Germline TP53 Mutations and the Changing Landscape of Li–Fraumeni Syndrome
For the TP53 Special Issue
ABSTRACT
Since its description by Li and Fraumeni over 40 years ago, Li–Fraumeni syndrome (LFS) remains one of the most striking familial cancer predisposition syndromes. Children and adults are affected by a wide array of cancers that occur predominantly at younger ages. This review discusses LFS, describes its association with TP53, and examines the classic and evolving definitions of the syndrome. The potential implications of multigene assessments of individuals at increased cancer risk, which have already begun to identify those with very little personal or family cancer history carrying germline TP53 mutations, are considered. Newer options in the management of individuals with LFS are also discussed, highlighting the importance of further clinical trials for cancer detection, prevention, and management. Finally, we observe how the clinical criteria for TP53 mutation screening appear to be evolving as our understanding of the impact of germline TP53 mutations continues to expand.
Li–Fraumeni Syndrome
Initial Description and Epidemiology
In 1969, Frederick P. Li and Joseph F. Fraumeni Jr. described four families with the cancer syndrome that would come to be known as Li–Fraumeni syndrome (LFS) [Li and Fraumeni, 1969]. These families were identified from the examination of over 600 medical records and death certificates from children with rhabdomyosarcoma. Each family had two siblings or first cousins with childhood soft tissue sarcomas, diverse young-onset cancers, and multiple primary tumors in close family members [Li and Fraumeni, 1969]. Further epidemiological studies and segregation analyses confirmed that LFS is a rare familial cancer predisposition syndrome with autosomal-dominant inheritance [Lynch et al., 1978; Strong et al., 1987; Li et al., 1988].
Syndrome Definition
The clinical definition of LFS as described by Li and Fraumeni and now known as “classic LFS” includes a proband with a sarcoma under the age of 45 years, a first-degree relative with a cancer prior to the age of 45 years, and a first- or second-degree relative with any cancer before 45 years or a sarcoma at any age [Li et al., 1988].
Since the initial characterization of LFS, families have been identified who share features of LFS without fully meeting classic LFS criteria. These families have come to be known as “LFS-like” or “Li–Fraumeni-like (LFL).” Two sets of criteria proposed by Birch and Eeles, respectively, have been subsequently used to identify these families. The Birch criteria do not require the proband to have a sarcoma, but are otherwise close to classic LFS [Birch et al., 1994]. The Eeles definition of LFL simply includes two first- or second-degree relatives with LFS component tumors at any age, rather than the three required by the classic criteria [Eeles, 1995]. Collectively, these clinical definitions have tried to permit broader recognition of individuals and families with LFS.
Characteristic tumors in the LFS spectrum, often referred to as “core” or component LFS tumors, include soft tissue and bone sarcomas, premenopausal breast cancers, brain tumors (especially choroid plexus carcinomas), adrenal cortical carcinomas (ACCs), and leukemias [Li et al., 1988; Olivier et al., 2003; Gonzalez et al., 2009b]. Component LFS tumors comprise about 70% of LFS-related malignancies and are distinguished from their sporadic counterparts by their tendency to occur at significantly earlier ages [Nichols et al., 2001; Olivier et al., 2003; Gonzalez et al., 2009b; Ruijs et al., 2010]. A broad range of other tumors including lymphomas, gastrointestinal malignancies, melanoma, and lung cancer have been described in LFS families and also found to occur at earlier ages and as part of multiple primary neoplasms [Nichols et al., 2001; Gonzalez et al., 2009b].
TP53 and LFS
In 1990, observations of the inactivation of the TP53 gene in the sporadic forms of many LFS-associated tumors led to the hypothesis and then discovery of a definitive association between TP53 and LFS [Malkin et al., 1990; Srivastava et al., 1990]. Cellular TP53 had been previously recognized as one of the most prominent tumor suppressors, whose activation as a transcription factor stimulates downstream pathways that lead to protective cellular processes including cell cycle arrest and apoptosis [Vogelstein et al., 2000].
