Clinical and genetic findings in a large cohort of patients with ryanodine receptor 1 gene-associated myopathies†
Communicated by Jürgen Horst
Abstract
Ryanodine receptor 1 (RYR1) mutations are a common cause of congenital myopathies associated with both dominant and recessive inheritance. Histopathological findings frequently feature central cores or multi-minicores, more rarely, type 1 predominance/uniformity, fiber-type disproportion, increased internal nucleation, and fatty and connective tissue. We describe 71 families, 35 associated with dominant RYR1 mutations and 36 with recessive inheritance. Five of the dominant mutations and 35 of the 55 recessive mutations have not been previously reported. Dominant mutations, typically missense, were frequently located in recognized mutational hotspot regions, while recessive mutations were distributed throughout the entire coding sequence. Recessive mutations included nonsense and splice mutations expected to result in reduced RyR1 protein. There was wide clinical variability. As a group, dominant mutations were associated with milder phenotypes; patients with recessive inheritance had earlier onset, more weakness, and functional limitations. Extraocular and bulbar muscle involvement was almost exclusively observed in the recessive group. In conclusion, our study reports a large number of novel RYR1 mutations and indicates that recessive variants are at least as frequent as the dominant ones. Assigning pathogenicity to novel mutations is often difficult, and interpretation of genetic results in the context of clinical, histological, and muscle magnetic resonance imaging findings is essential. Hum Mutat 33:981–988, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
Mutations in the ryanodine receptor type 1 gene (RYR1; MIM# 180901) cause the well-characterized, dominantly inherited congenital myopathy central core disease (CCD; MIM# 117000) and the malignant hyperthermia susceptibility (MHS) trait (MIM# 145600), a pharmacogenetic reaction to volatile anesthetics and muscle relaxants. In recent years, a wide and increasing range of additional histopathological variants have been associated with RYR1 mutations—namely, multi-minicore disease (MmD), congenital fiber-type disproportion, and centronuclear myopathy (CNM). King Denborough syndrome, a dysmorphic syndrome with associated MHS, has also been recently associated with RYR1 mutations in a number of patients [D'Arcy et al., 2008; Dowling et al., 2011]. Taken together, RYR1-related myopathies are probably the most frequent form of congenital myopathies [Monnier et al., 2008; Sewry et al., 2008]. Typical CCD and the MHS trait are usually dominantly inherited, but recessive mutations have been recognized only relatively recently [Clarke et al., 2010; Jungbluth, 2007b; Jungbluth et al., 2002, 2005; Kossugue et al., 2007; Monnier et al., 2008; Wilmshurst et al., 2010; Zhou et al., 2007]. Clinically, there is a wide spectrum of severity ranging from severely weak patients never achieving independent ambulation to individuals with MHS but no muscle weakness. Patients with dominant CCD typically present with hypotonia, proximal weakness pronounced in the hip girdle, mild facial weakness, often marked joint laxity, and orthopedic complications such as congenital hip dislocation and scoliosis [Gamble et al., 1988; Jungbluth, 2007a; Voermans et al., 2009]. Respiratory involvement may be a feature in patients at the severe end of the spectrum, but overall is rare. In patients with recessive mutations, a more diffuse involvement comprising extraocular and facial muscle weakness, as well as more pronounced bulbar and respiratory impairment, has been described [Clarke et al., 2010; Jungbluth et al., 2005; Wilmshurst et al., 2010].
On muscle biopsy, the classical picture of dominantly inherited CCD features predominance or uniformity of hypotrophic type 1 fibers and central cores running along the longitudinal extent of the muscle fiber. The histopathological spectrum associated with recessive RYR1 mutations is much wider, comprising fiber-type disproportion, increased internal and/or central nuclei, and a range of oxidative abnormalities ranging from subtle unevenness of stain to multiple cores of variable size [Bevilacqua et al., 2011; Clarke et al., 2010; Jungbluth et al., 2008; Sewry et al., 2002, 2008; Wilmshurst et al., 2010]. The histopathological appearance can vary between different members of the same family or between consecutive biopsies of the same patient [Ferreiro et al., 2002; Monnier et al., 2008; Wilmshurst et al., 2010]. Cores may not always be present, and type 1 predominance or uniformity may be the only histological finding [Sato et al., 2008], and there is probably an age-related appearance of the cores [Sewry et al., 2002].
