Novel mitochondrial DNA mutations responsible for maternally inherited nonsyndromic hearing loss†
Communicated by Mireille Claustres
Abstract
Some cases of maternally inherited isolated deafness are caused by mtDNA mutations, frequently following an exposure to aminoglycosides. Two mitochondrial genes have been clearly described as being affected by mutations responsible for this pathology: the ribosomal RNA 12S gene and the transfer RNA serine (UCN) gene. A previous study identified several candidate novel mtDNA mutations, localized in a variety of mitochondrial genes, found in patients with no previous treatment with aminoglycosides. Five of these candidate mutations are characterized in the present study. These mutations are localized in subunit ND1 of complex I of the respiratory chain (m.3388C>A [p.MT-ND1:Leu28Met]), the tRNA for Isoleucine (m.4295A>G), subunit COII of complex IV (m.8078G>A [p.MT-CO2:Val165Ile]), the tRNA of Serine 2 (AGU/C) (m.12236G>A), and Cytochrome B, subunit of complex III (m.15077G>A [p.MT-CYB:Glu111Lys]). Cybrid cell lines have been constructed for each of the studied mtDNA mutations and functional studies have been performed to assess the possible consequences of these mutations on mitochondrial bioenergetics. This study shows that a variety of mitochondrial genes, including protein-coding genes, can be responsible for nonsyndromic deafness, and that exposure to aminoglycosides is not required to develop the disease, giving new insights on the molecular bases of this pathology. Hum Mutat 33:681–689, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
Mitochondria are intracellular organelles of eukaryotic organisms that produce energy in the form of ATP. This ATP is synthesized by oxido-reduction reactions (respiratory chain) coupled to ATP synthesis (ATP synthase). This essential mechanism for cellular energy production is called oxidative phosphorylation [Mitchell, 1961]. These reactions are carried out by enzymatic complexes constituted by polypeptides, which are encoded both by nuclear DNA and mitochondrial DNA. The ATP produced is used by cells for a number of metabolic reactions such as biochemical synthesis and the maintaining of cell function.
Because of its implication in several biochemical pathways, mitochondria are in constant interaction with cellular metabolism. As a result, any defect in oxidative phosphorylation could cause cellular dysfunction, resulting in pathologies known as mitochondrial cytopathies. These pathologies have their origin in mutations both in the mtDNA and in nuclear DNA. Several mtDNA point mutations and rearrangements (deletions, insertions, duplications, depletion) of mitochondrial DNA are known to be the origin of some of these pathologies [DiMauro and Schon, 2003; Wallace, 2005].
Syndromic deafness is one of these pathologies associated with mtDNA mutations, namely mutation m.3243A>G, located in the MTTL1 gene encoding tRNALeu (UUR). This mutation is frequently associated with the systemic MELAS syndrome [Reardon et al., 1992; van den Ouweland et al., 1992]. Not only can syndromic deafness be caused by mtDNA mutations, so can nonsyndromic deafness, as it has been shown that some isolated deafness with a maternal inheritance are also caused by mtDNA mutations. This type of deafness has been frequently related to exposure to aminoglycosides.
Until now, mutations in only two mitochondrial genes have been clearly associated with nonsyndromic deafness. These are the ribosomal RNA 12S gene (MT-RNR1), with mutations m.1494C>T and m.1555A>G [Prezant et al., 1993; Zhao et al., 2004], proposed to cause increased susceptibility to aminoglycoside antibiotic-induced hearing loss as well as nonsyndromic sensorineural hearing loss [Usami et al., 1998]; and the transfer RNA Serine (UCN) gene (MT-TS1), with mutations m.7511T>C, m.7445A>G, and m.7472_7473insC [Sue et al., 1999; Toompuu et al., 2004; Vernham et al., 1994], which lead to a reduction of tRNA level [Kokotas et al., 2007]. Currently, no other gene has been described harboring any mtDNA mutation that could cause this pathology, least of all any protein-coding gene.
The tissue affected in this pathology is most probably the cochlear hair cells, which are essential for hearing functions since they are responsible for maintaining the ionic gradients necessary for sound signal transduction [Dallos and Evans, 1995]. These cells can be easily compromised by a mitochondrial dysfunction because they are highly metabolically active and rich in mitochondria [Chinnery et al., 2000].
In order to search for novel candidate mtDNA mutations that could be responsible for nonsyndromic deafness, Leveque et al. sequenced the whole mitochondrial DNA of 29 families which exhibited a clear maternal pattern of inheritance (three to five generations) and did not carry four of the known mutations. This sequencing has been done using a microarray resequencing chip [Leveque et al., 2007]. This study identified several candidate mtDNA mutations that were selected because they were localized in highly conserved regions, and, for those localized in protein-coding genes, because they were also nonsynonymous mutations.
