High-throughput sequencing of a 4.1 Mb linkage interval reveals FLVCR2 deletions and mutations in lethal cerebral vasculopathy†
Communicated by Claude Fèrec
Abstract
Rare lethal disease gene identification remains a challenging issue, but it is amenable to new techniques in high-throughput sequencing (HTS). Cerebral proliferative glomeruloid vasculopathy (PGV), or Fowler syndrome, is a severe autosomal recessive disorder of brain angiogenesis, resulting in abnormally thickened and aberrant perforating vessels leading to hydranencephaly. In three multiplex consanguineous families, genome-wide SNP analysis identified a locus of 14 Mb on chromosome 14. In addition, 280 consecutive SNPs were identical in two Turkish families unknown to be related, suggesting a founder mutation reducing the interval to 4.1 Mb. To identify the causative gene, we then specifically enriched for this region with sequence capture and performed HTS in a proband of seven families. Due to technical constraints related to the disease, the average coverage was only 7×. Nonetheless, iterative bioinformatic analyses of the sequence data identified mutations and a large deletion in the FLVCR2 gene, encoding a 12 transmembrane domain-containing putative transporter. A striking absence of alpha-smooth muscle actin immunostaining in abnormal vessels in fetal PGV brains, suggests a deficit in pericytes, cells essential for capillary stabilization and remodeling during brain angiogenesis. This is the first lethal disease-causing gene to be identified by comprehensive HTS of an entire linkage interval. Hum Mutat 31:1–8, 2010. © 2010 Wiley-Liss, Inc.
Introduction
Cerebral proliferative glomeruloid vasculopathy (PGV) is a severe autosomal recessive disorder of brain angiogenesis, resulting in abnormally thickened and aberrant perforating vessels, forming glomeruloids with inclusion-bearing endothelial cells. This peculiar vascular malformation was delineated by Fowler in 1972 in relation to a stereotyped, lethal fetal phenotype (MIM♯ 225790), associating hydranencephaly and hydrocephaly with limb deformities [Fowler et al., 1972]. PGV disrupts the developing central nervous system (CNS) but the reason for which abnormal angiogenesis is restricted to the CNS parenchyme remains unknown. Arthryogryposis, when present, appears to be a secondary result of CNS motoneuron degeneration, itself one potential outcome of perfusion failure. Since its earliest description, 42 PGV cases from 26 families have been reported on the basis of histological criteria [Bessieres-Grattagliano et al., 2009; Williams et al., 2010].
Identification of a causative gene for a very rare lethal syndrome is a challenge at many levels. The first issue is to find a family that allows the identification of a linkage interval. Such an interval may contain too many genes to make the classical subsequent strategy practical, consisting in designing primers that will permit sequencing of each exon of all the genes of the region. The second difficulty is that sequencing of all the exons is sometimes vain in light of the growing number of noncoding regions identified as pathogenic alleles [Benko et al., 2009; Kleinjan and van Heyningen, 2005; Lettice et al., 2003]. Finally, for prenatally lethal syndromes such as PGV, technical constraints such as poor-quality genomic DNA samples are added. Recent advances in biotechnology permit the sequencing of all the DNA, including the noncoding regions, in most genomic intervals. After homozygosity mapping of a 4.1-Mb region, we applied targeted genome capture by using a NimbleGen array and high-throughput Roche 454 GS FLX sequencing to the genomic DNA of the proband of six families. Bioinformatic analysis of the data allowed us to identify FLVCR2 (MIM♯ 610865) as the gene responsible for Fowler syndrome (FS). High-throughput sequencing (HTS) generated false positive and false negative results, in part due to insufficient sequencing coverage, and unless care is taken, these can engender the risk of missing mutations during the analysis.
Materials and Methods
Patients
The seven families analysed have been previously reported (Families I to VII) [Bessieres-Grattagliano et al., 2009]. Genomic DNA was extracted from frozen tissue or cultured amniocyte cells in fetal cases and from peripheral blood samples for parents and unaffected siblings.