The RYR1 gene is located on chromosome 19q13.1, contains 106 exons [Phillips et al., 1996], and encodes Ryr1, the principal sarcoplasmic reticulum Ca release channel with a crucial role in excitation–contraction coupling. Mutations identified to date in association with classical CCD and the MHS trait have largely been dominant missense mutations, with only a few small deletions and duplications identified [Davis et al., 2003; Levano et al., 2009; Robinson et al., 2006; Wu et al., 2006; Zhou et al., 2007]. Recessively inherited RYR1 myopathies are often associated with compound heterozygosity for one or more missense and a nonsense mutation, splice-site or frameshift mutation [Clarke et al., 2010; Jungbluth, 2007a; Monnier et al., 2008].
Due to the technical challenges associated with the screening of this large gene, only sequencing of the mutational hotspots was available in our diagnostic setting until September 2007, when full genomic sequencing was introduced. To date we have screened 310 families, 17 of which have been reported separately in a recent paper on congenital myopathies with centronuclear myopathies [Wilmshurst et al., 2010].
In the present study, we report the genetic results and clinical presentation of 92 patients from 71 families, in whom we found pathogenic RYR1 mutations.
Patients and Methods
Patients
Patients tested for mutations in the RYR1 gene were included because of the presence of a congenital myopathy, and muscle biopsy and/or muscle magnetic resonance imaging (MRI) findings compatible with the diagnosis of an RYR1-related myopathy. Written informed consent was obtained from each family prior to testing, and this project was approved by the relevant local ethics committee.
Molecular Genetic Studies
The entire coding regions (exons 1–106) of the RYR1 gene, including splice sites, were sequenced at the genomic level in all patients. For the patients in whom we identified mutations, we tested available family members for the familial mutations and any variants of unknown significance (VUSs) by targeted sequencing.
Linkage analysis was performed using the following microsatellite markers flanking the RYR1 gene: D19S224, D19S896, D19S896, D19S570, D19S220, D19S897, D19S422, D19S881, D19S47, and D19S200 in addition to the RYR1_IVS89 intragenic marker.
The RYR1 nucleotide numbering is based on transcript variant NM_00540.2, where the nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, according to journal guidelines (www.hgvs.org/mutnomen). The initiation codon is codon 1. The variants reported have been submitted to the Leiden RYR1 locus-specific database (http://www.lovd.nl/RYR1).
All the variants identified were investigated by in silico analysis using Alamut v1.5 (Interactive Biosoftware, http://www.interactive-biosoftware.com/). This software incorporates several prediction algorithms including SpliceSiteFinder, MaxEntScan, NNSPLICE, GeneSplicer, as well as variant scoring methods—namely, PolyPhen-2, SIFT, and Align GVGD. It examines the conservation of both the nucleotide and amino acid residue across 11 species, and includes a search of previously reported variants in the literature.
Based on the Alamut findings, literature searches, and National Centre of Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov), all variants were divided into “mutations,” “polymorphisms,” or “VUSs.” Variants were classified as polymorphisms if they were listed on NCBI as a polymorphism with an allele frequency of greater than 5% in a sufficient number of normal controls. Variants were classified as VUSs if there was no convincing evidence that they had a causative effect and if there was not enough evidence to class as a polymorphism. VUSs included both synonymous and nonsynonymous changes not listed on NCBI; variants listed on NCBI, but with an allele frequency of less than 5%; and also variants where the allele frequency was derived from testing only a very small number of chromosomes (e.g., c.13317C>T; p.Ala4439Ala, where only two chromosomes were tested). VUSs also included some intronic variants that were not predicted to affect splicing.