Five of these candidate mutations were characterized in the present study: m.3388C>A (p.MT-ND1:Leu28Met), in subunit ND1 of complex I of the respiratory chain; m.4295A>G, localized in the tRNA for Isoleucine (MT-TI); m.8078G>A (p.MT-CO2:Val165Ile), affecting subunit COII of complex IV; m.12236G>A, located in the tRNA of Serine 2 (AGU/C) (MT-TS2); and m.15077G>A (p.MT-CYB:Glu111Lys), located in Cytochrome B, subunit of complex III (Table 1). All of these mutations are homoplasmic and were found in patients with a clear maternal lineage, with deaf relatives present in at least three generations (or two if deaf individuals were more than three per family), when maternal inheritance could be assessed and paternal inheritance excluded [Leveque et al., 2007]. For each patient, no other potential mtDNA pathogenic variant has been found.
| Mutation | Gene locus | Accession number in the MITOMAP mtDNA sequence database |
|---|---|---|
| m.3388C>A (p.MT-ND1:Leu28Met) | MT-ND1 HGNC ID: 7455 | 20110704001 |
| m.4295A>G | MT-TI HGNC ID: 7488 | 20110704002 |
| m.8078G>A (p.MT-CO2:Val165Ile) | MT-CO2 HGNC ID: 7421 | 20110704003 |
| m.12236G>A | MT-TS2 HGNC ID: 7498 | 20110704004 |
| m.15077G>A (p.MT-CYB:Glu111Lys) | MT-CYB HGNC ID: 7427 | 20110704005 |
- a The studied mtDNA mutations are presented indicating the gene where they are located and the gene's accession number according to HGNC (http://www.genenames.org/) as well as the mutation's accession number in the MITOMAP mtDNA sequence database (http://www.mitomap.org/MITOMAP).
In order to determine if these novel mutations could be responsible for nonsyndromic deafness, we constructed cybrid cell lines for each of the studied mtDNA mutations. These cybrids allowed us to perform functional studies to assess the possible consequences of these mutations on mitochondrial bioenergetics. We found that some of these mutations caused respiratory chain dysfunction in our model. Particularly m.3388C>A (p.MT-ND1:Leu28Met) and m.4295A>G, which lead to clear dysfunctions of complexes I and III activity, respectively, linked to quantitative diminutions of the steady state of these complexes and a decline of mitochondrial O2 consumption. These results show that these mutations are good candidates for being responsible for nonsyndromic deafness.
Materials and Methods
Pathogenicity Prediction
To analyze the possible impact of the amino acid substitutions caused by m.3388C>A (p.MT-ND1:Leu28Met), m.8078G>A (p.MT-CO2:Val165Ile), and m.15077G>A (p.MT-CYB:Glu111Lys) on the three-dimensional protein structure and its consequence on protein function, we used the PolyPhen program (http://genetics.bwh.harvard.edu/pph/), which allows to predict whether an amino acid change is likely to be deleterious to protein function [Sunyaev et al., 2001]. PSIC (Position-Specific Independent Counts) Profile scores of >2.0 indicate the polymorphism is probably damaging to protein function. Scores of 1.5–2.0 are possibly damaging, and scores of <1.5 are likely benign.
In order to confirm these results, we used the MutPred Server (http://mutpred.mutdb.org/), which gives the probability of a mutation being deleterious for protein function [Thusberg et al., 2011].
For the mutations affecting tRNA genes, m.4295A>G (MT-TI) and m.12236G>A (MT-TS2), we used the pathogenicity scoring system devised by McFarland's team in 2004, and revised in 2011 [McFarland et al., 2004; Yarham et al., 2011].
Phosphorylation Motifs Analysis
In order to determine if the studied nonsynonymous mutations had any consequences on the phosphorylation motifs present on the respiratory chain proteins (Tyrosine or Serine-binding motifs, and Tyrosine or Serine kinase/phosphatase motifs), we used the PhosphoMotif Finder (http://www.hprd.org/FAQ/PhosphoMotif_finder) of the Human Protein Reference Database.
Cell Lines and Culture Conditions
Blood platelets from patients carrying the studied mutations and one control individual of haplogroup H (representative of >50% of the french population) were used for the generation of cybrid cell lines. The patient's haplogroups were as follows: the patient with mutation m.3388C>A (p.MT-ND1:Leu28Met) belongs to haplogroup H2, the patient with mutation m.4295A>G belongs to haplogroup K, the patient with mutation m.8078G>A (p.MT-CO2:Val165Ile) belongs to haplogroup U, the patient with mutation m.12236G>A belongs to haplogroup H, and the patient with mutation m.15077G>A (p.MT-CYB:Glu111Lys) belongs to haplogroup U.
Since the genealogical tree of the family harboring mutation m.4295A>G was not presented in the previous article [Leveque et al., 2007], we report it in Figure 1.