Genome Linkage Screening and Linkage Analysis
Genome-wide homozygosity mapping was performed using 250 K Affymetrix single nucleotide polymorphism (SNP) arrays in five affected and three unaffected individuals of two Turkish and one French multiplex, consanguineous families. Data were evaluated by calculating multipoint lod scores across the whole genome using MERLIN software, assuming recessive inheritance with complete penetrance.
NimbleGen Sequence Capture and High-Throughput Sequencing
A custom sequence capture array was designed and manufactured by Roche NimbleGen (Madison, WI). Twenty-one micrograms of genomic DNA was used for sequence capture in accordance with the manufacturer's instructions (Roche NimbleGen) and a final amount of 3 µg of amplified enriched DNA was used as input for generating a ssDNA library for HTS; 25% lane of a Roche 454 GS FLX sequencer with Titanium reagents) yielding 135 Mb of sequence data per sample.
Capillary Sequencing of FLVCR2
Primers were designed in introns flanking the 10 exons using the “Primer 3” program (http://fokker.wi.mit.edu/primer3/input.htm) and are listed in Supp. Table S1. PCR were all performed in the same conditions, with a touchdown protocol consisting of denaturation for 30 sec at 96°C, annealing for 30 sec at a temperature ranging from 64 to 50°C (decreasing 1° during 14 cycles, then 20 cycles at 50°) and extension at 72°C for 30 sec. PCR products were treated with Exo-SAP IT (AP Biotech, Buckinghamshire, UK), and both strands were sequenced with the appropriate primer and the “BigDye” terminator cycle sequencing kit (Applied Biosystems Inc., Bedford, MA) and analyzed on ABI3130 automated sequencers. Mutation numbering is based on cDNA reference sequence NM_017791.2.
Immunohistochemistry
Immunohistochemistry was carried out on 6-µm selected sections using antisera directed against smooth muscle actin (diluted 1:800). Immunohistochemical procedures included a classical microwave pretreatment protocol in citrate buffer to aid antigen retrieval. Incubations were performed for 1 hr at room temperature, using the TECHMATE system (DAKOPATTS, Trappes, France). After incubation, histological slides were processed using the LSAB detection kit (DAKOPPATTS). Peroxidase was visualized by means of either 3-3′ diaminobenzidine or amino-ethyl carbazole.
Results
We have collected DNA from fetuses of seven families reported earlier (Families I to VII) [Bessieres-Grattagliano et al., 2009]. All 14 fetal cases bore the brain-specific angiogenic anomalies characteristic of PGV, resulting in thickened and aberrant perforating vessels and glomeruloids, as exemplified in Figure1A. Endothelial cells (ECs) were positive for CD34 in both control fetal brains (Fig. 1B) and in the tortuous glomerular capillaries (Fig. 1C). VEGF-A, although not normally expressed by small brain capillaries (Fig. 1D), was strikingly found in the glomerular ECs of PGV fetuses (Fig. 1E, arrowhead). Like normal ECs though, PGV ECs expressed VEGFR2 and, weakly, Glut-1 (not shown). CD68, characteristic of macrophages, was completely absent (data not shown). Numerous GFAP-positive astrocytes were observed throughout the cerebral mantle, with immunoreactive endfeet justaposed to glomeruloids (Fig. 1F). An antibody to alpha-smooth muscle actin (αSMA) stained vessels within the outer leptomeninges and the walls of perforating vessels in normal fetal brains (Fig. 1G). In contrast, although PGV meningeal vessels had similar aSMA expression, the dysplastic intraparenchymous vessels were irregularly stained, if at all (Fig. 1H), while most glomeruloid vessels were negative for aSMA (Fig. 1I).