We classified novel variants as mutations if they affected a moderate to highly conserved nucleotide, a highly conserved amino acid, and if the resulting physiochemical difference was at least moderate.
Clinical information of patients in whom mutations were identified was taken from the notes or from the referral forms; if information was insufficient, specific questionnaires were sent to the referring clinicians. Muscle MRI findings from a subgroup of 17 patients were also included in a report on muscle MRI findings in RYR1-related myopathies [Klein et al., 2011], patients indicated with “*” in Supp. Tables S1 and S2. Also included were five patients previously reported to show epigenetic silencing, as we identified additional mutations in four of the five patients [Zhou et al., 2006, 2007], marked “+” in Supp. Tables S1 and S2.
Results
In the 71 families included in this study, we identified 27 dominant mutations, five of them novel; and 55 recessive mutations, 35 of them novel. The mutations, the evidence for their pathogenicity, and key clinical details are listed in Supp. Table S1a (dominant) and Supp. Table S1b (recessive). Additional clinical details, including the affected family members, are listed in Supp. Table S2a (dominant) and Supp. Table S2b (recessive). Supp. Table S3a lists the VUSs for those patients who had additional VUS. Supp. Table S3b gives further details of each VUS identified.
Dominant RYR1 Mutations
We identified 27 putative dominant mutations in 35 families (comprising 45 patients; this includes the five patients with uncertain inheritance given below). Clear dominant segregation could be shown in eight families, two mutations had arisen de novo; in two families, only one parent was tested; in 15 families, parental DNA was not available; and in eight families, an asymptomatic parent carried the change. Twenty-six of the 27 mutations were missense mutations; one patient had a deletion of three nucleotides, resulting in a single amino acid deletion. Of the 27 dominant mutations identified, 14 were found in MHS/CCD region 3 (amino acid residues 4,550–4,940), previously identified as a mutational hotspot for CCD, two in MHS/CCD region 2 (amino acid residues 2,163–2,458), and one in MHS/CCD region 1 (amino acid residues 35–614); the latter two regions were previously mainly associated with MHS mutations.
We found seven recurrent dominant mutations: three patients with c.4178A>G; p.Lys1393Arg, previously reported in Scandinavian MHS patients and shown to have an effect on Ryr1 function [Broman et al., 2009; Vukcevic et al., 2010]. The variants c.7354C>T; p.Arg2452Trp identified in three patients, and c.14582G>A; p.Arg4861His in two patients have also been previously reported in MH and CCD, and demonstrated to have a functional effect [Bannister et al., 2007; Monnier et al., 2001; Shepherd et al., 2004; Tilgen et al., 2001].
Of the five novel mutations identified, three were in the RYR1 CCD hotspot region 3. For evidence of pathogenicity, see Supp. Table S1a.
Five of the previously reported dominant mutations have so far only been found in MHS; the references of these are indicated in Supp. Table S1a with “*”. Of these, two were also found in patients with recessive inheritance. The c.7063C>T, p.Arg2355Trp variant, previously described in MH, was detected as the only mutation in a patient with a mild neuromuscular phenotype and a dominant family history of MH [Carpenter et al., 2009b; Robinson et al., 2006]. The same mutation was also found in conjunction with a frameshift mutation in a patient with recessive inheritance in our cohort. The c.13513G>C; p.Asp4505His dominant mutation was detected in two patients with a relatively severe and a mild phenotype. Interestingly, the same variant was also found in two other patients with an apparently recessive inheritance, in conjunction with a splice-site mutation in a patient with a moderate phenotype and together with a second missense mutation in the other patient. This mutation (c.13513G>C; p.Asp4505His) has been previously reported together with c.7085A>G; p.Glu2362Gly in a MHS patient with hyperCkemia, but no information was provided if those variations were in trans or cis [Malandrini et al., 2008]. The variant c.14423C>T was very recently reported in MHS in cis with the reported c.14422C>T variant [Kraeva et al., 2011].
