Confirmation of association of FCGR3B but not FCGR3A copy number with susceptibility to autoantibody positive rheumatoid arthritis†
Communicated by Christopher Mathew
Abstract
The FCGR locus encoding the low-affinity Fcγ receptors (FcγR) for immunoglobulin G has largely been missed by genome-wide association studies due to complications with structural variation and segmental duplication. Recently identified copy number variants (CNVs) affecting FCGR3A and FCGR3B have been linked to a number of autoimmune disorders. We have developed and validated a novel quantitative sequence variant assay in combination with an adapted paralogue ratio test to examine independent CNVs carrying FCGR3A and FCGR3B in rheumatoid arthritis (RA) compared with healthy volunteers (n = 1,115 and 654, respectively). Implementation of a robust statistical analysis framework (CNVtools) allowed for systematic batch effects and for the inherent uncertainty of copy number assignment, thus avoiding two major sources of false positive results. Evidence for association with neither duplications nor deletions of FCGR3A was found; however, in line with previous studies, there was evidence of overrepresentation of FCGR3B deletions in RA (odds ratio [OR] 1.50, P = 0.028), which was more apparent in rheumatoid factor positive disease (OR 1.61, P = 0.011). The level of FcγRIIIb, encoded by FCGR3B, expression on neutrophils was shown to correlate with gene copy number. Thus, our results may highlight an important role for neutrophils in the pathogenesis of RA, potentially through reduced FcγRIIIb-mediated immune complex clearance. Hum Mutat 33:741–749, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
Rheumatoid arthritis (RA; MIM# 180300) is a chronic inflammatory autoimmune condition characterized by destructive polyarthritis of the synovial joints, with an estimated prevalence of 0.8% in the UK [Symmons et al., 2002]. In the majority of patients, RA is characterized by the presence of autoantibodies (rheumatoid factor [RF] and anti-citrullinated peptide antibodies). Genome-wide association studies (GWAS) have yielded numerous positive disease-associated loci in Caucasians [The Wellcome Trust Case Control Consortium, 2007], although only HLA-DRB1 and PTPN22 reached genome-wide significance. Subsequent studies have replicated numerous loci that were originally identified by GWAS, with more modest significance levels and effect sizes [Stahl et al., 2010]. Emerging data suggest that different genetic and environmental associations may be observed in autoantibody-positive and autoantibody-negative RA, although this must be distinguished from the increased statistical power in the more prevalent autoantibody-positive subgroup [Morgan et al., 2009].
Immunologically important genes appear to be particularly prone to copy number variation [Bailey et al., 2002; Redon et al., 2006; Tuzun et al., 2005], which may be an important evolutionary mechanism conveying immunological diversity. Correspondingly, various copy number variants (CNVs) have been shown to be associated with autoimmune conditions and affect expression levels with dose dependency [Aldred et al., 2005; Morris et al., 2010; Stranger et al., 2007; Willcocks et al., 2008]. The coverage of GWAS platforms is limited to unique regions of the human genome where probes designed to interrogate genomic variation map to discrete targets. Segmental duplications (SDs) and complex CNVs pose significant analytical challenges to the interpretation of GWAS data sets, particularly where most simple nucleotide polymorphism (SNP) records in the National Institute for Biotechnology Information database (dbSNP) in SDs are actually paralogous sequence variants (PSVs) [Estivill et al., 2002]. A recent study of common CNVs in The Wellcome Trust Case Control Consortium (WTCCC) cohorts [The Wellcome Trust Case Control Consortium, 2010] found no new associations with CNVs in RA other than those already identified in GWAS through linkage disequilibrium (LD) with SNPs. The WTCCC study used a custom high-resolution oligonucleotide array [Conrad et al., 2010] to interrogate over 11,500 loci, 3,432 of which passed quality control. One of the CNVs that provided good calls was CNVR383.1, carrying FCGR3A (MIM# 146740) and FCGR3B (MIM# 610665), for which four copy number classes could be distinguished. However, oligonucleotide probes mapping to CNVR383.1 have multiple targets due to the high sequence identity between the gene paralogues in the FCGR gene locus. There are reports of several independent CNV-affected portions of this locus that were detected in whole-genome screens utilizing various platforms (Database of Genomic Variants, http://projects.tcag.ca/variation/), with independent deletions and duplications of FCGR3B and FCGR3A being described, although the precise limits and breakpoints remain unknown [Breunis et al., 2009; Niederer et al., 2010; Willcocks et al., 2008]. FCGR3B and FCGR3A share 98% sequence identity [Ravetch and Perussia, 1989], which introduces considerable difficulties in generating robust assays. CNVR383.1 probes from the custom high-resolution oligonucleotide array used in the WTCCC study would therefore have mapped to both FCGR3B and FCGR3A and detected the total number of copies of the FCGR3 genes. Given these complexities, copy number variation within a single gene at the FCGR locus has therefore not been adequately assessed using the commercially available CNV platforms.