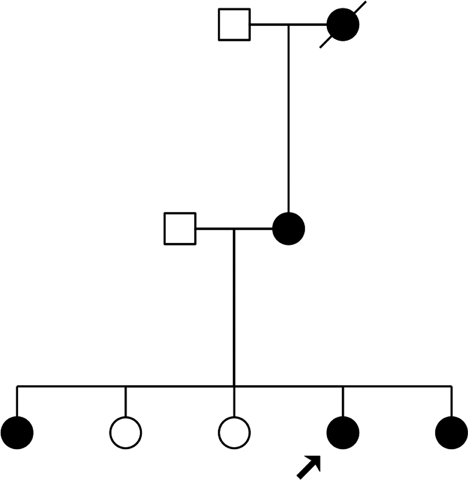
Geneological tree of the family harboring mutation m.4295A>G. The proband is indicated by an arrow.
Cybrids were produced by polyethylene glycol fusion of platelets with human osteosarcoma 143B cells lacking mtDNA (rho0 cells) as described, elsewhere [Chomyn, 1996], followed by selection in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% dialyzed fetal bovine serum and 100 mg/L 5-bromo-2′-deoxyuridine (BrdU) (Sigma-Aldrich).
Cybrids carrying the studied mutations and control cybrids were grown in DMEM (containing 4.5 mg glucose and 0.11 mg pyruvate per ml), supplemented with 10% FBS, Uridine (50 μg/ml) (Sigma-Aldrich), and antibiotics (penicillin 100 U/ml and streptomycin 100 mg/ml) (Gibco). Cultures were grown at 37°C in a humidified atmosphere of 5% CO2 in air.
DNA Extraction and RFLP Tests
Total genomic DNA was isolated from patient's blood platelets and from cybrid cell lines using a phenol-chloroform protocol, as described elsewhere [Sambrook et al., 1989]. Fragments of interest were amplified by PCR using specific primers, as follows: for m.3388C>A (p.MT-ND1:Leu28Met, MIM# 516000, NC_012920), the primers used were hmtL3323: 5′ tcctactcctcattgtaccc 3′ and hmtH3500: 5′ gtagagggtgatggtagatg 3′; for m.4295A>G (MT-TI, MIM# 590045, NC_012920) the primers used were hmtL4294mis: 5′ tgtctgataaaagagttactttCat 3′ and hmtH4501: 5′ tgtgcctgcaaagatggtag 3′; for m.8078G>A (p.MT-CO2:Val165Ile, MIM# 516040, NC_012920) a couple of primers were hmtL8057mis: 5′ gacgtcttgcactcatCagct 3′ and hmtH8182: 5′ aactgtggtttgctccacag 3′; for m.12236G>A (MT-TS2, MIM# 590085, NC_012920) the primers were hmtL12213mis: 5′ cacaagaactgctaactcGt 3′ and hmtH12281: 5′ ctaagaccaatggatagctg 3′; and for m.15077G>A (p.MT-CYB:Glu111Lys, MIM# 516020, NC_012920), the primers used were hmtL14894 : 5′ ttcctagccatgcactactc 3′ and hmtH15142 : 5′ gacatagcctatgaaggctg 3′.
To confirm the presence of the mutations of interest in our cybrids, RFLP tests were undertaken with the following restriction enzymes (New England Bioloabs, Inc, United Kingdom): BfaI for m.3388C>A (p.MT-ND1:Leu28Met); NlaIII for m.4295A>G; PvuII for m.8078G>A (p.MT-CO2:Val165Ile); BssSI for m.12236G>A; and DdeI for m.15077G>A (p.MT-CYB:Glu111Lys).
Respirometric Analyses
Cellular oxygen consumption was measured by high-resolution respirometry with the OROBOROS Oxygraph-2k (OROBOROS Instruments, Innsbruck, Austria), with 106 cells per assay. Cells were permeabilized with 0.3 μg of digitonin (Sigma-Aldrich). Respiratory State 3 was induced adding Pyruvate (5 mM), Malate (2 mM), and ADP (3 mM), or Rotenone (0.5 μM), Succinate (10 mM), and ADP (3 mM) (all chemicals from Sigma-Aldrich). Each measurement was repeated at least five times and means and standard deviation were calculated. Student's t-test was performed to assess the probability of a difference being statistically significant or not.
Biochemical Studies
107 cells were used to measure the enzymatic activities of the respiratory chain complexes II, III, and IV spectrophotometrically as described elsewhere [Medja et al., 2009; Rocher et al., 2008]. The enzymatic activity of complex I was determined as described elsewhere [Chretien et al., 2003]. Normalization was done by citrate synthase (CS) activity. Each measurement was repeated at least five times and means and standard deviation were calculated. Student's t-test was performed to assess the probability of a difference being statistically significant or not.
Electrophoretic Techniques
For BN-PAGE, mitoplast-enriched pellets were prepared as described [Klement et al., 1995], using a solution of 4 mg/ml digitonin. Mitoplast fractions were then solubilized with n-dodecyl-β-D-maltoside, as described elsewhere [Schagger et al., 1996; Tiranti et al., 1999], and mixed 1:1 with protein solubilizing solution (1M 6-aminocaproic acid, 50 mM bistris, 1% Servablue G) and 15 μg of protein/lane were resolved by 4–12% blue native gels [Schagger, 1995].