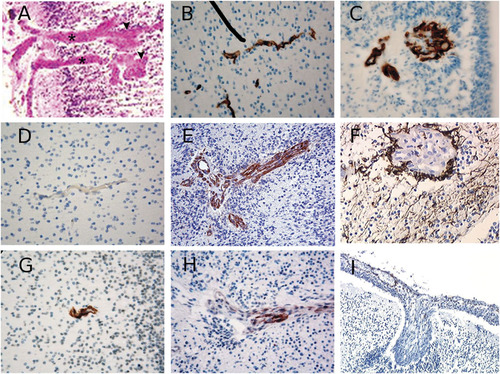
Marker analysis in Fowler syndrome fetal brain. A: Cortical plate of Fowler syndrome (FS) fetal brain (family IV) showing abnormal perforating vessels. Note the characteristic thickened vessels (asterisks), ending in glomeruloid formations (arrowheads), often devoid of recognizable lumina. CD34 capillary staining in (B) on a brain from a control, stage matched fetus and (C) from a FS fetus (family I). VEGF immunostaining arround (D) a brain parenchymal capillary from a control fetus in which it is essentially absent, and (E) from a FS fetus in which it appears markedly increased. F: GFAP astroglial immunostaining on a FS fetal brain. Alpha SMA immunostaining of pericytes on (G) a brain section from a control fetus versus (H and I) from two FS fetuses.
To find the molecular basis for this phenotype, we first undertook a genome-wide SNP analysis using an Affymetrix 250 K SNP chip with five affected and three unaffected members of two Turkish and one French multiplex, consanguineous families. Informed consent was obtained from all patients and their relatives; clinical data of all families have previously been reported [Bessieres-Grattagliano et al., 2009]. Genome-wide linkage analysis conducted with the MERLIN program revealed a 13-Mb genomic region on chromosome 14 from rs10151019 to rs12897284, with a lodscore of 5.4. Moreover, four affected sibs from the two Turkish families shared the same alleles for 280 consecutive SNPs, suggesting a founder effect and reducing the interval to 4.1 MB, from rs2803958 to rs11159220. These two families originated from villages 12 km apart in Khramanmaraps (central Turkey). Microsatellite marker analysis further confirmed the same disease allele in both families, and showed linkage in three additional families (Fig. 2).
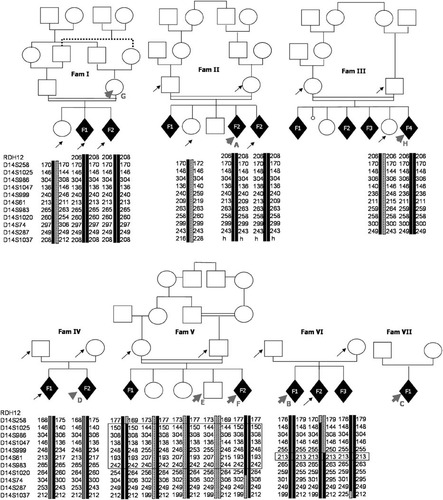
Pedigree and linkage analysis results. Pedigrees of families included in this study. Arrows indicate individuals for whom DNA was available, and arrowheads indicate the samples sequenced by HTS. Homozygosity or linkage was analyzed by microsatellite markers analysis and confirmed a founder effect by haplotype identity in two Turkish families (I and II) that were later discovered to carry the same FLVCR2 exon 2 to 10 deletion.
To identify the causative gene, we applied array-based sequence capture of the complete 4.1-MB region followed by high-throughput sequencing. DNA from one proband of six families, the heterozygous mother from family I, and a healthy brother not carrying the at-risk allele were selected (Fig. 2). Coverage varied from 2× to 12× in individuals depending on the integrity of their DNA (Table 1), with an average coverage depth of 7×; 60% (851,147) of the enriched reads were located on the targeted regions. Only 25% of the targeted regions reached 10× coverage depth.