The low-affinity FCGR locus at 1q23.3 contains a set of five genes with high sequence identity (FCGR2A, FCGR3A, FCGR2C, FCGR3B, FCGR2B, centromere to telomere) encoding proteins with discrete functions, expressed on different cell types that bind immunoglobulin (Ig)G-containing immune complexes. Fcγ receptor (FcγR) cross-linking results in numerous immunological responses of direct relevance to autoimmune and inflammatory disorders such as immune complex capture and presentation, phagocytosis, and cytokine and proinflammatory mediator release, thus making them excellent candidate genes for autoantibody-associated diseases such as RA. Indeed, a common functional SNP in FCGR3A (rs396991) confers a phenylalanine to valine amino acid substitution at position 158. FcγRIIIa (encoded by FCGR3A) is expressed on natural killer (NK) cells, macrophages, some monocyte subsets, and γδ T cells and signals through associated γ-or ζ-chain immunoreceptor tyrosine-based activation motifs (ITAMs). Homozygosity for the higher affinity V allele has consistently been shown to be associated with susceptibility to autoantibody-positive RA [Robinson et al., 2009; Thabet et al., 2009]. Thus, one may postulate that FCGR3A duplications may display association with RA, although this was not observed in a small study that used multiplex ligation-dependent probe amplification (MLPA) [Thabet et al., 2009]. FcγRIIIb (encoded by FCGR3B), on the other hand, is selectively expressed on neutrophils and eosinophils and lacks a cytoplasmic domain, being linked to the cellular membrane by a glycosylphosphatidylinositol (GPI) anchor. It signals through associated receptors, predominantly the ITAM-bearing intracellular portion of FcγRIIa [Chuang et al., 2000] and the complement receptor CR3 [Poo et al., 1995]. FCGR3B has two common polymorphic forms, NA1 and NA2 (HNA1a/HNA1b or *1/*2), which differ by five nucleotides that encode four amino acid differences. This alters the number of glycosylation sites, and neutrophils from individuals homozygous for the FCGR3B-NA2 allele have been found to exhibit lower levels of phagocytosis than FCGR3B-NA1 homozygotes [Salmon et al., 1990]. Previous studies in RA have shown an association with the FCGR3A-FCGR3B 158V-NA2 haplotype, which includes the lower affinity variant of FCGR3B [Morgan et al., 2006]. This is consistent with the recent observation of low FCGR3B copy number in systemic lupus erythematosus (SLE) and RA [Aitman et al., 2006; Fanciulli et al., 2007; McKinney et al., 2010; Willcocks et al., 2008] and the observation that neutrophils from individuals with low FCGR3B copy number display reduced uptake and adherence to immune complexes [Willcocks et al., 2008], which are known to be deposited in the joints in RA [Cooke et al., 1975; Vetto et al., 1990].
Initial FCGR3B CNV association studies in SLE and RA used quantitative PCR (qPCR) [Aitman et al., 2006; Fanciulli et al., 2007; Willcocks et al., 2008], which generated concerns over assay accuracy and reliability, partly as a consequence of lack of internal controls that could be used for standardization. Hollox et al. (2009) subsequently optimized a paralogue ratio test (PRT) to analyze both FCGR3A and FCGR3B copy number and presented the results from a set of reference samples. Recent publications using variants of PRT have confirmed the association between low FCGR3B copy number and SLE [Morris et al., 2010; Niederer et al., 2010].
Despite the recent technical improvements in genotyping platforms, copy number measurement data are not usually whole integer values, but estimates that tend to cluster around expected integer copy number values. Methods that aim to assign absolute copy number values to each sample tested, ignoring uncertainty or discarding borderline samples, are therefore prone to error [Carter, 2007]. As a consequence, many of the previously reported studies have been limited by assignment into discrete copy number categories prior to statistical analysis. Additional complexity may also be introduced when using DNA samples that have been handled differently, with batch effects potentially leading to strong bias. Barnes et al. (2008) suggested a robust statistical framework that could be used to analyze raw copy estimates without the need to assign individual samples into copy number classes or discard uncertain calls while also taking batch effects into account. Methodologies sympathetic to minimizing bias such as those described above have not been adequately utilized in the analysis of CNV at the FCGR locus to date.
In this manuscript, we describe the development and use of a novel quantitative sequence variant (QSV) assay. This assay used quantitative data extracted from sequencing electropherogram peak heights using the QSVanalyser software [Carr et al., 2009] to determine the number of copies of FCGR3B relative to the total number of copies of FCGR3B and FCGR3A. When combined with a modified PRT, which measured the total number of copies of FCGR3B and FCGR3A, this assay allowed independent assessment of the FCGR3B and FCGR3A CNVs. The analytical framework within CNVtools was fully utilized to test association in RA. Furthermore, the methodologies developed for this study may be readily adaptable to the investigation of CNV within other homologous gene families subject to CNV.