In the germline, over 250 mutations have been described throughout the TP53 gene [see http://p53.iarc.fr; Petitjean et al., 2007; Leroy et al., 2013]. Initial studies focused on mutations within exons 5–8, the region that encodes the DNA-binding domain of the protein [Olivier et al., 2003; Petitjean et al., 2007]. Mutations at specific hot-spot codons have subsequently been described that either interfere with DNA binding or disrupt the structure of the binding surface, thus interfering with its ability to modulate transcription of target genes [Olivier et al., 2010]. Similar hot-spot mutations are found among somatic TP53 mutations, although within the germline a greater proportion of mutations are found to occur at mutation-prone CpG residues [Olivier et al., 2010]. A large number of mutations are missense mutations that lead to a codon change, posing challenges to the functional interpretation of new variants [Leroy et al., 2013]. Notably, there are mutations that have been found outside of the DNA-binding domain as well as rearrangements and deletions that have expanded the scope of TP53 mutations defining LFS.
Chompret Criteria for TP53 Testing
A series of criteria to identify the best candidates for TP53 genetic testing have been proposed by Chompret et al. [Chompret et al., 2001] based on the data from a sequential analysis of French childhood cancer patients, early-onset breast cancer patients, and their family members. Approximately 29%–35% of families meeting these criteria were shown to have TP53 mutations [Bougeard et al., 2008; Gonzalez et al., 2009b]. These criteria were subsequently revised in 2008 [Bougeard et al., 2008] and 2009 [Tinat et al., 2009]. The current 2009 Chompret criteria, shown in Box 1, include a proband with an LFS tumor before the age of 46 years and a first- or second-degree relative with an LFS tumor before the age of 56 years. LFS tumors include soft tissue or osteosarcomas, brain tumors, premenopausal breast cancers, leukemias, ACCs, and bronchoalveolar lung carcinomas. Breast cancer in the first-or second-degree relative is excluded if the proband had breast cancer. Alternatively, the proband can have multiple tumors (except multiple breast cancers) of which at least two belong to the LFS spectrum, the first of which occurs before the age of 46 years. Finally, a proband with an ACC or choroid plexus carcinoma irrespective of family history would also meet the criteria for TP53 testing [Tinat et al., 2009]. 21% of families meeting these revised criteria (22 of 105 families) were shown to have TP53 mutations in an independent series [Ruijs et al., 2010].
Box 1. Chompret criteria for TP53 testing [Tinat et al., 2009]
| Proband with LFS tumor* before 46 years old AND |
| first- or second-degree relative with an LFS tumor** before 56 years old or with multiple tumors |
| OR |
| Proband with multiple tumors (except multiple breast cancers), the first of which occurs prior to the age of 46 years, with at least two belonging to the LFS spectrum. |
| OR |
| Proband with an ACC or choroid plexus carcinoma regardless of family history. |
- *Including soft tissue or osteosarcomas, brain tumors, premenopausal breast cancers, leukemias, ACCs, and lung bronchoalveolar carcinomas.
- **Except breast cancer if proband had breast cancer.
Cancer Risk Among LFS Families
Estimates of cancer risk among LFS family members are consistently high in so-called “classic” families. Early data estimated that 50% of carriers in classic LFS kindreds develop cancer by the age of 40 years, and 90% by the age of 60 years [Lustbader et al., 1992]. However, these figures are changing as the syndrome definition is broadened. Female TP53 mutation carriers have a significantly higher cancer risk and earlier age of cancer development than do male mutation carriers [Hwang et al., 2003]. While this excess has been largely attributed to early-onset breast cancer, the trend is preserved even after excluding breast, ovarian, and prostate cancers from the analysis, and the effect begins in childhood [Hwang et al., 2003; Gonzalez et al., 2009b]. One study demonstrated a 57% (+/− 10% SE) cumulative probability of developing a second cancer at a follow-up of 30 years, with childhood cancer survivors harboring the highest relative risk [Hisada et al., 1998]. This phenomenon is not attributable solely to the carcinogenic effects of treatment, although individuals with LFS are thought to have a greater tendency to develop secondary malignancies in the field after therapeutic radiation [Li et al., 1988; Hisada et al., 1998]. This issue is currently being re-evaluated as new technologies for the delivery of radiation have been developed.