The c.8360C>G; p.Thr2787Ser mutation was detected in cis with c.14578_14580del, previously reported in MHS and CCD, respectively, in a family with two affected siblings with severe phenotype, who inherited both changes from the unaffected mother (see pedigree in Fig. 1).

Family 22 (patient 22 in Supp. Table S1a and family members 22 in Table S2a): The c.8360C > G; p.Thr2787Ser was detected in cis with c.14578_14580del in a family with two affected siblings, who inherited both changes from an unaffected mother. They had a severe phenotype, nonambulant with proximal, axial, facial, and bulbar weakness, and rigid spine and scoliosis. Muscle biopsy of the older sibling shows type 1 predominance, cores and core-like areas, and increase of connective tissue. These two mutations have been previously reported in MHS and CCD families respectively.
Clinically, the 40 patients (family members with sufficient clinical information included) with dominant mutations had variable onset of symptoms ranging from reduced fetal movements prenatally, or polyhydramnios, to adult onset weakness. Severity was also very variable: 14 of the 40 patients were able to run; all patients who were old enough, except two (able to sit; age, 4 years), were able to walk; of these, six had difficulty in walking up stairs, while three were not able to do so. Facial weakness was present in 15 patients, mostly mild. Two index cases had restricted upward gaze, one with a King Denborough phenotype, as previously reported [D'Arcy et al., 2008], while the other and his similarly affected mother carried a change that was previously reported in dominant CCD [Monnier et al., 2001]. This family had an unusual phenotype with arthrogryposis and arachnodactyly in addition to the proximal weakness, and it was possible that genetic variations in other genes were contributing to the phenotype observed in this family.
RYR1 Mutations Associated with Uncertain Inheritance Pattern (marked “?” in Supp. Table S1a)
In five patients, the only pathogenic change identified had been previously reported as recessive mutation in conjunction with other mutations. One of those, c.10616G>A; p.Arg3539His, was indeed found in another family of our cohort with clear recessive inheritance. The c.12884C>T; p.Ala4295Val variant was detected in two of our patients, one with ophthalmoplegia, the other with a moderate CCD phenotype without ophthalmoplegia. In both patients, the mutation was inherited from an asymptomatic parent. This amino acid change (p.Ala4295Val; c.12891C>T; NM_000540) has been previously reported in cis with c.7304G>A; p.Arg2435His in a large family with MH and MmD [Jeong et al., 2008]. In these cases, there is the possibility that a second large rearrangement of the other allele, as reported recently, was missed by our detection methods [Monnier et al., 2009]. The patient with the c.14126C>T, p.Thr4709Met mutation has been reported by us before, with the mutation hypothesized to be expressed monoallelically in skeletal muscle [Zhou et al., 2007]; in this patient, no further mutation was found in the diagnostic setting, and there remains the possibility of epigenetic silencing, or that a large deletion or duplication, or a mutation affecting the promoter, was undetected.
In the 35 families, a total of six MH reactions were reported either in the index case or in one of the relatives. All of these carried a heterozygous missense mutation (see Supp Tables S1a and S2a). All but c.2677G>A; p.Gly893Ser have been reported before to cause MHS (but are not listed as causative on the European malignant hyperthermia group webpage [www.emhg.org]). Cosegregation of the mutation with the disease in at least two pedigrees, the absence of the sequence change from 100 control samples, and functional characterization are required to be listed as causative.
Recessive RYR1 Mutations
In the 36 families with recessively inherited RYR1 mutations, the combination of a missense mutation with a second RYR1 mutation expected to result in a reduced amount of functional RyR1 protein was observed in 17 families. In particular, we found the combination of a RYR1 missense mutation with a nonsense mutation in nine of the 36 families, with a splice-site mutation in five of the 36 and with a frameshift mutation in three of the 36 families. Six of the 36 families were found to be homozygous for a missense mutation. In eight of the 36 families, two missense mutations, and in four of the 36 families, three missense mutations (the same combination of two mutations proven to be in cis in three families; see below) were detected. In 23 of the 36 families, the parents and other relatives were tested, and the mutations were found to be in trans (indicated in Supp. Table S1b).