Materials and Methods
Subjects
Human reference DNA samples from the International HapMap project were obtained from the Coriell Institute (Camden, New Jersey) for use as a comparison in assessing the performance of the copy number assays. All samples used were from plate HAPMAPPT01.
The Biologics in Rheumatoid Arthritis Genetics and Genomics Syndicate (BRAGGSS) provided 1188 DNA samples extracted in two batches (BRAGGSS1 and BRAGGSS2), which were considered to be independent throughout this study due to the known influence of DNA handling as a major contributor to batch effects in copy number assays [Barnes et al., 2008]. All RA patients were Caucasian of Northern European descent and fulfilled the 1987 American College of Rheumatology classification criteria [Arnett et al., 1988]. A total of 735 healthy controls were recruited from Sheffield [Mewar et al., 2006]. Informed consent was obtained from all study participants, which was approved by the respective Research Ethics Committees. All DNA samples were isolated using standard phenol chloroform methods and were normalized to 20 ng/µl after being subjected to quantity and quality checks by spectrophotometry at 260 and 280 nm. Duplicate samples were assayed as part of the quality control process. All duplicates were in agreement, and where two quantitative measurements were available, the mean of them was used in the analysis.
Copy Number Assays
The experimental approach of Hollox et al. (2009) was adopted in a two-part assay. The first element measured the copy number of FCGR3B relative to FCGR3A; however, instead of the restriction enzyme digest variant ratio (REDVR), we used an alternative sequencing-based method based on electropherogram peak heights [Carr et al., 2009]. The second element recorded the total number of FCGR3 gene copies (FCGR3B + FCGR3A) using a dispersed stable repeat on chromosome 18 [Hollox et al., 2009]. These assays were validated by comparing with publicly available genome-wide platform and gene-specific data, using a set of HapMap reference samples (see Supporting Information). Each group of samples (BRAGGSS1, BRAGGSS2, and healthy controls) was treated as a separate batch. All samples in a batch were amplified using the same master mix of reagents on the same day, and all downstream processing was carried out maintaining batch identity.
GPI QSV analysis: Determination of the relative copy number of FCGR3A and FCGR3B
PCR primers were designed (forward, dGTTTGGCAGTGTCAACCATCTCATCATTC; reverse, dGGTTGCAAATCCAGAGAAATGTTCAGAG) to coamplify a 257-bp fragment of FCGR3A and FCGR3B surrounding the gene-defining PSV [Ravetch and Perussia, 1989], which determines the cell membrane anchorage (GPI). This fragment included five resequencing-confirmed PSVs, four of which appeared on sequencing electropherograms and had the potential to serve as QSVanalyser targets when sequenced with the forward primer (Figure 1). The optimized master mix for the GPI QSV assay is shown in Supp. Table S1. Thermal cycling started with initial denaturing at 95°C for 30 sec, followed by 30 cycles of 95°C for 15 sec, 55°C for 15 sec, and 72°C for 30 sec, terminating with 72°C for 5 min. Each PCR product (40 µl) was purified using Charge Switch PCR cleanup kits (Life Technologies, Carlsbad, CA) using 1 µl of magnetic beads, resuspended in 10 µl of elution buffer, which effectively normalized and concentrated the amplicons. Purified PCR product (1 µl) was used as template in BigDye terminator sequencing reactions using 1 µl of BigDye (Life Technologies) and 2.4 pmol of the forward primer per reaction. Sequencing electropherograms were basecalled using the Sequence Analyzer software (Life Technologies). Examples of representative electropherograms are shown in Supp. Figure S1. Processed electropherograms were analyzed using QSVanalyser [Carr et al., 2009]. Reference templates were generated from tiling path clones (RP11-5K23 for FCGR3A and RP11-474I16 for FCGR3B).
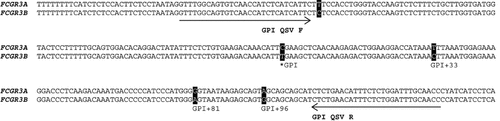
Alignment of the GPI PSV region of FCGR3A and FCGR3B showing forward and reverse primers used in the QSV assay. Also indicated are the PSV targets for the QSVanalyser algorithm (GPI, GPI + 33, GPI + 81, and GPI+96).