Beyond Classic LFS
While the clinical definitions of LFS initially led to somewhat narrowly focused criteria for TP53 germline testing, the definition of the syndrome continues to evolve as testing becomes more widely available and further unbiased testing is performed in both research and clinical settings. A TP53 mutation may be identified in patients who lack the classic family history features. This may be due to insufficient family history information, germline mosaicism, or a de novo TP53 mutation [Sorrell et al., 2013].
De Novo Mutations
The de novo mutation rate of TP53 has been estimated among select cohorts of individuals. In one study, among a group of patients selected for the presence of multiple primary tumors in the proband or the occurrence of a cancer in a first- or second-degree relative or first cousin before the age of 46 years, four of 17 cases, or 24% of the cohort were estimated to have a de novo mutation [Chompret et al., 2000]. In another study, a de novo rate of 7%–20% was estimated among patients with early-onset cancer referred for TP53 testing (five to 15 of 75 cases) [Gonzalez et al., 2009a]. Together, these data highlight one way in which LFS clinical criteria alone may not be sufficient to identify all individuals with a TP53 mutation, and that conversely, the absence of family history does not exclude the possibility of a germline TP53 mutation.
Early-Onset Breast Cancer
Another cohort of patients among whom germline TP53 testing may be undertaken regardless of family history includes women with early-onset breast cancer, particularly women diagnosed under the age of 30 years. Approximately 3%–8% of women in this age group with an otherwise negative family history are estimated to carry a germline TP53 mutation [Lalloo et al., 2006; Mouchawar et al., 2010; McCuaig et al., 2012]. Recently, breast cancers associated with germline TP53 mutations have been shown to be more likely hormone receptor and/or HER2 positive [Masciari et al., 2012; Melhem-Bertrandt et al., 2012]. Among less than 50-year-old women with HER2+ breast cancer and unselected for family history, 1.4% (three of 213) demonstrated to carry a TP53 mutation [Rath et al., 2013]. Among less than 35-year-old women in that cohort, the prevalence was 4.9% (three of 41, 95% CI 1%–17%). Malignant phyllodes breast tumors have been associated with LFS and also reported in Brazilian cohorts [Birch, 2001, Giacomazzi, 2013]. Based on these data, some have called for the addition of TP53 to BRCA1 and BRCA2 to the genes routinely evaluated in women with young-onset breast cancer [McCuaig et al., 2012; NCCN, 2013].
Additional Phenotypes Associated with Germline TP53 Mutations
LFS definitions and indications for TP53 testing are likely to continue to evolve as further evidence emerges to elucidate the role of germline TP53 mutations in different cancer types. For example, while leukemias were included among the original descriptions of LFS, subsequent studies suggested that leukemias may be a less common manifestation of germline TP53 mutations [Birch et al., 2001; Nichols et al., 2001; Gonzalez et al., 2009b]. However, emerging work now suggests that at least a certain subtype of pediatric acute lymphoblastic leukemia, low hypodiploid, is associated with germline TP53 mutations [Holmfeldt et al., 2013].
Similarly, a specific subtype of rhabdomyosarcoma, the anaplastic subtype, has been found among germline TP53 mutation carriers. In a recent report, 11 of 15 (73%) individuals with anaplastic rhabdomyosarcoma had germline TP53 mutations. Interestingly, only five of these individuals had family histories of LFS cancers [Hettmer et al., 2013].