Proteins were electrotransferred from the gels onto HybondTM-P (polyvinylidene difluoride) membranes (Amersham). Mitochondrial complexes protein were detected using mouse monoclonal antibodies: anticomplex I/NDUFB6, anticomplex IV/COII, anticomplex II/70 kD, anticomplex III/Core2, from MitoSciences, Eugene, OR. Protein loading was assessed by reprobing the blot with a complex II specific antibody. Rho0 143B cells were used as a negative Control.
Proteins were detected by chemiluminescence (ECLTM Western Blotting Analysis System, Amersham) and revealed by autoradiography. Densitometric analysis was performed with NIH Image J as described elsewhere [Faustin et al., 2004]. Each measurement was repeated at least five times and means and standard deviation were calculated. Student's t-test was performed to assess the probability of a difference being statistically significant or not.
Results
Predicted Consequences on Protein Function, Three-Dimensional Protein Structure, and Phosphorylation Motifs
We used PolyPhen to determine whether the studied nonsynonymous mutations (m.3388C>A (p.MT-ND1:Leu28Met), m.8078G>A (p.MT-CO2:Val165Ile), and m.15077G>A (p.MT-CYB:Glu111Lys) could have any consequences on protein function and three-dimensional protein structure.
This program predicted that m.3388C>A (p.MT-ND1:Leu28Met) is potentially deleterious, with a PSIC profile changed from +1.540 to +0.025 (score = 1.515). It also predicted that in the affected region, there were 10 three-dimensional protein structures possible, but this number falls to 0 in the presence of the mutation. To confirm this prediction, we used the MutPred Server, which gave a high probability of m.3388C>A (p.MT-ND1:Leu28Met) being a deleterious mutation (g = 0.817).
Moreover, the PhosphoMotif Finder predicted a decrease in both numbers of Tyrosine-binding motifs (10–9) and Tyrosine kinase/phosphatase motifs (27–26) for this mutation.
Polyphen analysis for the other mutations predicted them to be benign. MutPred gave g = 0.574 and g = 0.684 for m.8078G>A (p.MT-CO2:Val165Ile) and m.15077G>A (p.MT-CYB:Glu111Lys), respectively. In addition, PhosphoMotif Finder predicted no consequences on phosphorylation motifs for m.8078G>A (p.MT-CO2:Val165Ile). Though, it did predict a decrease in the number of serine kinase/phosphatase motifs (128–126) for m.15077G>A (p.MT-CYB:Glu111Lys).
Concerning the mutations localized in tRNA genes, m.4295A>G (MT-TI) and m.12236G>A (MT-TS2), the pathogenicity scoring system devised by McFarland's team in 2004, and revised in 2011 [McFarland et al., 2004; Yarham et al., 2011] gave a score of 11 for m.4295A>G (MT-TI), predicting it to be definitely pathogenic, and of 7 for m.12236G>A (MT-TS2), predicting it to be possibly pathogenic.
Cybrids Construction and RFLP
Following cybrid construction and selection, the presence of mutations of interest was established by RFLP with specific primers and restriction enzymes for each mutation. Cybrid cells were compared to patient blood platelets (containing the mutation) and 143B cells (that do not contain the mutation). In this way, we could confirm the correct transfer of mtDNA from patient's platelets to our cybrid cell lines, and the presence of the studied mtDNA mutations in these cell lines.
For mutation m.4295A>G, the restriction enzyme cuts in the presence of the mutation while for the other mutations (m.3388C>A [p.MT-ND1:Leu28Met], m.8078G>A [p.MT-CO2:Val165Ile], m.12236G>A, and m.15077G>A [p.MT-CYB:Glu111Lys]) the enzymes cut in the absence of the mutation (Fig. 2).
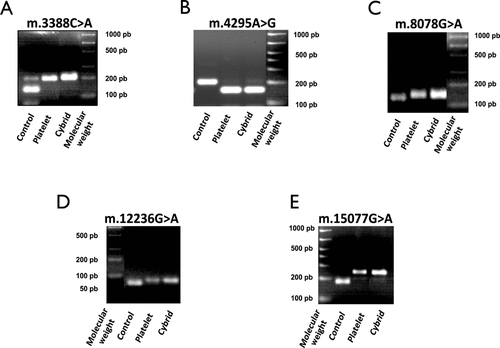
Presence of the studied mtDNA mutation in the cybrid cell line. The presence of mutations of interest was established by RFLP with specific primers and restriction enzymes for each mutation. Cybrid cells were compared to patient blood platelets (containing the mutation) and 143B cells (that do not contain the mutation). (A) RFLP for m.3388C>A (p.MT-ND1:Leu28Met); (B) RFLP for m.4295A>G (MT-TI); (C) RFLP for m.8078G>A (p.MT-CO2:Val165Ile); (D) RFLP for m.12236G>A (MT-TS2); and (E) RFLP for m.15077G>A (p.MT-CYB:Glu111Lys).