PRT: Chromosome 18 repeat
The PRT described by Hollox et al. (2009) was adapted. Briefly, a short fragment of FCGR3B, FCGR3A, and a dispersed repeat, 11 bp longer, on chromosome 18 were coamplified using oligonucleotide primers complementary to all three loci (FAM forward dCATGATCTGGCCCTGAAAC, reverse FAM-TGAGTTCAAGAAAGCAGTTTG; HEX forward dTGCCCTTCATGATCTGGCC, reverse HEX-TGAGTTCAAGAAAGCAGTTTG). Master mix components for the PRT are detailed in Supp. Table S2. For this study, the running of both FAM and HEX fragments down the same capillary was enabled by moving the forward primer for the HEX-labeled PCR by seven nucleotides, thereby avoiding spectral overlap between FAM- and HEX-labeled electropherogram peaks of equal size (Supp. Fig. S2). This modification was rigorously tested against the published method in the reference samples, demonstrating assay equivalence (Supp. Fig. S3). Additionally, instead of using peak area to calculate the total copy number, we used peak height, as previously described, to facilitate rapid automation using a proprietary piece of software developed for this purpose (http://dna.leeds.ac.uk/peakheights/).
Quality Control (QC) and normalization
For the GPI QSV, the “ABI QC” program (http://www.insilicase.co.uk/Desktop/ABIQC.aspx) was applied to each data folder in “no blank” mode. This program returned a table of signal-to-noise ratios for the *.ab1 files in each folder. Any signal-to-noise ratios dropping below 100 indicated that samples were discarded.
The QSVanalyser program was then run on each folder and the data were reviewed. Traces giving no results (where sequences surrounding each QSV were not found by the software) were discarded. These traces may have interfered with the final QSVanalyser score, so the program was run again with the failed traces removed.
These QC steps resulted in 72 discards from the BRAGGSS samples and 81 from the healthy controls. The main reason for data exclusion was the absence of either the FAM- or HEX-labeled amplicon in the PRT.
Detailed descriptions of the experimental and data handling methods are available in the Supporting Information and a workflow schematic is provided in Supp. Figure S4.
Estimates of gene copy number
To analyze copy number on a gene-specific basis, two continuous measures were created, representing the estimated copy number for each gene. This was achieved by calculating the product of the PRT score (which measures FCGR3A + FCGR3B) and the GPI score (which measures FCGR3B /[FCGR3A + FCGR3B]) to give the estimate of FCGR3B copy number and subtracting this value from the PRT value to estimate FCGR3A copy number.
Immunoassays
The majority of RA cases were recruited from NHS Rheumatology Clinics throughout UK, and IgM RF status was measured in all cases using standard nephelometric assays. Patients who had ever had titers of ≥40 units/µl were defined as RF positive.
Statistical analysis using CNVtools
Two important potential sources of false positive association in copy number analysis are incorrect handling of the uncertainty in copy number calls and ignoring batch effects that arise when samples are handled separately. To avoid both of these, we used the CNVtools package in R to analyze the continuous estimates of copy number for each gene separately. The method fits a mixture of normal distributions to the data, each corresponding to a different copy number class, which is treated as a latent variable in the model, and allows for batch effects by permitting the means of the distributions to vary according to batch. Evidence for association is assessed by carrying out a likelihood ratio test comparing the likelihood when disease status is allowed to depend on discrete copy number class with the likelihood under the null hypothesis of no association. The method differs from other commonly used methods in which there is no assignment of individuals to copy number classes, although posterior probabilities of class membership can be derived.
CNVtools was applied to the data for each gene, treating the controls and the two case cohorts as three separate batches, after first rescaling the data to mean of 0 and variance of 1. The number of components in the mixture model was fixed at 3, representing normal copy number (class 2), less than two copies (class 1 including hemizygotes and double deletions) and more than two copies (class 3 including heterozygous and homozygous duplications). For both genes the software was unable to fit higher component models to the data, probably due to the low numbers in the minor groups. Statistical power to detect significant associations with specific copy number subgroups (i.e., 0 or 1 copy) would in any case be too low.
Statistical association tests
- 1.
General model—Initially, we examined evidence for an effect of copy number on disease risk by testing whether disease risk varies between the three copy number categories (2 degree of freedom [df] test). This model makes no assumptions about the direction of effect with respect to the effect of CNV, but has the disadvantage of low power.
- a.
For FCGR3A, we considered a more restricted version of this model, comparing duplications versus normal copy number/deletions, hypothesizing that duplication is the risk factor for disease and deletions have no increased risk (1 df).
- b.
For FCGR3B, we considered a more restricted version of this model, comparing deletions versus normal copy number/duplications, following previous work that suggested an effect on risk of RA of deletions at this locus [McKinney et al., 2010].
- a.
As a final analysis, we compared duplications and deletions versus normal copy number, hypothesizing that disease risk is increased with any deviation in copy number from 2 (1 df).