The study of TP53 mutations in the context of medulloblastoma has also become an area of interest. One study found TP53 mutations in 28 of 133 (21%) sonic hedgehog subtype (SHH) medulloblastoma tumors of which 56% with evaluable germline data had germline TP53 mutations [Zhukova et al., 2013]. Genomic sequencing also demonstrated that a SHH medulloblastoma in an LFS patient demonstrated catastrophic chromosomal rearrangements, or chromothripsis [Rausch et al., 2012]. Together, the broader utilization of genomic technologies across cancers will likely continue to identify more specific manifestations of TP53 germline mutations.
R337H and LFS in Brazil
While the majority of cancer-associated TP53 mutations have been reported to occur in exons 5–8, one mutation outside of this region, R337H, plays a prominent role among carriers from Brazil. This mutation, occurring in exon 10 in the tetramerization domain, was initially found among 97% of a series of 36 patients with ACC from Southern Brazil, where the incidence of ACC is 10–15 times higher than in the United States [Ribeiro et al., 2001]. These cases of ACC occurred in individuals without strong family histories of cancer suggestive of LFS. Further studies confirmed the presence of the R337H mutation in Brazilian patients with ACC and choroid plexus carcinoma without classic LFS family histories [Giacomazzi et al., 2013]. Since that time, systematic genetic epidemiologic studies of the R337H mutation in Brazil have revealed a broader range of LFS diagnoses, including breast cancer, brain tumors, and leukemias [Achatz et al., 2007]. The relatively low penetrance of this allele and observed propensity for the development of certain tumor types have been suggested to be related to the biology of the R337H mutation. This mutation affects the stability of the TP53 tetramer in a pH-sensitive manner, thus leading to the hypothesis that destabilization may be promoted in some tissue-specific milieu [DiGiammarino et al., 2002].
While the R337H mutation has been described in a few reports from Europe [Chompret et al., 2000; Herrmann et al., 2012; Waldmann et al., 2012], the vast majority of cases have been described from Brazilian families. Although some controversy remains, recent haplotype mapping of the region surrounding the R337H mutation suggests identity by descent with the possibility of a founder mutation from a single common ancestor [Ribeiro et al., 2001; Pinto et al., 2004; Garritano et al., 2010]. The prevalence of the R337H mutation in Brazil and several studies demonstrating the association with early breast cancer has led to calls for the routine evaluation of TP53 with BRCA1 and BRCA2 in early-onset breast cancer in Brazil as well [Assumpcao et al., 2008; Carraro et al., 2013; Cury et al., 2014].
As a result of the high frequency of this mutation among patients with ACC in Southern Brazil, a neonatal screening program was recently completed among 171,649 participants, of whom 0.27% were found to carry the R337H mutation [Custodio et al., 2013]. Eleven of the 461 carriers who underwent further surveillance developed ACC detected at earlier stages compared with the nonsurveillance group suggesting a potential clinical benefit of early detection. The complexities of conducting a population-based newborn screening program for a strong and pluripotent cancer susceptibility gene have been discussed [Achatz et al., 2009].
Genetic Modifiers of the LFS Phenotype
Germline TP53 mutations have been reported in about 80% of families who meet classic LFS criteria, and about 20%–40% of families who meet LFL criteria [Olivier et al., 2002; Varley, 2003]. Despite intense investigation, thus far TP53 is the only gene that has been definitively associated with LFS. Though there has not been conclusive data supporting mutation genotype correlation to phenotype, there is some evidence suggesting missense mutations with dominant-negative effects have correspondingly earlier ages of tumor development compared with loss-of-function events from rearrangements [Zerdoumi et al., 2013]. There has also been great interest in the identification of genetic modifiers of the LFS phenotype, both within the TP53 gene as well as in related genes. For example, SNP309 (T>G), rs2279744, in the gene for MDM2, an E3 ubiquitin protein ligase that promotes proteosomal degradation of TP53, has been reported to be associated with earlier age of tumor diagnosis [Bond et al., 2004]. This effect was found to be enhanced in the presence of one of the better studied TP53 polymorphisms, P72R (p.Pro72Arg) at a locus affecting MDM2 affinity [Bougeard et al., 2006]. R72 has been previously shown to promote apoptosis more effectively than P72, although the clinical significance of this finding has not been validated [Olivier et al., 2010].