| Individual | A (Fam II) | B (Fam VI) | C (Fam VII) | D (Fam IV) | F (Fam V) | G (Fam I) | H (Fam III) | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Origin | Turkish | French | French | French | Maroccan | Turkish | French | ||
| Coverage | 8,8X | 4X | 8,6X | 2,3X | 11,8X | 11,6X | 6,6X | 7X | |
| All | 2852 | 1804 | 2639 | 823 | 3067 | 3841 | 2005 | 17 031 | |
| Number of Variations (total) | Variations in E removed | 1379 | 790 | 1154 | 282 | 1527 | 2075 | 1182 | |
| SNP removed | 565 | 380 | 608 | 112 | 695 | 872 | 821 | ||
| Variations in E and SNPs removed | 546 | 300 | 465 | 80 | 569 | 750 | 747 | ||
| Number of variations on mRNA | Total | 100 | 74 | 105 | 41 | 87 | 139 | 58 | |
| Variations in E and SNP removed | 23 | 14 | 17 | 6 | 20 | 26 | 29 | ||
| Total | 41 | 22 | 44 | 13 | 42 | 60 | 25 | ||
| Number of variations on CDS | Variations in E and SNP removed | 8 | 2 | 4 | 2 | 11 | 12 | 15 | |
| Non synonymous | 22 | 8 | 23 | 9 | 23 | 28 | 13 | ||
| Non synonymous and SNP removed | 8 | 2 | 4 | 2 | 9 | 8 | 9 | 42 | |
| Next generation sequencing | Del exons 2–10 hmz | c.402C>G, p.Tyr134Stop hmz | c.251G>A, p.Arg84His htz | c.1056C>G, p.Thr352Arg hmz | c.1234G>C, p.Gly412Arg hmz | (mother) | – | ||
| FLVCR2 variations | Capillary sequencing | Del exons 2–10 hmz | c.402C>G, p.Tyr134Stop hmz | c.251G>A, p.Arg84His htz c.1192C>G, p.Leu398Val htz | c.1056C>G, p.Thr352Arg htz c.1289C>T, p.Thr430Met htz | c.1234G>C, p.Gly412Arg hmz | Del Ex 2–10 htz in mother | c.1076T>C, p.Leu359 Pro hmz | |
| Comparison and reason for discrepancy | confirmation | confirmation | Arg84His: confirmation Leu398Val: 7 reads, 4 with the mutation, but excluded for unidirectionality | T352R: 4 reads of only the mutated allele,T430M: no reads | confirmation | Deletion confirmed in foetuses (hmz), htz in parents | 2 reads of only the mutated allele |
- E is a healthy brother in family V not carrying the disease allele by haplotyping, and taken as healthy control. Mutation numbering is based on cDNA sequence with a “c.” symbol before the number, where +1 corresponds to the A of ATG translation codon (codon 1) of the cDNA reference sequences (NM_017791.2). Mutation names were checked by the Mutalyzer program [Wildeman et al., 2008]. SNP, single nucleotide polymorphisms; hmz, homozygous; htz, heterozygous.
The number of the detected variations was too large to handle them manually. To facilitate the analysis of these variations a specific genome browser was set up to visualize the locations of variations on the genome, and at the same time an analysis tool has been developed. This analysis tool applied a series of filters to the identified variations. These filters were based on the following criteria: (1) the quality of the sequence variant measured as the number of reads that detected the variant, (2) the presence or absence of variants in public databases such as dbSNP and HapMap, (3) the presence or absence of the variants among the studied samples, (4) annotation of the sequence variants based on their location (intron, exon, etc.), and the characteristics of the resulting change such as synonymous, nonsynonymous or stop mutation. Filtered results were visualized in an interactive table permitting us to sort and analyze the results. Thus, initial analysis of the sequence data that met an arbitrary threshold of at least three reads, of which at least one was required to be in the opposing orientation, detected a total of 23,262 variations, 17,031 of which were on chromosome 14 (73%, Supp. Table S2). Of these, 3,457 variants were found to not correspond to known SNPs, and were absent from the normal control individual (E). After initial exclusion of nonexonic and synonymous variants, 42 variants in 29 candidate genes remained. In 20 of these genes, a single variation was found in one individual, whereas two and three variations were found in six and two genes, respectively (Fig. 3).