Flow Cytometry
CD16b expression was measured on neutrophils of RA patients selected for different FCGR3B copy number. Ammonium-chloride-lysed heparin peripheral blood samples were stained with antibodies against CD16 (clone 3G8; Caltag, Buckingham, UK) or appropriate IgG1 isotype control. Neutrophils were identified on the basis of forward and side scatter characteristics (BD FACSCalibur; BD Biosciences) and the mean fluorescence intensity of the CD16 expression determined.
Results
Validation of FCGR3A and FCGR3B Copy Number Assays
Assay parity was observed when comparing PRT data from this study with the PRT methods from the original study, and in comparison with hybridization-based methods (whole genome tiling path array CGH and Affy 6.0) undertaken on the same HapMap reference samples (Supp. Fig. S5). For the GPI QSV assay, agreement between GPI and GPI + 96 QSVs is presented in Supp. Figure S6. The mean of these two QSV scores was used as a measure of relative abundance of FCGR3B against the total of FCGR3A and FCGR3B, and was compared to the REDVR assay [Hollox et al., 2009], which measured the ratio of FCGR3A copies to FCGR3B. Data for the HapMap reference samples are plotted in Figure 2, which demonstrates grouping of sample values along the y-axis, suggesting that the QSV method is more sensitive to relative gene copy number than REDVR. Subjective copy number calls for the HapMap samples based on PRT and relative copy number plots for both Hollox et al. (2009) and this study are shown in Supp. Figure S7. Interpretation of these copy number calls is shown against data from other platforms for the same samples in Supp. Table S3. Concordance between the different data sets is shown in Supp. Table S4.
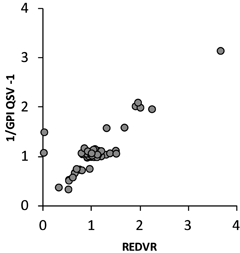
Relationship between REDVR FCGR3A–FCGR3B data and GPI QSV values for 90 HapMap CEU samples. The sequencing-based GPI QSV value was converted to the REDVR equivalent parameter (1/GPI QSV-1) to allow direct comparison.
Estimation of Population Frequencies of FCGR3A and FCGR3B Copy Number
For each gene, the distributions of copy number estimates are shown in Figure 3, based on the 1115 cases and 654 controls passing quality control. One sample was excluded from further analysis due to a measurement of more than seven copies of FCGR3A, which was thought to be anomalous. Four samples had FCGR3B copy number estimates lower than 0.5. These were presumed to be homozygous deletions and were recoded to fit into class 1 (deletions) with probability 1. Ten samples had FCGR3B copy number estimates above 3.7 and these were assumed to represent three or more copies. Thus, these were recoded into class 3 (duplications) with probability 1. For FCGR3A, there were no apparent double deletions. There were, however, 11 samples with estimated copy number above 3.5, and these were recoded into class 3 (duplications) with probability 1.
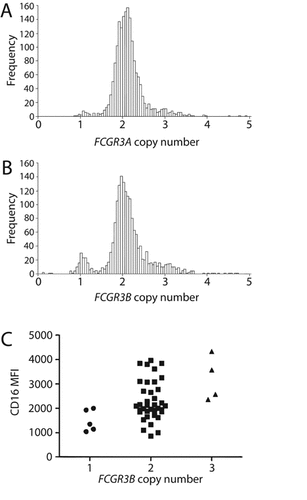
Frequency distribution of copy number estimates of A: FCGR3A and B: FCGR3B in patients and controls based on GPI QSV and PRT data. Samples with very low and very high values were recoded to be included in the low and high copy number classes, respectively. These data were subsequently analyzed using CNVtools. C: CD16 (FcγRIIIb) expression on neutrophils of individuals stratified by FCGR3B copy number. MFI, mean fluorescence intensity.
Mixture models were fitted to the standardized data sets for each gene. For FCGR3B, the three-component model could only be fitted by constraining the variances to be proportional to the copy number. However, for FCGR3A, a three-component model was fitted allowing for independent variance within each copy number group. Parameter estimates were calculated for each copy number category for each gene and are presented in Table 1. Healthy control copy number frequencies of FCGR3B were compared with previously published frequencies derived from various experimental methodologies (Supp. Table S5). In general, the FCGR3B copy number frequencies observed in our healthy controls were consistent with Caucasian cohorts measured using PRT [Hollox et al., 2009] and MLPA [Breunis et al., 2009]. However, we noted the high degree of variability in estimates generated from qPCR platforms, which have yielded frequency estimates of the FCGR3B deletion between 5% [McKinney et al., 2010] and 41% [Aitman et al., 2006] in Caucasian healthy controls. Our observed frequencies of FCGR3A copy number were broadly comparable with those observed in other populations studied using PRT, except our cohort exhibited a slightly higher frequency of duplications (Supp. Table S6).