Another polymorphism found in intron 3 (PIN3) of the TP53 gene consisting of a 16-bp duplication has also been the focus of a number of investigations as a cancer risk modifier in various populations and in LFS kindreds. The presence of the duplication (A2 allele) has been associated with the susceptibility to different cancers including colon, breast, and ovarian cancers outside of LFS families [Wang-Gohrke et al., 1999; Gemignani et al., 2004; Costa et al., 2008]. A recent meta-analysis suggested increased cancer risk in individuals with the A2A2 genotype versus the A1A1 genotype among Indian, Mediterranean, and northern European populations for breast and colon but not lung cancer [Sagne et al., 2013a]. While the biological function of this polymorphism has not yet been validated, some in vitro evidence suggests that the presence of the A2 allele leads to a decrease in TP53 mRNA expression [Gemignani et al., 2004]. The effects of potential genetic modifiers within the TP53 gene (e.g., PIN3) or elsewhere in the genome are subjects of intense interest at this time as possible approaches to accounting for the remarkable variation in patterns of cancer and age at diagnosis in LFS [Sagne et al., 2014].
The clinical observation of younger age of onset and increased disease severity in successive generations of TP53 families has raised the possibility of genetic anticipation in LFS [Trkova et al., 2002]. While sampling biases and enhanced detection may contribute to these differences, one mechanism by which genetic anticipation has been hypothesized to occur is via successive telomere degradation. Telomere length of TP53 carriers has been demonstrated to be shorter than their wild-type counterparts, and there is an association with earlier development of cancers in children with shorter telomeres [Tabori et al., 2007]. The possibility of following telomere length as a clinical biomarker has thus been raised, although further investigation is necessary for clinical confirmation.
There has also been interest in understanding the apparent enrichment of copy-number variations (CNVs) seen in the germline of LFS patients [Shlien et al., 2008; Silva et al., 2012a]. Recent work has highlighted the accumulation of rare CNVs in individuals with DNA-binding domain mutations [Silva et al, 2012a]. Together, the accumulation of CNVs may reflect progressive genomic instability that may translate to a method for risk stratification, but further investigation will be required to fully understand the impact of CNV changes in LFS phenotypes.
Management of Individuals with Germline TP53 Mutations/LFS: Unaffected Individuals and Cancer Survivors
Clinical Screening Guidelines in LFS
Current practice guidelines established by the National Comprehensive Cancer Network (NCCN) recommend yearly physical examinations including skin and neurologic examinations for all individuals with LFS, with special attention to the possibility of rare malignancies, secondary malignancies, and/or pediatric cancers, depending on the at-risk population. They also advise consideration of colonoscopy every 2–5 years beginning at the age of 25 years. For women, recommendations include breast cancer surveillance, as outlined in detail below. Further surveillance is recommended based on family history of the individual [NCCN, 2013]. Finally, consideration of entry into a clinical trial investigating novel screening approaches is suggested.
Breast Cancer Screening
Breast cancer is one of the most common cancers occurring among women with germline TP53 mutations. Breast cancer surveillance programs for women with LFS are based on data that established the value of breast MRI in women with BRCA mutations. The complete screening program includes a clinical breast examination once or twice yearly beginning at the age of 20–25 years. This can be performed earlier depending on the earliest age of onset of breast cancer in the family. Imaging should be used as a screening modality annually starting at the age of 20–25 years, or earlier, depending on family history [NCCN, 2013]. While the NCCN guidelines recommend MRI and mammogram, they also acknowledge the ongoing debate regarding the best screening modality for this age group, and consideration of MRI alone is often made. Screening mammograms carry the risk of adding to cumulative radiation exposure and mammographic densities can be difficult to interpret in this young premenopausal age group. Prophylactic mastectomies are also offered to women in LFS families to reduce risk. Counseling should be performed around the psychosocial and physical aspects of undergoing bilateral prophylactic mastectomies and the appropriate age at which this surgery might be considered and possibly undertaken.