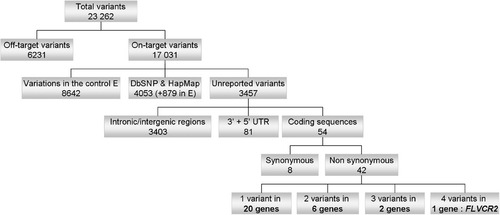
Summary of HTS data analysis. This diagram illustrates the flow chart of HTS data analysis. After elimination of variants found outside of the mapping region (27% of total variants) and those corresponding to known SNPs (29% of on-target variants) or shared with the control individual E (50% of on-target variants), HTS identified 54 variants in coding sequences, eight of which were synonymous. The remaining 46 variants were located in 29 candidate genes, 20 of which were excluded because only one variant was identified. Finally, only one gene, FLVCR2, presented four variants.
FLVCR2 was the only gene with variations identified in four out of seven individuals. In addition, careful examination of the FLVCR2 locus in the proband of family II revealed a homozygous deletion of exons 2 to 10, as the absence of both nucleotide variations and reads over a 46.8-kb genomic region (Fig. 4A). The deletion was confirmed to segregate in families I and II, and cloning of the breakpoints revealed the inclusion of the last two exons of the neighboring C14orf1 gene, with no repeated DNA sequences at the boundaries. It is noteworthy that this deletion was not detected by Affymetrix 250 K SNP chip. Indeed, only one SNP was located in the nondeleted portion of intron 1. Direct sequencing of the 10 exons of FLVCR2 (Supp. Table S2), identified mutations in two additional families (Table 1), such that mutant FLVCR2 alleles were identified in each of the seven families studied (five homozygotes and two compound heterozygotes; Table 1 and Fig. 4B).
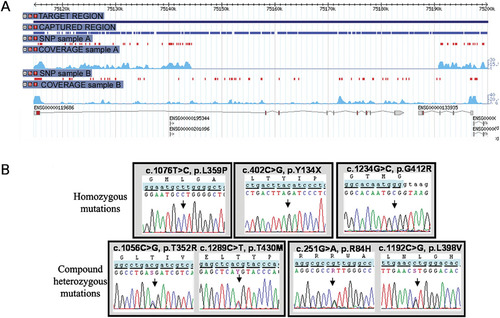
FLVCR2 deletion and mutations. A: Genome browser view centered on the FLVCR2 locus (ENSG00000119686) showing all variations (red dots) and reads coverage (light blue) in individuals A (fetus, family II) and B (fetus, family V). Note the absence of variations and reads in individual A, suggesting a homozygous deletion of exons 2 to 10, as well as the two final exons of the adjacent c14orf1 transcript (ENSG00000133935). B: Chromatograms of FLVCR2 homozygous (upper panel) and compound heterozygous mutations (lower panel).
Reasons for false-negative results using HTS approaches are summarized in Table 1, and emphasize the need for complementary confirmation. In particular, in family IV, a second heterozygous mutation was found by direct resequencing, although it had an apparently homozygous mutation as indicated by the HTS analysis. In family III, the homozygous mutation found with Sanger sequencing had only been read two times in the HTS and had thus been excluded by the stringency of the filter. As a third example, the second heterozygous mutation in family VII had been read four times but was excluded for unidirectionality. Interestingly, in family VI, not known to be consanguineous, the identical nonsense mutation was found in the three affected sibs (homozygous in fetuses and heterozygous in parents), suggesting more distant consanguinity or a founder effect.
FLVCR2 is a member of the major facilitator superfamily (MFS) of transporter proteins, that shuttle small molecules in response to ion gradients [Pao et al., 1991]. Like other MFS members, FLVCR2 is predicted to contain 12 membrane-spanning segments and six extracellular loops. As shown in Figure 5A, the three homozygous mutations are predicted to alter an amino acid localized to one transmembrane domain (TM): TM2 in family VI, TM8 in family III, and TM10 in family V. In family IV, one of the two mutations alters an amino acid predicted to be localized in TM8 and the other in the intracellular loop 5.