| Category | α | µ1 | µ2 | µ3 | σ2 |
|---|---|---|---|---|---|
| FCGR3A ≤ 1 | 0.020 | −2.693 | −2.844 | −2.965 | 0.122 |
| 2 | 0.881 | −0.269 | −0.080 | −0.147 | 0.337 |
| ≥3 | 0.099 | 1.986 | 2.277 | 2.010 | 0.769 |
| FCGR3B ≤1 | 0.085 | −2.127 | −2.077 | −2.022 | 0.051 |
| 2 | 0.732 | −0.173 | −0.097 | −0.085 | 0.169 |
| ≥3 | 0.184 | 1.286 | 1.621 | 1.445 | 0.562 |
- α is the proportion of subjects in each copy number category. µi is the mean for batch i, where i = 1 is BRAGGSS1, 2 is BRAGGSS2, and 3 is healthy controls. σ2 is the common variance within each category. For FCGR3A, the log likelihood for this model, ln L = −3,370.48. For FCGR3B, the log likelihood for this model, ln L = −3,281.28.
Association of FCGR3A and FCGR3B Copy Number with RA
Table 2 shows the association analysis results for the general model and the more restricted model considered for each of the two genes. For FCGR3A, there was no evidence of association using either model. For FCGR3B, there was borderline evidence of association (P = 0.078) when testing very generally for a difference between three groups (deletions, normal copy number, and duplications; test 1). The best-fitting model compared FCGR3B deletions with all other copy number states, where there was evidence of overrepresentation of low copy number in RA samples (odds ratio [OR] 1.50, P = 0.028; test 2). This was in line with our prior hypothesis for the effect of FCGR3B, based on previous reports in the literature [Fanciulli et al., 2007; McKinney et al., 2010; Willcocks et al., 2008]. The magnitude of this association was greater in the subgroup of 932 RA patients who were RF positive (OR 1.61, P = 0.01; Table 3).
| Category | α | µ1 | µ2 | µ3 | σ2 | p(D) | OR | ln L | χ2 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| FCGR3A | ||||||||||
| Test 1 ≤ 1 | 0.020 | −2.694 | −2.844 | −2.940 | 0.131 | 0.572 | 0.80 | −3,369.8 | 1.33 (2 df) | 0.514 |
| 2 | 0.884 | −0.270 | −0.080 | −0.136 | 0.340 | 0.626 | 1 | |||
| ≥3 | 0.096 | 1.992 | 2.283 | 2.128 | 0.730 | 0.676 | 1.24 | |||
| Test 2a ≤ 1 | 0.020 | −2.696 | −2.844 | −2.967 | 0.121 | 0.625 | 1 | −3,370.0 | 0.98 (1 df) | 0.323 |
| 2 | 0.885 | −0.269 | −0.080 | −0.137 | 0.341 | |||||
| ≥3 | 0.095 | 2.000 | 2.288 | 2.139 | 0.723 | 0.677 | 1.25 | |||
| FCGR3B | ||||||||||
| Test 1 ≤ 1 | 0.085 | −2.127 | −2.077 | −2.024 | 0.051 | 0.712 | 1.53 | −3,278.7 | 5.10 (2 df) | 0.078 |
| 2 | 0.732 | −0.175 | −0.098 | −0.083 | 0.169 | 0.618 | 1 | |||
| ≥3 | 0.183 | 1.268 | 1.613 | 1.468 | 0.561 | 0.641 | 1.10 | |||
| Test 2b ≤ 1 | 0.085 | −2.126 | −2.076 | −2.024 | 0.051 | 0.712 | 1.50 | −3,278.9 | 4.80 (1 df) | 0.028 |
| 2 | 0.732 | −0.172 | −0.097 | −0.085 | 0.169 | 0.622 | 1 | |||
| ≥3 | 0.183 | 1.287 | 1.622 | 1.446 | 0.561 | |||||
- α is the estimated proportion of subjects in each copy number category. µi is the mean for batch i, where i = 1 for BRAGGSS1, 2 for BRAGGSS2, and 3 for healthy controls. σ2 is the variance within each category, common across cohorts. ln L is the log likelihood for each model. p(D) is the proportion of cases (given the case–control distribution) within each copy number class. OR is odds ratio and P is P value. df is degree of freedom for the given χ2. Tests 1 and 2 as in text.