Of the recurrent mutations, the known c.11315G>A; p.Arg3772Gln variant was found in the homozygous state in five families. The other recurrent mutation was c.11798A>G found in four families, either in conjunction with two other missense mutations (three of the four families) or a splice-site mutation (one of the four families). The extended family was tested in three of these four families, and it was shown that c.11798A>G and c.4711A>G always occurred in cis, as previously reported in a large MHS pedigree with four mutations not always segregating with MHS [Tammaro et al., 2011]. The c.4711A>G mutation has also been recently described in conjunction with c.10097G>A; p.Arg3366His in a patient with a mild adult onset core myopathy, scoliosis and respiratory weakness [Duarte et al., 2011].
Of the 35 novel recessive mutations, 22 were missense, six were stop, three were splice site, three were frameshift, and one was a single amino acid deletion. The evidence to support the pathogenicity of these mutations is shown in Supp. Table S1b. Included in the group of patients with recessive inheritance are four patients previously reported (marked in Supp. Tables S1a and S1b with “+”) to have mutations expressed monoallelically in the skeletal muscle [Zhou et al., 2006, 2007]; in these, a further stop mutation was detected. No further mutation was identified in a fifth patient, also with monoallelic expression [Zhou et al., 2007]. Functional studies of the mutations of patient 36 have been reported very recently [Treves et al., 2011].
Clinically, we found more pronounced but overall variable severity associated with recessive inheritance: all patients presented this in the first 10 years of life, most at birth (16 of the 46) or prenatally (10 of the 46), and followed a course that was stable or even showed some improvement in childhood. All patients had proximal, some additional distal, and most had axial and facial weakness. In nine patients, weakness was severe; of those, seven were not able to walk unaided, three were not able to sit, and three severely affected patients with respiratory weakness and feeding difficulties died in infancy. Within the group of the severe patients, five were either compound heterozygous or homozygous for two missense, and four were compound heterozygous for a missense and a stop or frameshift mutation, respectively. At the milder end of the clinical spectrum, 13 patients were able to run.
Feeding difficulties were present in 14, necessitating gastrostomy insertion in eight patients.
Extraocular eye muscle involvement was present in 12, ranging form marked limitation of vertical and horizontal eye movements with or without ptosis to mild restriction of abduction or upward gaze. Within the group with extraocular muscle involvement, patients were compound heterozygous for a stop and a missense mutation (six of the 12), a splice mutation and a missense mutation (three of the 12), two missense mutations (two of the 12), and one patient with a single amino acid deletion in combination with a missense mutation.
In the five families with the homozygous c.11315G>A; p.Arg3772Gln mutation, the phenotype varied, ranging from an MHS susceptible individual, a relatively severe presentation with prenatal onset and markedly delayed motor milestones, to a King Denborough phenotype. In one family, the index case and his brother had a King Denborough phenotype with mild proximal and facial weakness, and the older sibling had a MH reaction at the age of 9 years. Interestingly, the father was also found to be homozygous for the change and was diagnosed to be MHS positive, but did not show any dysmorphic features or weakness (see pedigree in Fig. 2)
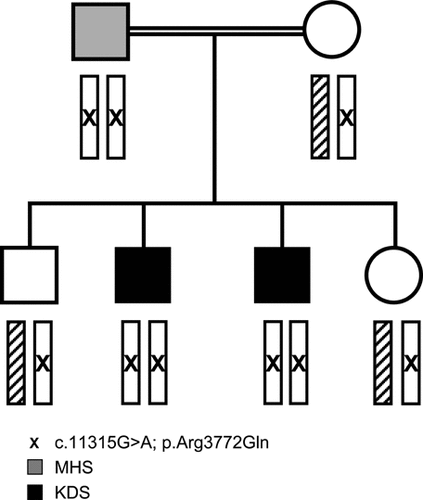
Family 61 (patient 61 in Supp. Table S1b and family members in Table S2b): The homozygous c.11315G > A; p.Arg3772Gln was found in a large consanguineous family; two brothers had a King Denborough phenotype with mild proximal and facial weakness, complicated in the older sibling by an MH reaction. Muscle biopsy of this latter individual only revealed mild myopathic changes with variability of fiber size, but no cores or type 1 predominance. Muscle MRI showed mild unspecific changes, not typical for RYR1. Testing of the family revealed that both siblings had each inherited a RYR1 variant from their parents; interestingly, the father was also found to be homozygous for the change and was diagnosed to be MHS.
