when SmB is increased exogenously, and knockdown of SmB/B′ leads to a strikingly reduced inclusion of the PTC-introducing exon [Saltzman et al., 2008, 2011]. Such AS-NMD has also been described for splicing regulatory factor autoregulation, including serine/arginine-rich proteins [Lareau et al., 2007]. Consistent with their functional importance, PTC-introducing alternative exon of splicing factors often lies within highly conserved or ultraconserved sequence region [Ni et al., 2007; Lareau et al., 2007].
Increased levels of SNRPB transcript 3 should be expected to be associated with decreased levels of coding transcripts. However, we did not detect decreased transcripts 1 and 2 levels in CCMS leukocytes. This may be explained by the variable functional consequences of the variants depending on the cell type. Indeed, the AS is known to vary across tissues and cell types [Pan et al., 2008, Supp. Table S3]. Moreover, the restricted phenotype of CCMS, mainly involving skeleton development and ossification process, further supports tissue-specific consequences of the pathogenic mechanism.
The identification of SNPRB variants in CCMS suggests an impairment of the core splicing machinery in the pathogenesis of this rare disorder. SmB/B′ is one of the nuclear proteins that are common to U1, U2, U4/U6, and U5 small nuclear ribonucleoprotein particles (snRNPs) involved in pre-mRNA splicing [review: Wahl et al., 2009]. AS is a fundamental feature in regulating eukaryotic transcriptome, as ∼95% of multiexon human transcripts are subject to this process [Pan et al., 2008]. Importantly, AS is differently regulated across cell types or developmental stages. Thus, its regulation controls the temporal and spatial expression of various isoforms, and notably, on–off regulation by NMD. AS enhances the complexity of proteome of higher organisms, but this increase in complexity leads to an increased susceptibility to malfunction. Indeed, cis- and trans-acting variants disrupting the splicing code or machinery required for splicing and regulation are involved in various diseases.
Splicing can be affected by cis-acting point mutations within exons and a large number of exonic mutations result in aberrant splicing [Wang and Cooper, 2007]. It is likely that the primary consequence of the SNRPB mutations in CCMS is a loss of ESSs or a gain of exonic splicing enhancers, strengthening the PTC-introducing alternative exon recognition and inclusion.
Mutations in core splicing machinery components or other trans-acting splicing factors and their regulators have the potential to affect expression of multiple genes and it has been demonstrated that SmB/B′ levels promote inclusion of alternative exons in many other genes [Saltzman et al., 2011]. Our finding of normal expression level of NABP1 without a significant decrease in the level of NABP2 in CCMS does not support the involvement of these two genes in CCMS. However, one cannot exclude tissue-specific consequences limited to the chondro-osseous tissues.
Recently, disease causing trans-acting mutations have been reported [Singh and Cooper, 2012], particularly in protein components of snRNP complexes as in Taybi Linder syndrome [Edery et al., 2011; He et al., 2011], Nager syndrome [Bernier et al., 2012; Petit et al., 2014], or Guion-Almeida syndrome [Lines et al., 2012]. Yet, the scarcity of reported trans-acting splicing factor mutations suggests that they are probably often lethal during embryonic development or at the cellular level.
In this study, we report CCMS as a novel developmental disease caused by defects in the core splicing machinery. Heterozygote mutations in SNRPB PTC-introducing alternative exon are responsible for this unique chondro-osseous phenotype, supporting a specific role of SNRPB in the ossification process. Future studies will hopefully explain why a defect in a ubiquitous splicing machinery has only limited, tissue-specific consequences.
Acknowledgment
Disclosure statement: The authors declare no conflict of interest.