| Category | α | µ1 | µ2 | µ3 | σ2 | p(D) | OR | ln L | χ2 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| FCGR3A | ||||||||||
| Test 1 ≤ 1 | 0.019 | −2.748 | −2.900 | −3.003 | 0.113 | 0.525 | 0.78 | −3,049.7 | 0.585 (2 df) | 0.746 |
| 2 | 0.889 | −0.241 | −0.082 | −0.136 | 0.348 | 0.587 | 1 | |||
| ≥3 | 0.091 | 2.083 | 2.327 | 2.113 | 0.803 | 0.607 | 1.09 | |||
| Test 2a ≤ 1 | 0.020 | −2.729 | −2.898 | −2.976 | 0.133 | 0.586 | 1 | −3,050.0 | 0.094 (1 df) | 0.760 |
| 2 | 0.884 | −0.245 | −0.086 | −0.139 | 0.341 | |||||
| ≥3 | 0.096 | 2.012 | 2.278 | 2.039 | 0.875 | 0.604 | 1.08 | |||
| FCGR3B | ||||||||||
| Test 1 ≤ 1 | 0.088 | −2.143 | −2.093 | −2.024 | 0.050 | 0.688 | 1.62 | −2,957.6 | 6.53 (2 df) | 0.038 |
| 2 | 0.737 | −0.147 | −0.122 | −0.083 | 0.169 | 0.578 | 1 | |||
| ≥3 | 0.175 | 1.326 | 1.585 | 1.461 | 0.571 | 0.579 | 1.00 | |||
| Test 2b ≤ 1 | 0.088 | −2.143 | −2.093 | −2.024 | 0.050 | 0.688 | 1.61 | −2,957.6 | 6.53 (1 df) | 0.011 |
| 2 | 0.737 | −0.147 | −0.122 | −0.083 | 0.169 | 0.578 | 1 | |||
| ≥3 | 0.175 | 1.327 | 1.586 | 1.460 | 0.571 | |||||
- α is the estimated proportion of subjects in each copy number category. µi is the mean for batch i, where i = 1 for BRAGGSS1, 2 for BRAGGSS2, and 3 for healthy controls. σ2 is the variance within each category, common across cohorts. ln L is the log likelihood for each model. p(D) is the proportion of cases (given the case-control distribution) within each copy number class. OR is odds ratio and P is P value. df is degree of freedom for the given χ2. Tests 1 and 2 as in text.
Neither locus showed clear evidence of association when comparing any type of copy number variation versus no copy number variation (P = 0.61 for FCGR3A and P = 0.07 for FCGR3B).
Neutrophil Expression of FcγRIIIb in Individuals Selected for Different FCGR3B Copy Number
Our flow cytometry data show a clear relationship between neutrophil FcγRIIIb expression and FCGR3B copy number (Mann–Whitney one vs. two copies P = 0.046, one vs. three copies P = 0.016, 2 vs. 3 copies P = 0.039; Fig. 3C), providing functional validation to support the accuracy of the copy number assay system employed in this study.
Discussion
We have confirmed association of low FCGR3B copy number with RA using a novel QSV assay and robust statistical methodology that circumvented exclusion of samples due to ambiguous calls. Although the initial general model shows no association at the nominal 5% significance level (P = 0.08; Table 2), when testing the more specific effect of deletions reported previously in the literature, the P value for association was 0.03. Although this finding would not remain significant if corrected for multiple testing, the evidence is substantially strengthened by the increased effect size and magnitude of association in the autoantibody (RF)-positive subgroup of patients (P = 0.01; Table 3). In addition, since our observation confirms previous reports using different technologies, we believe it is unlikely to be a false positive result.
Our methodology has significant advantages over qPCR and statistical methods that require assignment of discrete copy number categories that have previously been adopted [Aitman et al., 2006; Fanciulli et al., 2007; McKinney et al., 2010], and go some way to minimizing, and allowing for, the impact of batch effects. Additionally, with the QSV assay, we have introduced an alternative analysis method for the estimation of relative FCGR3A to FCGR3B copy number, which does not depend on the optimal restriction enzyme digestion that is a requirement of the REDVR component of the original PRT assay suite [Hollox et al., 2009], thereby minimizing a source of bias. It is likely that this technique is applicable to various other circumstances where there is a need for sequence variant quantification, especially where lack of recognition sequences precludes the use of restriction endonucleases.