Other screening guidelines also center around breast cancer surveillance. The Royal Marsden and the Institute of Cancer Research (ICR) in the UK recommend breast self-awareness and examination, with annual MRI screening from the age of 20 years with discussion of potential mastectomy for risk reduction. While individuals who carry germline TP53 mutations are encouraged to have an “open door policy” with a pediatrician or pediatric oncologist, no other focused screening is recommended before the age of 20 years [ICR, 2013]. Frebourg et al. (2001) have proposed annual monitoring starting in childhood with physical examinations by clinicians familiar with LFS. They also recommend breast cancer screening for women by yearly ultrasound beginning at age 20 or later with MRI [Frebourg et al., 2001]. In the Netherlands, regular breast cancer surveillance from the age of 20–25 years, as well as consideration of an annual history and physical examinations are recommended [Lammens et al., 2010b]. In Brazil, screening recommendations are based upon 2008 NCCN guidelines. Breast cancer screening guidelines include clinical breast examinations, mammography/MRI, as well as discussion regarding the possibility of mastectomy. For other tumor types, screening is recommended with annual clinical examinations, consideration of colonoscopy, and patient education and awareness of family history of cancer [Brazil, 2009].
Comprehensive Cancer Screening Studies
Given that tumors in the LFS spectrum can occur in multiple different sites with unpredictable timing, how best to perform comprehensive screening for at-risk individuals remains an area of great interest. Small numbers of affected individuals prohibit a traditional randomized trial design and furthermore consenting at-risk individuals to randomization in this setting would be challenging. The first prospective study that used F18-FDG-PET/CT as a screening modality found three malignant tumors among 15 asymptomatic individuals from known LFS families with TP53 mutations or obligate carrier status. Two papillary thyroid cancers and one esophageal adenocarcinoma were identified [Masciari et al., 2008]. The significant radiation exposure with this technology limits its role in the LFS population.
A subsequent study incorporated whole body MRI into a comprehensive screening protocol that also included clinical examinations and laboratory measures [Villani et al., 2011]. Individuals from eight LFS families were included in the study. Participants who elected to enroll in this enhanced screening protocol were compared with those who decided not to undergo screening. Among the individuals who underwent surveillance, five malignant lesions (two ACCs, two choroid plexus carcinomas, and one malignant fibrous histiocytoma) and five low-grade or premalignant lesions (three low-grade gliomas, one myelodysplastic syndrome, and one thyroid adenoma) were identified in otherwise asymptomatic individuals. The group without screening presented with tumors when they became symptomatic. The striking finding was the difference in outcome between the two groups, with individuals in the group who underwent screening shown to have a significant survival advantage with 100% at 3 years compared to 21% in the nonsurveillance group (95% CI 4%–48%) [Villani et al., 2011].
The use of MRI in this setting has the distinct advantage of avoiding ionizing radiation, and as technology improves, faster whole-body screens have become possible. Special consideration should be made for children who require sedation for MRIs. The risks of recurrent sedation should be weighed against the benefit of early screening and underscores the importance of defining the true long-term benefits of screening in children as well as in adults. The existing data, while provocative and hopeful, are neither randomized nor complete. Multiple centers around the world are currently working toward the design and implementation of prospective whole-body MRI protocols for LFS families that will contribute to further our understanding regarding the risks and benefits of such screening. In addition, a pilot trial is funded in the UK, the SIGNIFY study, comparing whole-body MRI in LFS carriers to population controls, in preparation for the prospective randomized trial necessary to prove whether or not whole-body MRI is an effective early detection tool in LFS. A similar study (LIFSCREEN) is currently underway in France. Carriers of germline TP53 mutations should be encouraged to participate in such clinical trials that are critical for identifying the best care and management strategies for individuals with LFS.