An interesting observation concerns a patient with recessive inheritance of two reported RYR1 mutations in combination with a dominantly inherited, previously reported mutation in the α-tropomyosin (TPM3) gene, (c.503G>A, p.Arg168His). Her daughter, with the same phenotype of a stable early onset proximal, axial, and mild facial weakness and ptosis, carries the TPM3 mutation and the known recessive RYR1 mutation c.10616G>A, p.Arg3539His [Monnier et al., 2008].
For detailed information on an unusual family with three novel mutations in different combinations, see pedigree in Fig. 3.
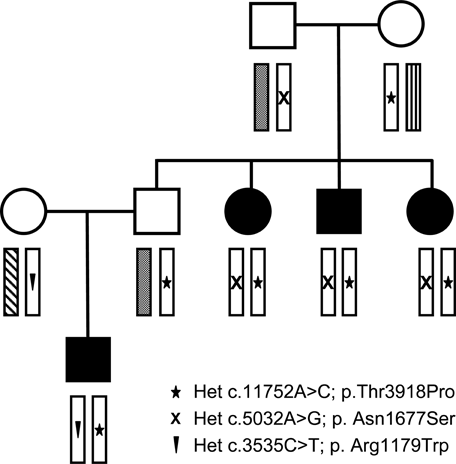
Family 51 of the 53 families (patient number 51 and 53 in Supp. Table S1b, and family members in Supp. Table S2b): Three RYR1 mutations were found in different combination in this family; the index case was a severely affected boy with prenatal onset, needed ventilation in the neonatal period, and nocturnal noninvasive ventilation since a few months of age. He had marked feeding difficulties, which required a gastrostomy insertion. He developed a severe early onset scoliosis (45° at the age of 1 year). At the age of 20 months, he had generalized weakness, predominantly proximal, axial, and facial, ptosis, and restricted gaze abduction. He was able to roll, but could not sit unsupported. Muscle biopsy had myopathic signs, but nor cores or type 1 predominance. The father is unaffected, but two of his siblings are affected. One sister was floppy at birth, had delayed motor milestones, cannot run, and has difficulties climbing stairs. She has proximal and facial weakness, but no ophthalmoplegia; the brother is similarily affected. These two individuals had minicores on muscle biopsy. The other sister has mild weakness and a history of talipes.
MH reactions were reported in two families with the homozygous c.11315G>A; p.Arg3772Gln mutation. In vitro contraction test (IVCT) results were only available in one of these two families and confirmed MHS.
In 14 families with putative recessive inheritance, no parental DNA was available and the phase of the mutations could not be determined with certainty.
Discussion
Since the introduction of full gene sequencing for RYR1-related myopathies in our diagnostic setting in 2007, we have identified 82 causative mutations in 71 families. Altogether 41of the 82 mutations were novel mutations, six dominant, and 35 recessive.
It is well documented that dominant mutations involved in CCD are mostly confined to the C terminal region of the gene, MHS/CCD region 3 (amino acid residues 4,550–4,940), whereas mutations involved in MH are mostly detected in region 1 and 2 within the N terminal, (amino acid 35–614 and 2,163–2,458, respectively). Of the 27 dominant mutations in our study, 17 of the 27 (62%) were found in the hotspot regions, 14 in region 3, indicating that the classical phenotype of CCD is closely but not exclusively associated with the previously identified mutational hotspot region. Consistent with previous reports, most dominant mutations in our cohort (26 of the 27) were missense mutations. A single amino acid deletion was only found in one patient, in conjunction with a missense mutation in cis, complicating the assignment of the contribution of each variation to the resulting phenotype.