Effect of Studied mtDNA Mutations on Mitochondrial O2 Consumption
In order to determine the consequences of the studied mtDNA mutations on mitochondrial bioenergetics, we measured mitochondrial O2 consumption on digitonin permeabilized cybrid cells, using an O-2K oxygraph developed by Oroboros, which permits high-resolution respirometry. Using Pyruvate, Malate, and ADP as substrates, we studied respiratory State 3 starting the electron transfer with complex I, studying the global activity of complexes I, III, and IV. In these conditions, two of the cybrid cell lines showed a decrease in oxygen consumption: 25% decline compared to control (40.11–30.63 pmol of O2/(s × 106 cells), P < 0.006) for cybrids containing mutation m.3388C>A (p.MT-ND1:Leu28Met) (3388), and 50% decline compared to control (40.11–20.16 pmol of O2/(s × 106 cells), P < 0.001) for cybrids with mutation m.4295A>G (4295) (Fig. 3A).
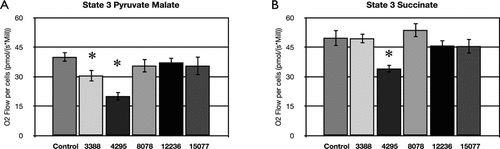
Effect of studied mtDNA mutations on mitochondrial O2 consumption. Mitochondrial O2 consumption was measured on digitonin permeabilized Cybrid cells, using an O-2K oxygraph developed by Oroboros. Respiratory State 3 was obtained using as substrates (A) Pyruvate, Malate, and ADP or (B) Succinate, Rotenone, and ADP. Means bearing * are significantly different (P < 0.05) from control according to Student's t-test (n = 5).
We also used succinate, rotenone (specific inhibitor of complex I), and ADP as substrates to reach respiratory State 3, bypassing complex I, in order to study the ensemble of complexes II, III, and IV. Under these conditions, cybrids 4295 showed a decrease in O2 consumption of 32% compared to control (49.86–34.17 pmol of O2/(s × 106 cells), P < 0.001), while the other cybrids had normal O2 consumption (Fig. 3B).
Consequences on Respiratory Chain Enzymatic Activities
Enzymatic activities of complexes I, III, and IV, as well as citrate synthase, were studied by spectrophotometry. Cybrids 3388 showed an important activity decrease of 55% compared to control (P < 0.001) for complex I activity, while the activity of the other complexes was normal. Cybrids 4295 showed a diminution of 37% compared to control (P < 0.001) in complex III activity. The activity of the other complexes showed no significant difference with the control. Cybrids harboring mutation m.8078G>A (p.MT-CO2:Val165Ile) (8078) presented an activity decrease of 22% compared to control (P < 0.002) of complex IV and normal activities for the remaining complexes. Cybrids with mutation m.12236G>A (12236), showed a slight activity decline of both complexes I (17% decline compared to control, P < 0.002) and IV (15% decline compared to control, P < 0.003) while the activity of complex III was normal. Finally, cybrids with mutation m.15077G>A (p.MT-CYB:Glu111Lys) (15077) showed normal activities for all three enzymatic complexes (Fig. 4).
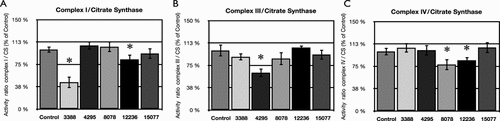
Consequences on respiratory chain enzymatic activities. Enzymatic activities of respiratory chain complexes were studied by spectrophotometry, and normalized by citrate synthase (CS). (A) Enzymatic activity of complex I normalized by CS, represented as a percentage of control; (B) enzymatic activity of complex III normalized by CS, represented as a percentage of control; (C) enzymatic activity of complex IV, normalized by CS, represented as a percentage of control. Means bearing * are significantly different (P < 0.05) from control according to Student's t-test (n = 5).
Assembly and Quantification of Respiratory Chain Complexes
In order to analyze whether the studied mtDNA mutations have any consequences on complexes assembly and quantity, a BN-PAGE electrophoresis was performed, normalizing results with complex II quantity. The outcome of this study showed a significant decrease in complex I protein for Cybrids 3388 (43% compared to control, P < 0.03), although this decrease is not as pronounced as the decrease in activity showed by this cybrid for complex I (55% compared to control, P < 0.001). Cybrids 4295 showed a significant decrease in complex III protein (32% compared to control, P < 0.02), while other complexes are normal. Cybrids 8078 showed a small decrease in complex IV quantity (26% compared to control, P < 0.03), while cybrids 12236 presented decreased protein levels of complexes I (20% compared to control, P < 0.02) and IV (17% compared to control, P < 0.04). Since cybrids 15077 presented normal enzymatic activities for all of the respiratory chain complexes, we did not measure protein levels in these cybrid cell lines (Fig. 5).