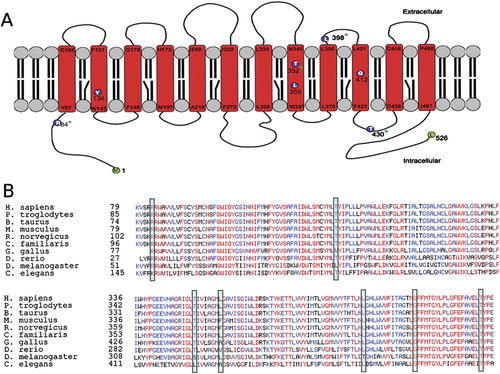
Localization of mutations in FLVCR2 and conservation of mutated FLVCR2 amino acids. A: Localization of mutations on a secondary structure prediction of the FLVCR2 transporter. The three homozygous mutations are predicted to alter an amino acid localized in one of the 12 transmenbrane (TM) domains: p.Y134X is located in TM2, p.L359P in TM8, and p.G412R in TM10. Compound heterozygous mutations in family VI alter amino acids at the N-terminal cytoplasmic end and in the extracellular loop 5 (blue asterisk). Compound heterozygous mutations in family IV alter an amino acid predicted to be localized in TM8 and in the intracellular loop 5 (green asterisk). B: Alignment and conservation of mutated FLVCR2 amino acids. Sequences for FLVCR2 from 10 different species have been aligned using the Multialin tool (“mnultiple sequence alignment with hierarchical clustering”) [Corpet, 1988]. Highly conserved amino acids are represented in red, moderately conserved amino acids are in blue and nonconserved ones are in black. Mutated amino acids are boxed.
Amino acid sequence alignment for FLVCR2 from 10 different species showed that T430 and G412 have been conserved because our common ancestor with Caenorhabditis elegans, whereas R84 has been conserved in common with Drosophila melanogaster (Fig. 5B). T352R and L398V alter residues less evolutionary conserved, especially L398V. However, those mutations are absent from both the dbSNP and the 1000 Genome database not yet integrated in dbSNP. Although the L398V mutation was predicted to be benign by the Polyphen algorithm (http://genetics.bwh.harvard.edu/pph/), the T352R mutation as well as the other missense mutations identified in this study were predicted to be damaging to protein function. Thus, the pathogenicity of these two last mutations is likely but not totally proven. In total, eight different mutations including one nonsense mutation (homozygous in family VI), six missense mutations, and one homozygous deletion in two families (I and II) have been found in FLVCR2.
Discussion
PGV is a very rare and lethal genetic condition. Since its first description, 42 cases from 26 families have been reported on the basis of histological criteria of PGV [Bessieres-Grattagliano et al., 2009; Williams et al., 2010]. In the 16 fetuses of our series born to eight unrelated families, neuropathological analysis defined a diffuse form of encephaloclastic prolifrative vasculopathy (EPV), affecting the entire CNS and resulting in classical PGV with pterygia and a severe fetal akinesia deformation sequence in 14 cases. In contrast, two cases from the single family IV presented a more focal form of EPV, without spinal cord involvement and subsequent arthrogryposis/pterygia. Identification of FLVCR2 mutations in this family suggests that the anteroposterior extent of CNS degeneration can be variable, and that PGV may be an extreme phenotype of a broader spectrum of proliferative vasculopathies. Stabilization of newly formed capillary sprouts during angiogenesis requires interactions of endothelial cells with mural support cells, known as pericytes. The regionally restricted distribution of PGV in family IV might be linked to the embryonic lineage of the telencephalic pericytes, of a distinct neural crest cell origin from those of the spinal cord [Etchevers et al., 2001]. Interestingly, immunostaining for αSMA (a marker for mature pericytes) in fetal PGV brains was drastically reduced in the PGV within the CNS while normal aSMA expression was found in the leptomeninges (Fig. 1I). Further studies should elucidate whether this observed effect on pericytes is the primary cause or an effect of this disease.
Recently, FLVCR2 mutations were also reported in five families with Fowler syndrome [Meyer et al., 2010], with the same homozygous Thr430Arg mutation in three families, and two compound heterozygous cases. Interestingely, Thr430Arg is associated with both forms of the disease, namely, with or without spinal cord involvement, suggesting no genotype phenotype correlations. It is noteworthy that the mutation concerned the same codon (Thr430) as in our family IV, the only one of our series without spinal cord involvement. More recently, Lalonde et al. [2010] also reported four FLVCR2 compound mutations in two FS families with spinal cord involvement. Interestingly, the only missense mutation predicted to be “benign” in our study (L398V) was identified by two distinct approaches in a common case reported by both Lalonde et al. [2010] and Meyer et al. [2010], adding to the likely pathogenicity of this variation. To sum up, 15 different FLVCR2 mutations (including those described in our study) have now been reported in 13 cases: one large deletion, two nonsense mutations, one splice site mutation, one insertion/deletion change, and 10 missense variations.