Five dominant novel variants were found, four localizing to hotspot region 3, one of which affects the same amino acid residue as a previously reported mutation [Davis et al., 2003].
The finding of a child with CCD, who inherited a previously reported dominant MH mutation from an as yet asymptomatic parent, could be in keeping with the known variable expression of the disease. We found five mutations in patients with dominant CCD, previously only described in MH, suggesting a more extensive continuum between RYR1-related MH and congenital myopathy phenotypes than has been previously assumed; this is also in keeping with the recent observation of late myopathic manifestations of some MH-related RYR1 mutations. [Jungbluth et al., 2009] It also raises the question of additional genetic modifiers or allelic RYR1 mutations that may have remained undetected on routine genomic sequencing or the modifying effect of VUSs, which could have an additional effect. Although in our cohort we did not find a correlation between the number of VUSs and the phenotype in families with variable severity, it is not possible to conclude whether the VUSs did have an additional effect.
In some patients, the only pathogenic change detected was a mutation previously reported in patients with recessive central core or CNM, inherited from an unaffected parent. In these cases, there remains the possibility of recessive inheritance with a genomic rearrangement on the other allele, undetectable on routine genomic DNA sequencing. This has been recently described in a severely affected neonate [Monnier et al., 2009].
In the patients with recessive inheritance, 55 different mutations were found, 35 of them novel. The relatively larger number of novel recessive compared with novel dominant RYR1 mutation may be explained by the fact that recessive RYR1 mutations are distributed widely throughout the coding sequence, which, until recently, was not systematically screened in its entirety. Of the 55 recessive mutations identified, 19 were in the hotspot regions, in contrast to the dominant CCD-associated RYR1 mutations more frequently in MHS hotspot regions 1 and 2 (n = 14), compared with CCD hotspot region 3 (n = 5) (see Fig. 4). This is in keeping with previous reports, indicating that recessive RYR1-related myopathies may be due to compound heterozygosity or homozygosity for MH mutations and that recessive RYR1 mutations are widespread across the entire RYR1 gene [Ferreiro et al., 2002; Jungbluth et al., 2005; Zhou et al., 2007]. In contrast to the literature, where most patients with RYR1-related recessive central core myopathy [Monnier et al., 2008], CNM [Wilmshurst et al., 2010], fiber-type disproportion [Clarke et al., 2010], or myopathy with myofibrillar disorganization [Bevilacqua et al., 2011] had a combination of a null mutation and a missense mutation, in our cohort we found a number of families (12 of the 36) with two recessively inherited missense mutations. When comparing the clinical severity of patients with the combination of a null and a missense mutations with those with two missense mutations, no difference in clinical severity could be found, as both combinations gave rise to either mild to moderate or severe weakness. Unfortunately, we do not have any functional or protein expression data from these patients to show a mutation-specific effect on muscle RyR1 protein expression in these patients. When comparing the genetic findings of the 14 patients with extraocular muscle involvement, a symptom usually observed in patients with recessive inheritance, we found three patients (two in the same family) in the dominant group who had mildly restricted upward gaze and only one patient who had more marked extraocular eye muscle involvement. As the only mutation detected in this family was previously reported in a large family with MH together with a second missense mutation (c.7304G>A; p.Arg2435His) [Jeong et al., 2008], it is possible we are missing a second change, for example, a genomic rearrangement, also suggested by the fact that it was inherited from an asymptomatic parent. All other 13 patients of 11 families with extraocular eye muscle involvement had recessive inheritance. Also in the group with recessive inheritance, some patients had only mild involvement with restricted abduction, which might be missed on examination if not specifically looked for.