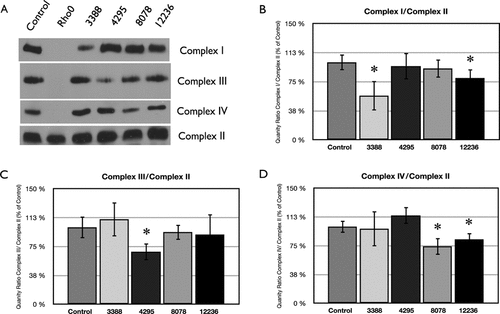
Quantification of respiratory chain complexes. A: BN-PAGE was undertaken, using the following specific antibodies: anticomplex I/NDUFB6, anticomplex IV/COII, anticomplex II/70 kD, anticomplex III/Core2, from MitoSciences, Eugene, OR. Densitometric analysis were performed with NIH Image J. B: Quantity means of complex I normalized by complex II. C: Quantity of complex III normalized by complex II. D: Quantity of complex IV normalized by complex II. Means bearing * are significantly different (P < 0.05) from control according to Student's t-test (n = 5). Since Cybrids 15077 presented normal enzymatic activities for all of the respiratory chain complexes, we deemed not necessary to perform BN-PAGE on these cybrid cell lines.
Discussion
Until now, nonsyndromic deafness has only been associated with mutations in two mitochondrial genes. These are the ribosomal RNA 12S gene (MT-RNR1) and the transfer RNA Serine (UCN) gene (MT-TS1). Four other genes were identified by Leveque et al. (2007), that could be involved in this pathology: subunit ND1 of complex I of the respiratory chain (MT-ND1); the tRNA for Isoleucine (MT-TI); subunit COII of complex IV (MT-CO2); and the tRNA of Serine 2 (AGU/C) (MT-TS2). We propose that mutations localized in these genes could be the cause of this pathology, and we examine this possibility here. Indeed, these homoplasmic mutations concerned highly conserved positions, and were nonsynonymous when localized in protein-coding genes. While these variants have previously been described, they have never been associated with nonsyndromic deafness. To study the effects of these mutations on mitochondrial bioenergetics, we constructed cybrid cell lines, which consist in placing the patient's mtDNA in a known nuclear background, using the osteosarcoma-derived 143B cell line that lacks its own mtDNA.
From the five mutations studied, two showed a significant defect in mitochondrial function (m.3388C>A [p.MT-ND1:Leu28Met] and m.4295A>G); two presented mild defects (m.8078G>A [p.MT-CO2:Val165Ile] and m.12236G>A), and one had no effect (m.15077G>A [p.MT-CYB:Glu111Lys]).
Exposure to Aminoglycosides is not Necessary to Initiate and Develop Nonsyndromic Deafness
Most cases of nonsyndromic deafness have been described to appear after exposure to aminoglycosides. Indeed, it has been proposed that these antibiotics target the 12S rRNA, the evolutionary equivalent to the 16S rRNA in bacteria, causing defects in mitochondrial translation [Guan, 2004]. Some mutations in this gene will increase the structural similarity between the mitochondrial rRNA 12S and the bacterial rRNA 16S, making it more sensitive to the action of aminoglycosides [Guan, 2004; Zhao et al., 2005]. However, most of the patients diagnosed for mitochondrial deafness by the French reference center of genetics deafness, even patients carrying the m.1555A>G in 12S rRNA, have never been treated with aminoglycosides.
We examined five of these patients in this study; none of them presented mutations in mitochondrial 12S ribosomal RNA. Their mutations affected OXPHOS complexes and their function, suggesting that the origin of nonsyndromic hearing loss with maternal inheritance could be indeed a direct dysfunction of mitochondrial bioenergetics in these individuals.
Mutation m.3388C>A (p.MT-ND1:Leu28Met) Causes Important Defects of Respiratory Chain Complex I
The mutation m.3388C>A (p.MT-ND1:Leu28Met), which affects the gene coding for subunit ND1 of complex I of the respiratory chain, causes a significant decrease in complex I protein levels, leading to a corresponding decrease in the activity levels of this complex.
This defect ultimately causes a decrease in mitochondrial respiration when complex I substrates are used (Pyruvate and Malate). However, when complex II substrates are used (Succinate), bypassing complex I, respiration levels are normal, confirming that complex I is the only complex affected.
According to PhosphoMotif Finder, m.3388C>A (p.MT-ND1:Leu28Met) is located in a Tyrosine-binding motif and a Tyrosine kinase/phosphatase motif, which are lost in the presence of the mutation. It has been shown that the enzymatic activity of mitochondrial respiratory chain complexes can be upregulated by phosphorylation [Arachiche et al., 2008; Papa et al., 1996], so the loss of these two motifs could lead to a lower activation of complex I by lack of phosphorylation. Moreover, a three-dimensional protein structure study, using the PolyPhen program, predicted the loss of the totality of the three-dimensional structures present in the substitution site of the protein, and an important decrease of the PSIC profile (from +1.540–+0.025) for a score of 1.515, which places the mutation in the category of possibly damaging. This was confirmed by the MutPred Server which gave a high probability of deleterious mutation for m.3388C>A (p.MT-ND1:Leu28Met) (g = 0.817).