Cancer Management
Generally, there have not been special recommendations for tumor-directed therapy for LFS patients. In general, minimizing radiation therapy is recommended where possible, but when it is necessary to achieve cure as an integral part of tumor-directed therapy, it may be used despite concerns about possible increased risks of subsequent treatment-related carcinogenesis. Similar concerns exist for systemic chemotherapies, with particular worries about alkylating agent-induced leukemias or myelodysplasias [Felix et al., 1996; Veldhoen et al., 1998; Inokuchi, 2005; Talwalkar et al., 2010; Schulz et al., 2012]. In breast cancer treatment, mastectomy may be recommended to avoid adjuvant radiation required in breast conservation. Recent data showing that breast cancers in patients with LFS are often hormone receptor positive and/or HER2 positive have raised hopes that targeted therapies will improve the outcome [Slamon et al., 2011; Masciari et al., 2012; Melhem-Bertrandt et al., 2012]. Therapies targeting TP53-deficient tumors may have particular impact on the outcome for cancers in LFS patients.
Genetic Counseling and Psychosocial Support
As the nature of genetic testing shifts from single gene to multiplex analysis, genetic counseling is also changing in the genomic era. With single gene testing, pretest counseling focusing on LFS and the psychosocial implications of discovering a TP53 mutation in a family could be thoroughly performed and allowed for each individual to opt in or out of testing. As next-generation sequencing becomes more widely available, while pretest counseling remains important, testing a large number of genes at once is shifting the burden of counseling families regarding individual genes from the pretest to the post-test setting. Pretest counseling should continue to address the potential impact of a positive result on an individual, including distress and anxiety as well as issues such as the potential for genetic discrimination [Mai et al., 2012].
When an individual tests positive for a pathogenic TP53 mutation, age-specific cancer risks should be discussed and these individuals and their families should be offered psychosocial support as needed. Other at-risk family members should be identified and offered testing, specifically for the previously identified mutation. In one study, approximately 50% (65 of 119) of presymptomatic individuals from LFS families chose to undergo testing [Lammens et al., 2010a]. Testing in minors is often especially controversial due to the issue of informed consent and should (as with all genetic testing) occur with comprehensive pretest and post-test counseling. The impetus to test individuals under the age of 18 may shift toward younger ages if screening strategies become more standardized and begin to show a survival benefit. Individuals who test positive are advised to avoid known carcinogens when possible, especially smoking, as the risk of lung cancer among TP53 carriers who smoke has been shown to be higher compared with the risk among mutation carriers who are nonsmokers [Schulz et al., 2012].
The LFS association, founded in 2010 by families affected by LFS, can provide useful educational and support resources for individuals affected by germline TP53 mutations (http://lfsassociation.org).
Evolving Paradigms for TP53 Mutation Testing
As data begin to accumulate from the unbiased testing of individuals using multiplex gene panels and whole-exome or whole-genome sequencing, the spectrum of clinical phenotypes associated with germline TP53 mutations is expected to evolve even further. Below, we summarize the clinical guidelines for identifying persons at risk for germline TP53 mutations and present some observations on how our understanding of the syndrome associated with TP53 mutations appear to be evolving as new genotype–phenotype correlations emerge.
Clinical Guidelines for Testing at-Risk Individuals
Currently, TP53 mutation testing is generally offered to individuals with cancer whose families meet clinical criteria for classic LFS or LFL, and those who individually or as families meet Chompret criteria. Figure 1A provides an example illustrating an LFS pedigree fitting the classic definition of LFS with a known TP53 mutation among family members who have undergone testing. As noted above, since approximately 80% of classic LFS families have identifiable germline TP53 mutations, a family may fulfill criteria for classic LFS but ultimately test negative for TP53. One such family is depicted in Figure 1B. These families are important to efforts to identify other genes that could be contributing to the clinical syndrome.