The FLVCR2 gene encodes a transmembrane protein that belongs to the MFS of secondary carriers that transport small solutes such as calcium [Pao et al., 1991]. It is closely related in both sequence and topology to the better-known FLVCR1, sharing 60% amino acid identity [Lipovich et al., 2002]. FLVCR1 has been identified as the receptor for a feline leukemia virus (FeLV-C), and like FLVCR2 and other MFS members, is predicted to contain 12 membrane-spanning segments and six extracellular loops. A single mutation in the sixth extracellular loop is sufficient to confer FeLV-C receptor activity on FLVCR2, which does not otherwise bind the native virus [Brown et al., 2006]. However, FLVCR2 functions as a receptor for the FeLV-C variant FY981 [Shalev et al., 2009]. FLVCR1 is found only in hematopoietic tissues, the pancreas, and kidney [Tailor et al., 1999], but rodent Flvcr2 is widely expressed during embryonic development, in particular within the CNS and in the vessels of the maturing retina, and human FLVCR2, within the fetal pituitary [Brasier et al., 2004]. FLVCR1 has been shown to function as a heme exporter, essential for erythropoiesis [Quigley et al., 2004]. Interestingly, the five glutamate residues in the C-terminal putative coiled-coil domain of FLVCR2, not present in FLVCR1, may serve an analogous function to the same ferric ion-binding glutamate sequence in glycine-extended gastrin, by stimulating cell proliferation [He et al., 2004]. Based on the cell types in which it is expressed and MFS transport of chelated complexes of divalent metal ions, the FLVCR2 transporter was postulated to be a gatekeeper for the controlled entry of calcium into target cell types [Brasier et al., 2004]. Calcium signaling is involved in virtually all cellular processes and its homeostasis is tightly regulated. Angiogenic factors such as VEGF-A and FGF2 induce a transient increase of endothelial cell intracellular calcium concentrations, which acts as a second messenger to induce proliferation, among other effects [Tomatis et al., 2007]. Blood vessels are susceptible to responding to angiogenic signals and undergoing calcification when their pericytic coverage has been disrupted [Collett and Canfield, 2005], both of which signs we have observed in PGV patient brain sections.
HTS of the entire exome has been used so far to identify disease-causing genes in the rare Miller and Bartter syndromes, respectively [Choi et al., 2009; Ng et al., 2010]. Recently, targeted exon-specific sequencing within a restricted 40 MB linkage interval allowed the identification of an additional gene for Familial Exudative Vitreoretinopathy [Nikopoulos et al., 2010]. Our study underlines the use of HTS for the coverage of an entire linkage interval with no compelling candidate genes and no justification for the exclusion of noncoding regions. Our nested analysis approach led rapidly to the identification of a disease-causing gene. Although it further demonstrates the power of this new technology, it also highlights other potential risks of missing mutations during data analyses. The number of patients, diagnostic accuracy and genetic homogeneity allowed us to compensate for low capture efficiency due to suboptimal DNA quality, and in the future, as the technology develops, furthering the depth of coverage should ensure a better distinction of background from true mutations. Finally, identification of the gene for Fowler syndrome will permit accurate genetic counseling for PGV and prenatal diagnosis, in particular, for the late-onset forms of the disease without spinal cord involvement.
Acknowledgements
We are grateful to families and to the French Society of Fetal Pathology (SOFFOET) for participating in the study. We thank Chantal Esculpavit for technical help. Grant sponsor: GIS-Maladies Rares. Grant sponsor: U.S. National Institute of Health (NIH) (grant NS039818 to S.T.).