It remains unclear from the data presented herein whether low FCGR3B copy number per se is the true causal variant identified in this cohort. Previous studies have shown that FCGR2C and HSPA7 are also deleted in Caucasians [Breunis et al., 2008; Breunis et al., 2009; de Haas et al., 1995; Willcocks et al., 2008]. However, up to 60% of Caucasians do not express FcγRIIc due to a SNP that introduces a premature stop codon, effectively creating a null allele [Ernst et al., 2002], and HSPA7 appears to be a transcribed pseudogene [Parsian et al., 2000], suggesting that simultaneous deletion of these genes is unlikely to provide an explanation for the observed association with RA. There is some evidence that simple deletions may be tagged by SNPs [McCarroll et al., 2006], but detection methods cannot be easily extended to complex CNVs with simultaneous duplications and deletions. Assessment of extended SNP haplotypes and LD structure in the wider FCGR locus is therefore fraught with difficulties in view of the incomplete understanding of the structural diversity and presence of multiple CNVs. Until the breakpoints of the common CNVs are known, it remains unclear whether the reported deletions and duplications occurred simultaneously as balanced duplication and deletion events, as assumed in the literature, or whether these represent distinct CNVs with different break points that include some or all of the same genes. Therefore, it is likely that previous estimates of LD, which have not taken CNV into account at this locus, are inaccurate, but methods to fully incorporate the observed complexity of SNP and structural variation at this locus remain to be developed. Preliminary analyses, which remain far from ideal, have not identified strong LD between FCGR3B deletions and functional SNPs in FCGR2A (R131H; rs1801274) or FCGR3A (F158V; rs396990). However, there is reported weak LD between FCGR3B (NA1/NA2; HNA1a/HNA1b) and total FCGR3 copy number in a small Japanese cohort [Hollox et al., 2009], although not strong enough to be predictive, and there may be weak LD with a SLE-associated SNP in FCGR2B (I232T; rs1050501) [Niederer et al., 2010]. It is thus unlikely that the FCGR3B copy number association can be detected using whole-genome SNP typing platforms in recent landmark studies, especially considering the extremely limited inclusion of variants mapping inside the SD. From both a biological and genetic perspective, we therefore believe that FCGR3B deletions per se are the most likely explanation for the observed genetic association with RA, and that this is likely to be independent of the previously reported associations in FCGR3A [Robinson et al., 2009] and FCGR2A [Raychaudhuri et al., 2009].
The association of low FCGR3B copy number with RA is consistent with the previous findings in SLE [Aitman et al., 2006; Fanciulli et al., 2007; Morris et al., 2010; Willcocks et al., 2008]. FcγRIIIb is predominantly expressed on neutrophils, suggesting that this receptor may have a protective role in RA pathogenesis. Neutrophils are the most abundant cell in synovial fluid, where they are primed and activated, and secrete large amounts of proinflammatory mediators such as reactive oxygen species, cytokines, and various proteases [Wright et al., 2010]. Neutrophil responses to IgG immune complexes appear to be tightly regulated and context specific, making it difficult to compare and contrast some of the published literature. We have confirmed that neutrophil FcγRIIIb cell surface expression is directly related to FCGR3B copy number [Koene et al., 1996; Morris et al., 2010; Willcocks et al., 2008]. Previous studies have demonstrated that individuals with low copy number exhibit reduced soluble immune complex uptake and reduced adhesion to immune complexes under flow conditions [Willcocks et al., 2008], with no difference in superoxide release [Wagner and Hansch, 2004; Willcocks et al., 2008]. This suggests that FcγRIIIb plays an important role in the recruitment of neutrophils into inflamed tissues [Tsuboi et al., 2008] and subsequent clearance of immune complexes, rather than stimulating degranulation and proinflammatory mediator release.
We have previously reported an association between the higher affinity FCGR3A-158V allele with RA, which also supports a role for FcγRIIIa and macrophage and/or NK cell activation in the pathogenesis of RA. Previous studies have found no evidence of LD between this SNP and low FCGR3B copy number in UK Caucasians [Niederer et al., 2010]. We hypothesized that increased FCGR3A copy number may also contribute to RA susceptibility. We found no evidence for an association between FCGR3A copy number and RA, in this study, although the power to detect an association was substantially reduced due to the lower frequency of FCGR3A duplications. The frequency of duplications (9.6%) was more common in the control population than deletions (2%), consistent with the previous estimates from UK [Niederer et al., 2010; Hollox et al., 2009], Dutch [Breunis et al., 2009; Thabet et al., 2009], and Swedish [Niederer et al., 2010] populations. Furthermore, the functional consequence of two copies of a higher affinity allele compared with three copies of a lower affinity allele remains unknown.
This study highlights some of the methodological difficulties involved in studying genetic variation at this locus and how they may be overcome with the development of new genotyping strategies directed towards the analysis of PSVs that identify SDs. However, the breakpoints of the independent CNVs affecting FCGR3A and FCGR3B remain elusive and it is unlikely, in the absence of complete sequencing from multiple ethnic populations, that exact attribution of function can be assigned. It appears that there may be at least three independent genetic effects at this locus comprising SNPs in FCGR2A and FCGR3A and copy number variation in FCGR3B. Further genetic and functional studies will be required to confirm these findings and unravel the true biological significance with respect to RA pathogenesis, disease progression, and response to therapy.
Acknowledgements
We thank Arthritis Research UK and the NIHR—Leeds Musculoskeletal Biomedical Research Unit for their support. We are grateful to BRAGGSS for access to RA patient DNA samples. We are grateful to Dr. Ed Hollox for providing reference samples and informative discussion.
Disclosure Statement: The authors declare no conflict of interest.