This lower activation of complex I, as well as the complex level diminution caused by the mutation would explain the decrease in the enzymatic activity of complex I. Thus, confirming the pathogenic effect of the mutation observed in the enzymatic and protein studies undertaken. This mutation also has a high but not complete penetrance in the patient's family, since 10 of his 13 maternal relatives suffer from deafness.
This mutation was previously found in an individual who also had the mutation m.1555A>G, in the ribosomal RNA 12S gene (MT-RNR1), known to be responsible for nonsyndromic deafness [Herrnstadt et al., 2002]. In order to confirm that the observed mitochondrial dysfunction was caused only by the mutation m.3388C>A (p.MT-ND1:Leu28Met), we checked for the presence of m.1555A>G in this patient's mtDNA sequence, but this mutation was not found. Thus, confirming that m.3388C>A (p.MT-ND1:Leu28Met) is the only possible mutation responsible for nonsyndromic deafness in this patient.
Different mutations in this subunit have already been associated to other pathologies, such as MELAS, a systemic syndrome that includes syndromic deafness [Blakely et al., 2005; Jaksch et al., 1996; Kirby et al., 2004], which confirms the importance of this complexes activity in mitochondrial function and disease, especially in deafness.
Mutation m.4295A>G (MT-TI) Causes Important Defects of the Respiratory Chain Complex III
Mutation m.4295A>G affects the tRNA for Isoleucine (MT-TI). McFarland's team in 2004 provided a program that gives a pathogenicity score for mutation in tRNA. This program was revised in 2011 [McFarland et al., 2004; Yarham et al., 2011]. This program predicts this mutation to be definitely pathogenic.
Our studies showed that this mutation causes a consequent decrease in complex III protein levels, leading to a decrease of this complex activity, which is reflected in a decline of mitochondrial respiration, both with complex I and complex II substrates, confirming the pathogenicity prediction. Since this cybrid cell line only showed a dysfunction in complex III, we looked for any mtDNA mutation in the Cytochrome b gene that could explain this dysfunction, but the only nonsynonymous variants found in this gene were frequent polymorphisms. Hence, we confirmed that the observed consequences on complex III are caused by mutation m.4295A>G (MT-TI).
It has been proposed that this mutation reduces the efficiency with which tRNAIle can be processed by 3′-tRNase, reducing the level of functional tRNAIle [Levinger et al., 2003]. Thus, leading to translational defects that could explain the observed complex III quantity diminution. However, it seems that translation of complexes I and IV are less affected by this mutation, since activity of these complexes is normal. This is not necessarily surprising, as other mtDNA mutations on tRNA genes that affect only one of the respiratory chain complexes have been previously described [Blakely et al., 2009; Seneca et al., 2001]. However, why the translation defects caused by these mutations do not have the same consequences on all of the respiratory chain enzymatic complexes is not well understood.
This mutation also has a high but not complete penetrance in the patient's family since five of his seven maternal relatives suffer from deafness. This mutation has also previously been described as causing hypertrophic cardiomyopathy [Merante et al., 1996] and occipital stroke [Finnila et al., 2001], but this is the first study showing that it can also be responsible for nonsyndromic deafness.
Mutation m.8078G>A (p.MT-CO2:Val165Ile) Causes Mild Defects of the Respiratory Chain Complex IV
Mutation m.8078G>A (p.MT-CO2:Val165Ile), located in the gene coding for subunit COII of complex IV, causes a mild enzymatic activity dysfunction of this complex. This decrease can be explained by a small decrease in complex IV protein levels observed by BN-PAGE.
However, this enzymatic defect does not have any consequence on mitochondrial respiration in our model. Due to the tissue specificity which is characteristic of mitochondrial pathologies [Rossignol et al., 1999], it is possible that the dysfunction caused by this mutation could have more important consequences on mitochondrial respiration in the cochlear hair cells than those observed in our model. This mutation has a high, though not complete, penetrance in the patient's family, since eight of his 13 maternal relatives are affected by deafness.
Mutation m.12236G>A (MT-TS2) Causes Mild Defects of the Respiratory Chain Complexes I and IV
The second mutation in the “mild” group is m.12236G>A, which affects the second tRNA of Serine (AGU/C) (MT-TS2). This genetic variant has been described before as a polymorphism defining individuals belonging to L2 haplogroup. But it was included in this study because it was found in a patient with H2 haplogroup, and this genetic variant has never been reported in individuals of this haplogroup before. Moreover, it affects a highly conserved region in this tRNA, and two of the four known mutations causing nonsyndromic deafness affect the other tRNA for Serine (UCN). Thus, we considered m.12236G>A to be a good candidate for causing nonsyndromic hearing loss in the concerned patient [Leveque et al., 2007]. The pathogenicity scoring system devised by McFarland's team in 2004, and revised in 2011 [McFarland et al., 2004; Yarham et al., 2011] predicted this mutation to be possibly pathogenic and our study showed that this genetic variant causes a low but statistically significant decrease in complex I and IV enzymatic activities. On the other hand, mitochondrial respiration of the cybrid cell line containing this mutation seems slightly decreased both with complex I and complex II substrates, but is not significantly different from control in our model. In a quantitative aspect, there was a modest decrease in complex I and IV protein levels. This could explain the activity decrease that this cybrid presents. However, it seems that translation of complex III is less affected by this mutation, since activity of this complex is normal.