Box 1 contains the revised Chompret criteria for identifying persons at-risk for TP53 mutations. An important consideration when testing families for the presence of a germline TP53 mutation is the careful selection for testing of the family member who is most likely to carry a mutation. Given the range of phenotypes and variable penetrance associated with these mutations, a negative test result from a poorly selected family member could mislead one to not pursue testing in other family members who would otherwise test positive. Selecting a family member most likely to be a carrier (e.g., with a LFS component tumor occurring at an exceptionally young age) will provide the highest yield of testing. Subsequent family members can then have site-specific testing to determine whether they carry the same mutation. Of course, the cancer pattern in some families with histories suggestive of LFS will ultimately be found to be attributed to mutations in other cancer susceptibility genes [Evans et al., 2008; Silva et al., 2012b].
TP53 Germline Mutations Among Individuals Without LFS Family History
At times, TP53 germline mutations may be identified among individuals without “typical” LFS family cancer histories. These individuals may have LFS component tumors, multiple primary malignancies, or other early-onset malignancies, such as early-onset colon cancer [Wong et al., 2006] even in the absence of family history. This finding may be due to de novo mutations, mosaicism, incomplete family history information, or to the variable penetrance of TP53 germline mutations, particularly among male carriers. Figure 2 depicts two affected children of an unaffected male carrier that illustrates some of these points.
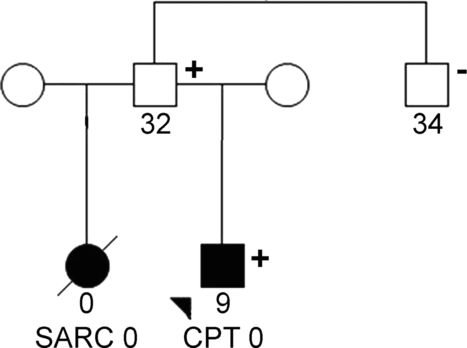
Testing Among Children
Testing for germline TP53 mutations among the pediatric population also seems to be evolving, as cancer screening protocols have become available. While genetic testing in minors remains a challenging issue, particularly with respect to informed consent, regular cancer screening may become more meaningful if available interventions are shown definitively to detect cancers early and improve outcome. While screening protocols do not yet have proven benefit for survival, they do offer the opportunity to address the development of potentially helpful interventions for children who carry TP53 mutations, and have been embraced by the LFS community.
For individuals of child-bearing age in families with TP53 germline mutations, reproductive genetic counseling should be offered. For these individuals, preimplantation genetic diagnosis is available [Rechitsky et al., 2002; Julian-Reynier et al., 2009]. Special attention to each individual's cultural and religious views must be considered when counseling on these topics [Schiffman et al., 2013].
Next-Generation Sequencing and TP53 Mutation Detection
Phenotypes outside of classic LFS associated with TP53 mutations have recently come into focus in the United States with the growing clinical use of multiplex panel tests that are beginning to replace single gene testing in many centers. The TP53 gene is included on a number of commercially available gene panels, including those for predisposition to breast, ovarian, and colorectal cancers [Domchek et al., 2013]. In addition to cancer panels, whole-exome and whole-genome sequencing of the germline of cancer patients alone and in combination with tumor sequencing are beginning to enter clinical care. Our understanding of LFS thus far has derived largely, though not exclusively, from original descriptions and selected series of patients. It remains to be seen whether broader, unbiased TP53 testing approaches will lead to the identification of novel ways in which TP53 genotype correlates with phenotype, thus challenging us to consider redefining or expanding the clinical definition of LFS. These approaches will also require modifications to the classic genetic counseling paradigms.
If more individuals with germline TP53 mutations are identified by the expanding use of multiplex testing panels and whole-exome/whole genome sequencing, we hypothesize that penetrance estimates for TP53 mutations are likely to change. Definitions of the syndrome are also likely to evolve to encompass new phenotypes associated with TP53 mutations.
Highlights of this review are summarized in Box 2.
Box 2. Highlights
|
Acknowledgments
The authors are grateful to the Li–Fraumeni families who kindly consented over the years to be part of the DFCI/NCI LFS registry and to Elizabeth Root for her technical assistance with the pedigrees.
Disclosure Statement: The authors do not have any conflicts of interest to declare.