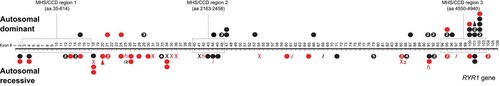
Overview of all detected mutations: autosomal dominant mutations above the line and autosomal recessive mutations below the line. Red/grey (in print), novel mutations; Black, reported mutations; Dot, missense mutation, X, stop mutation; Fs, frameshift; /, splice-site mutation; triangle, deletion; a number in the dot, the same mutation was found in many patients. The previously reported MHS/CCD regions are marked with a dotted line.
The King Denborough phenotype was found in a few patients of our cohort with either dominant or recessive inheritance. The genetic basis of this disorder is not entirely clear; MH is one of the characteristic features of the syndrome. De novo dominant mutations and recessive mutations in the RYR1 gene have been reported in some but not all patients [D'Arcy et al., 2008; Dowling et al., 2011], therefore suggesting further genetic heterogeneity.
Comparing the overall severity between patients with recessive and dominant inheritance in both groups, there is marked variability. In general, the most severe patients within our cohort had recessive mutations and none of the recessively inherited myopathies presented in adulthood. At the other end of the spectrum, some patients with recessive disease present like the previously described typical dominant CCD phenotype. MH was encountered more often in patients and family members within the group with dominant inheritance and only in patients with missense mutations, although, considering the lack of IVCT data, this information might be incomplete. For the homozygous c.11315G>A mutation, clinical variability associated with the homozygous and heterozygous state has been described before [Carpenter et al., 2009a].
Of our patients, five have been previously shown to have monoallelic expression, which was interpreted as allele silencing [Zhou et al., 2006, 2007]; however, in four of these, a further nonsense mutation was subsequently detected. Nonsense-mediated decay of the affected allele explains why only one allele was found to be expressed in the muscle. We do not have any other molecular explanation for the fifth patient in whom no other mutation was found, and hence allelic silencing remains one possibility in this case.
There are a number of diagnostic challenges in the diagnosis of RYR1-related myopathy, as there is marked variability of clinical presentation and histopathologic findings, as well as different modes of inheritance. In addition, the RYR1 gene is very large, analysis is expensive and time consuming, and detection of large genomic rearrangements is not possible with the techniques used in a routine diagnostic setting. Assigning pathogenicity to individual novel variants can be difficult, especially if the variants are multiple and if functional studies are not available. Assessing if variants are in trans or cis requires parental DNA and often large families to assess segregation of changes in affected and unaffected members; these are not always available. We cannot exclude that multiple VUSs found in some patients may have an additive effect. Also, the concept of MH being caused by a single dominant mutation has been challenged, as multiple changes have been found in MH patients, and discordant results between genetic results and IVCT have been reported [Levano et al., 2009; Tammaro et al., 2011]; the same might apply to RYR1-related myopathies.
The carrier frequency of a variant in RYR1 has been estimated to be 1:2,000 [Monnier et al., 2002; Wu et al., 2006]; the prevalence of an RYR1-related myopathy in Southeastern Michigan was calculated to be 1:90,000 [Amburgey et al., 2011]. Also, as reported recently [Pandey et al., 2011], digeny for mutations in two different genes is certainly a possibility, as supported by the combination of pathogenic TPM3 and recessive RYR1 mutations in one of our families. All these issues make genetic counseling challenging in individual families.
Conclusion
Our study provides further evidence that RYR1-related myopathies are common, and certainly by far the most common cause of congenital myopathies in UK (Muntoni and Jungbluth, personal observation). While the identification of previously identified, clearly pathogenic mutations, especially in families with dominant inheritance provides a secure basis for the diagnosis, our results suggest that the final diagnosis of a RYR1-related myopathy can often only be reached by integrating the clinical, muscle biopsy, imaging assessments, and genetic findings.
Acknowledgements
The University Children's Hospital in Zurich is gratefully acknowledged by A.K.
Disclosure Statement: S.L., N.T., and S.A. worked in the DNA Laboratory of GSTS Pathology, which is a Limited Liability Partnership joint venture offering clinical genetic testing of the RYR1 gene