This mutation has a high but not complete penetrance in the patient's family, since six of his eight maternal relatives are affected by deafness.
It is possible that with an L2 haplogroup mitochondrial background, this mutation is harmless, while with a different mitochondrial background (H2 in this case), it could have some consequence on the tRNA function. Moreover, other mutations in this gene (m.12258C>A and m.12262C>A) have already been described as causing syndromic hearing loss [Cardaioli et al., 2011; Mansergh et al., 1999], confirming the importance of this gene in deafness of mitochondrial origin.
As discussed above, it is possible that due to tissue specificity, the observed mild deficiencies of complexes I and IV in our model could be more important in the cochlear hair cells.
Other Factors, Like the Mitochondrial DNA Haplogroup Background, have an Important Influence on the Severity of the Phenotype
Our results show that four of the five studied mtDNA mutations have consequences on mitochondrial bioenergetics and particularly m.3388C>A (p.MT-ND1:Leu28Met) and m.4295A>G (MT-TI) that have very consistent effects.
Not all patient maternal relatives develop nonsyndromic deafness and these mutations have already been described in healthy individuals. This leads us to believe that these mutations do not have complete penetrance and probably require other factors to produce the phenotype. This is particularly meaningful for mutations m.4295A>G and m.12236G>A, which have been found at internal branches of the phylogenetic tree, meaning that they are relatively ancient mutations, especially m.12236G>A that is described as a polymorphism defining individuals belonging to L2 haplogroup. The fact that these genetic variants have not been removed by negative selection implies that normally they do not have a major negative impact on mitochondrial bioenergetics. But in our cybrid cell lines, these mutations cause significant defects in mitochondrial metabolism, particularly m.4295A>G. This suggests that other factors, like the mitochondrial DNA haplogroup background, have an important influence on the severity of the consequences of these mutations, explaining why they may not be pathological in some cases. Indeed, it has been shown that the mtDNA haplogroup background can influence the phenotype of a pathogenic mutation [Hudson et al., 2007].
Moreover, it has been observed that for some mtDNA mutations, the presence of the mutation is necessary but not sufficient to induce the pathology, and a two-locus genetic model involving a nuclear modifier has been proposed [Carelli et al., 2003], where the nuclear background would have an important influence on the clinical expression of an mtDNA mutation.
Finally, it has been shown that also environmental factors can have an impact on the pathogenic effect of a mitochondrial DNA mutation. For instance, the use of aminoglycosides can lead to nonsyndromic deafness if the mutation m.1555A>G, in the 12S rRNA gene is present. Recently, it was shown that aminoglycosides can lead to mitochondrial dysfunction in orangutans, where this pathological mutation is the wild-type allele, rendering pathological a normally neutral variant [Pacheu-Grau et al., 2011] demonstrating the influence that external factors can have on the phenotype caused by a mutation.
As the mutations studied in this article, many other mtDNA mutations do not have complete penetrance, meaning that they require other factors as the ones cited above to become pathological. This implies that pathological mtDNA mutations are not as simple to determine, as it was believed, and that even variants that are thought to be neutral, like population polymorphisms, can be rendered pathological depending on the genetic constitution and the environment.
Several Mitochondrial Genes, Including Protein-Coding Genes, could be Involved in Nonsyndromic Deafness
It is a known fact that one mitochondrial pathology can be caused by different mutations in different mtDNA genes. However, diagnosis and research on a disease is generally focused only to the same gene in which a mutation was first associated to the phenotype or the disease, and screening of other mitochondrial genes is frequently dismissed. This could negatively affect the discovery of novel mtDNA pathogenic mutations responsible for the disease.
This is the case with nonsyndromic deafness. Indeed, the genetic screening of patients for this pathology is usually concentrated on the ribosomal RNA 12S gene (MT-RNR1) and the transfer RNA Serine (UCN) gene (MT-TS1), overlooking other mitochondrial genes.
Thus, as we have shown here, several mitochondrial genes, including genes coding for rRNA, tRNA, and respiratory chain complex subunits, can be important for nonsyndromic deafness, and continued research could identify new mitochondrial mutations responsible for nonsyndromic deafness, as well as unveil the role of different mitochondrial genes in this disease.
Acknowledgements
This work was supported by AFM (to N.G.C.) and by AMMI.